Is There an Association between a Tonsillar Diffuse Large B-Cell Lymphoma Arising after a Neck Squamous Cell Carcinoma of Occult Primary? A Case Report and Extensive Literature Review
Abstract
1. Introduction
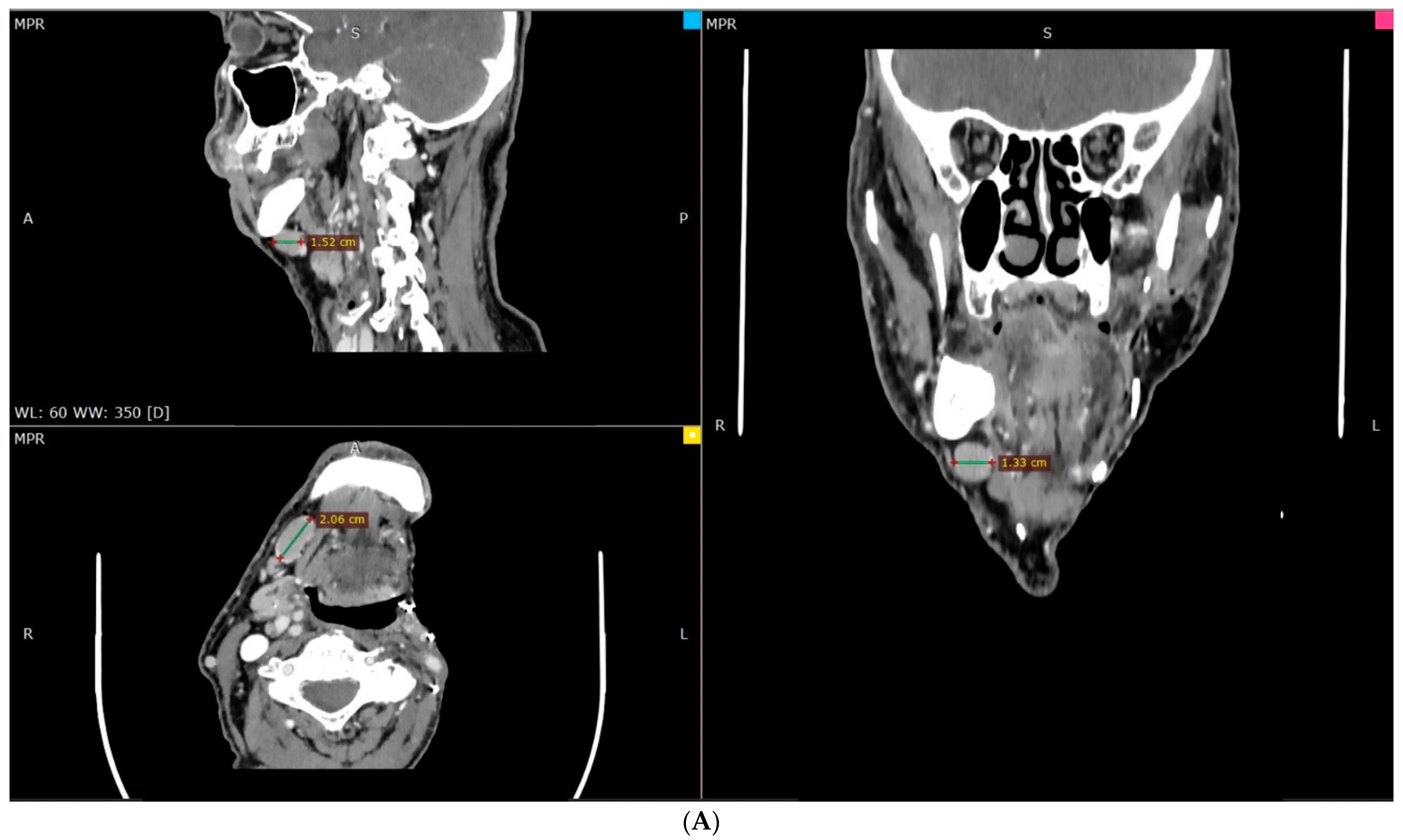
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; de Oliveira Araujo, I.B.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Cairo, M.S.; Perkins, S.L. Hematological Malignancies in Children, Adolescents and Young Adults; World Scientific: Singapore, 2012. [Google Scholar]
- Hartge, P.; Devesa, S.S.; Fraumeni, J.F. Hodgkin’s and non-Hodgkin’s lymphomas. Cancer Surv. 1994, 19–20, 423–453. [Google Scholar]
- Grulich, A.E.; Vajdic, C.M.; Cozen, W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 405–408. [Google Scholar] [CrossRef]
- SEER. Non-Hodgkin Lymphoma-Cancer Stat Facts [Internet]. National Cancer Institute, 2011. Available online: https://seer.cancer.gov/statfacts/html/nhl.html (accessed on 1 May 2022).
- Shipp, M.A. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N. Engl. J. Med. 1993, 329, 987–994. [Google Scholar]
- Tezer, M.S.; Tuncel, U.; Özlügedik, S.; Uzun, M.; Kulaçoğlu, S.; Ünal, A. Coexistence of laryngeal squamous cell carcinoma and non-Hodgkin’s lymphoma with nasopharyngeal involvement. J. Laryngol. Otol. 2006, 120, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Habermann, W.; Anderhuber, W.; Humer-Fuchst, U.; Stammberger, H. Simultaneous occurrence of metastatic tonsillar squamous cell carcinoma and angioimmunoblastic T-cell lymphoma in a cervical lymph node. J. Laryngol. Otol. 1997, 111, 580–582. [Google Scholar] [CrossRef]
- Watanabe, N.; Inohara, H.; Akahani, S.; Yamamoto, Y.; Moriwaki, K.; Kubo, T. Synchronous squamous cell carcinoma and malignant lymphoma in the head and neck region. Auris Nasus Larynx 2007, 34, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Kader, I.; Leavers, B.; Shashinder, S.; Wylie, B.; Chi, K.-K.; Sundaresan, P. Synchronous or metachronous lymphoma and metastatic cutaneous squamous cell carcinoma in the head and neck region: A diagnostic and management dilemma. J. Laryngol. Otol. 2016, 130 (Suppl. 4), S45–S49. [Google Scholar] [CrossRef] [PubMed]
- Millwaters, M.; Khan, N.; Halfpenny, W. Simultaneous lymphoma and squamous cell carcinoma presenting as a neck lump. Br. J. Oral Maxillofac. Surg. 2008, 46, 144–145. [Google Scholar] [CrossRef]
- Singh, N.J.; Tripathy, N.; Roy, P.; Manikantan, K.; Arun, P. Simultaneous Triple Primary Head and Neck Malignancies: A Rare Case Report. Head Neck Pathol. 2016, 10, 233–236. [Google Scholar] [CrossRef]
- Yildirim, M.; Belli, S.; Ozsoy, S.; Taskin, U. Primary triple head and neck tumors: Laryngeal squamous cell carcinomas, Kaposi’s sarcoma, and non-Hodgkin’s lymphoma. Indian J. Pathol. Microbiol. 2019, 62, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hamilton, L.; Montgomery, J.; Stewart, M. Head and neck squamous cell cancer associated with lymphoproliferative malignancies is aggressive. J. Laryngol. Otol. 2020, 134, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Shibuya, H.; Hayashi, K.; Ayukawa, F. Radiation-induced cancer after radiotherapy for non-Hodgkin’s lymphoma of the head and neck: A retrospective study. Radiat. Oncol. 2009, 4, 21. [Google Scholar] [CrossRef]
- Thakur, J.S.; Minhas, R.S.; Mohindroo, N.K.; Sharma, D.R.; Mohindroo, S.; Thakur, A. Primary non-Hodgkin’s lymphoma of the infratemporal fossa: A rare case report. Head Neck Oncol. 2009, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.J.; Sachs, R.K.; Wilson, D.J. Radiation-induced cancer: A modern view. Br. J. Radiol. 2012, 85, e1166–e1173. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A. Infectious Agents as Causes of Non-Hodgkin Lymphoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Hartge, P.; Smith, M.T.; Ferretti, G.; Felici, A.; Cognetti, F. Environmental and behavioral factors and the risk of non-Hodgkin lymphoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Bagan, J.V.; Carbonell, F.; Gómez, M.J.; Sánchez, M.; Navarro, A.; Leopoldo, M.; Bagán, L.; Zapater, E. Extra-nodal B-cell non-Hodgkin’s lymphomas of the head and neck: A study of 68 cases. Am. J. Otolaryngol. 2015, 36, 57–62. [Google Scholar] [CrossRef]
- Krishnan, B.; Morgan, G.J. Non-Hodgkin Lymphoma Secondary to Cancer Chemotherapy. Cancer Epidemiol. Biomark. Prev. 2007, 16, 377–380. [Google Scholar] [CrossRef]
- Takano, S.; Matsushita, N.; Oishi, M.; Okamoto, S.; Teranishi, Y.; Yokota, C.; Iguchi, H. Site-specific analysis of B-cell non-Hodgkin’s lymphomas of the head and neck: A retrospective 10-year observation. Acta Otolaryngol. 2015, 135, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Pileri, S.; Ponzoni, M. Pathology of nodal marginal zone lymphomas. Best Pract. Res. Clin. Haematol. 2016, 30, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, M.; Stryjewska-Makuch, G.; Janik, M.; Kolebacz, B.; Lisowska, G.; Ścierski, W. Head and neck lymphomas—A retrospective ten-year observation. Contemp. Oncol. 2017, 1, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kang, K.; Jung, H.; Park, Y.M.; Cho, J.-G.; Baek, S.-K.; Kwon, S.-Y.; Jung, K.-Y.; Woo, J.-S. Extranodal involvement of diffuse large B-cell lymphoma in the head and neck: An indicator of good prognosis. Auris Nasus Larynx 2019, 46, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidou, K.; Dimitrakopoulos, J.; Iordanidis, F.; Gkagkalis, A. Extranodal non-hodgkin lymphomas of the oral cavity and maxillofacial region: A clinical study of 58 cases and review of the literature. J. Oral Maxillofac. Surg. 2012, 70, 2776–2785. [Google Scholar] [CrossRef]
- Yan, S.; Ma, J.; Yang, M.; Liu, B.; Li, S.; Yang, L.; Zhang, Q.; Li, X. Analysis of the Clinicopathologic Characteristics and Prognosis of Head and Neck Lymphoma. Anal. Cell. Pathol. 2022, 2022, 4936099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Zheng, Y.M.; Xie, J.M.; Zhu, J.; Song, Y.; Teng, X.M.; Liu, W.M.; Ding, Y.M.; Huang, Y.M.; Zhou, X. Long-term Tumor-free Survival with Untreated Primary Diffuse Large B-cell Lymphoma of the Tonsil. Am. J. Surg. Pathol. 2015, 39, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.; Musthyala, B.; Ahmed, F.; Murthy, S.S.; Raju, K.V.V.N. A tale of synchronous lung carcinoma and diffuse large B-cell lymphoma of ileum: A rare combination. Lung India 2015, 32, 398–401. [Google Scholar] [CrossRef]
- Sugimoto, K.D.; Uejima, S.D.; Uchiyama, Y.D.; Yasue, R.D.; Nambu, K.D.; Ishikawa, J.D.; Koma, Y.D.; Akita, T.D.; Toh, T.D.; Fujimoto, T.D. Metachronous primary cancer of the tongue and malignant lymphoma of the small intestine. Medicine 2021, 100, e24806. [Google Scholar] [CrossRef]
- Choi, H.; Lee, H.W.; Ann, H.W.; Kim, J.K.; Kang, H.P.; Kim, S.W.; Ku, N.S.; Han, S.H.; Kim, J.M.; Choi, J.Y. A Case of Rectal Squamous Cell Carcinoma with Metachronous Diffuse Large B Cell Lymphoma in an HIV-Infected Patient. Infect. Chemother. 2014, 46, 257–260. [Google Scholar] [CrossRef][Green Version]



| Case | Age (in Years) | Gender | Squamous Cell Carcinoma (SCC) Diagnosis | Lymph Node Involvement in Neck | Lymphoma Diagnosis | Time Lapse between the 2 Diagnoses |
|---|---|---|---|---|---|---|
| Habermann et al., 1997 [9] | 64 | Male | Left tonsillar SCC | yes | T-cell NHL, lymph nodes | Simultaneously |
| Watanabe et al., 2007 [10] | 57 | Male | oropharyngeal SCC, cT2N2bM0 | yes | Nasopharyngeal ML, stage IE | Simultaneously |
| Kader et al., 2016 [11] | 8 patients, mean age 79.5 | - | Skin SCC (patients with oral SCC excluded from study) | yes | Low-grade non-Hodgkin lymphoma or chronic lymphoid leukaemia | Mean time 3.4 years, lymphoma first |
| Millwaters et al., 2008 [12] | 70 | Male | Left tongue base SCC | yes | Follicular non-Hodgkin lymphoma | Simultaneously |
| Sigh et al., 2016 [13] | 71 | Male | Laryngeal SCC (and papillary thyroid carcinoma) | yes | Nodal marginal zone lymphoma | Simultaneously |
| Yildirim et al., 2019 [14] | 74 | Male | Laryngeal SCC | yes (and Kaposi sarcoma in cervical lymph node) | Non-Hodgkin lymphoma in tongue base | Simultaneously |
| Li et al., 2020 [15] | 4 patients, mean age 56.5 | Male | Metachronous head and neck SCC | yes | Non-Hodgkin lymphoma or chronic lymphocytic leukaemia | 1–4 years, lymphoma first |
| Toda et al., 2009 [16] | 4 patients, mean age 53 | 3 Male, 1 Female | Metachronous tongue, gum and maxillary SCC | unknown | Non-Hodgkin lymphoma | 8.7–22.7 years, lymphoma first |
| Thakur et al., 2009 [17] | 41 | Female | Metachronous right retromolar SCC | Unknown | Non-Hodgkin lymphoma, right infratemporal fossa | 1 year, lymphoma first |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatsis, D.; Niakou, A.; Paraskevopoulos, K.; Papadopoulou, S.; Vahtsevanos, K. Is There an Association between a Tonsillar Diffuse Large B-Cell Lymphoma Arising after a Neck Squamous Cell Carcinoma of Occult Primary? A Case Report and Extensive Literature Review. Hematol. Rep. 2024, 16, 260-269. https://doi.org/10.3390/hematolrep16020026
Tatsis D, Niakou A, Paraskevopoulos K, Papadopoulou S, Vahtsevanos K. Is There an Association between a Tonsillar Diffuse Large B-Cell Lymphoma Arising after a Neck Squamous Cell Carcinoma of Occult Primary? A Case Report and Extensive Literature Review. Hematology Reports. 2024; 16(2):260-269. https://doi.org/10.3390/hematolrep16020026
Chicago/Turabian StyleTatsis, Dimitris, Athena Niakou, Konstantinos Paraskevopoulos, Stavroula Papadopoulou, and Konstantinos Vahtsevanos. 2024. "Is There an Association between a Tonsillar Diffuse Large B-Cell Lymphoma Arising after a Neck Squamous Cell Carcinoma of Occult Primary? A Case Report and Extensive Literature Review" Hematology Reports 16, no. 2: 260-269. https://doi.org/10.3390/hematolrep16020026
APA StyleTatsis, D., Niakou, A., Paraskevopoulos, K., Papadopoulou, S., & Vahtsevanos, K. (2024). Is There an Association between a Tonsillar Diffuse Large B-Cell Lymphoma Arising after a Neck Squamous Cell Carcinoma of Occult Primary? A Case Report and Extensive Literature Review. Hematology Reports, 16(2), 260-269. https://doi.org/10.3390/hematolrep16020026








