Additional Genetic Alterations and Clonal Evolution of MPNs with Double Mutations on the MPL Gene: Two Case Reports
Abstract
1. Introduction
2. Case Presentation
2.1. Case 1
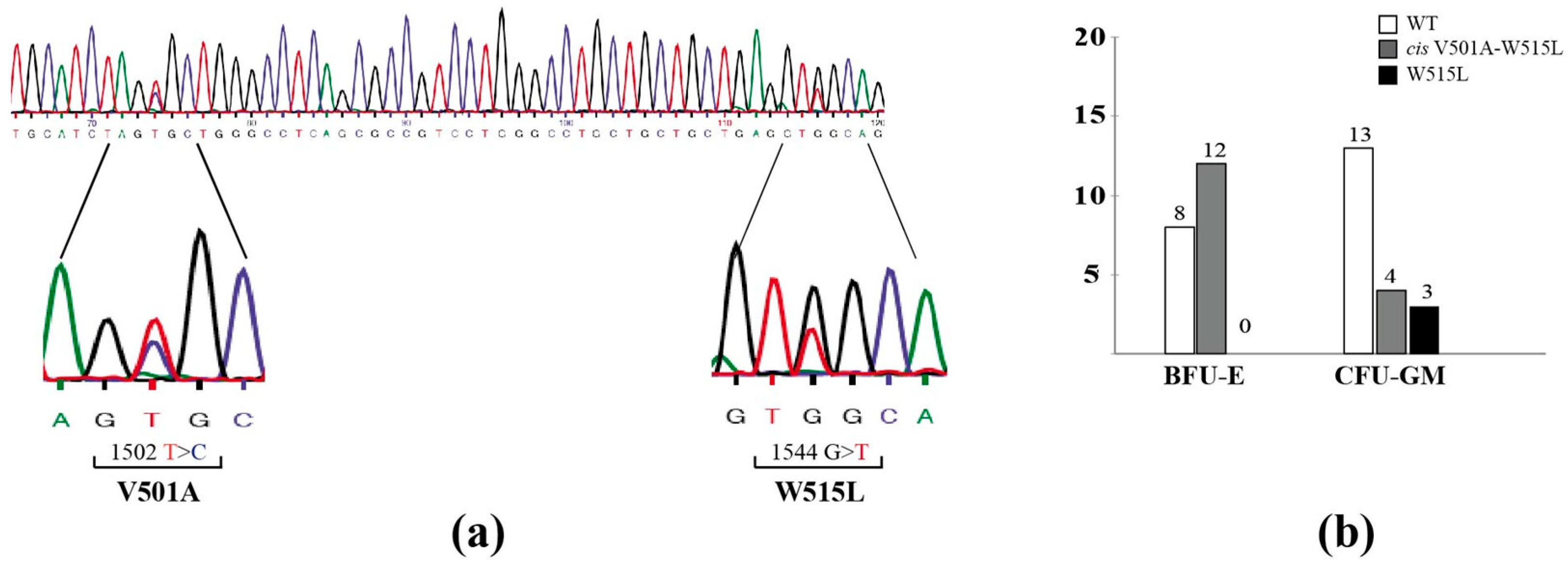
2.2. Case 2
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, G.A.; Stella, S.; Pennisi, M.S.; Pirosa, C.; Fermo, E.; Fabris, S.; Cattaneo, D.; Iurlo, A. The Role of New Technologies in Myeloproliferative Neoplasms. Front. Oncol. 2019, 9, 321. [Google Scholar] [CrossRef]
- Guglielmelli, P.; Vannucchi, A.M. Current management strategies for polycythemia vera and essential thrombocythemia. Blood Rev. 2020, 42, 100714. [Google Scholar] [CrossRef]
- Pandey, R.; Kapur, R. Targeting phosphatidylinositol-3-kinase pathway for the treatment of Philadelphia-negative myeloproliferative neoplasms. Mol. Cancer 2015, 14, 118. [Google Scholar] [CrossRef]
- Hammaren, H.M.; Virtanen, A.T.; Abraham, B.G.; Peussa, H.; Hubbard, S.R.; Silvennoinen, O. Janus kinase 2 activation mechanisms revealed by analysis of suppressing mutations. J. Allergy Clin. Immunol. 2019, 143, 1549–1559.e1546. [Google Scholar] [CrossRef]
- Jang, M.A.; Choi, C.W. Recent insights regarding the molecular basis of myeloproliferative neoplasms. Korean J. Intern. Med. 2020, 35, 1–11. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Mead, A.J. Heterogeneity in myeloproliferative neoplasms: Causes and consequences. Adv. Biol. Regul. 2019, 71, 55–68. [Google Scholar] [CrossRef]
- How, J.; Hobbs, G.S.; Mullally, A. Mutant calreticulin in myeloproliferative neoplasms. Blood 2019, 134, 2242–2248. [Google Scholar] [CrossRef]
- Plo, I.; Bellanne-Chantelot, C.; Mosca, M.; Mazzi, S.; Marty, C.; Vainchenker, W. Genetic Alterations of the Thrombopoietin/MPL/JAK2 Axis Impacting Megakaryopoiesis. Front. Endocrinol. 2017, 8, 234. [Google Scholar] [CrossRef]
- He, X.; Chen, Z.; Jiang, Y.; Qiu, X.; Zhao, X. Different mutations of the human c-mpl gene indicate distinct haematopoietic diseases. J. Hematol. Oncol. 2013, 6, 11. [Google Scholar] [CrossRef]
- Vainchenker, W.; Kralovics, R. Genetic basis and molecular pathophysiology of classical myeloproliferative neoplasms. Blood 2017, 129, 667–679. [Google Scholar] [CrossRef]
- Pietra, D.; Brisci, A.; Rumi, E.; Boggi, S.; Elena, C.; Pietrelli, A.; Bordoni, R.; Ferrari, M.; Passamonti, F.; De Bellis, G.; et al. Deep sequencing reveals double mutations in cis of MPL exon 10 in myeloproliferative neoplasms. Haematologica 2011, 96, 607–611. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, X.; Wang, X.; Zhang, Z.; Yeh, C.H.; Uyeji, J.; Albitar, M. MPL mutation profile in JAK2 mutation-negative patients with myeloproliferative disorders. Diagn. Mol. Pathol. 2011, 20, 34–39. [Google Scholar] [CrossRef]
- Elsayed, A.G.; Ranavaya, A.; Jamil, M.O. MPL Y252H anMd PL F126fs mutations in essential thrombocythemia: Case series and review of literature. Hematol. Rep. 2019, 11, 7868. [Google Scholar] [CrossRef] [PubMed]
- Grinfeld, J.; Nangalia, J.; Baxter, E.J.; Wedge, D.C.; Angelopoulos, N.; Cantrill, R.; Godfrey, A.L.; Papaemmanuil, E.; Gundem, G.; MacLean, C.; et al. Classification and Personalized Prognosis in Myeloproliferative Neoplasms. N. Engl. J. Med. 2018, 379, 1416–1430. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Nagaharu, K.; Ohishi, K.; Nakamura, M.; Ikejiri, M.; Nakatani, K.; Mizutani, M.; Tamaki, S.; Ikeda, T.; Tawara, I.; et al. MPL exon 10 mutations other than canonical MPL W515L/K mutations identified by in-house MPL exon 10 direct sequencing in essential thrombocythemia. Int. J. Hematol. 2021, 113, 618–621. [Google Scholar] [CrossRef]
- Usseglio, F.; Beaufils, N.; Calleja, A.; Raynaud, S.; Gabert, J. Detection of CALR and MPL Mutations in Low Allelic Burden JAK2 V617F Essential Thrombocythemia. J. Mol. Diagn. 2017, 19, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, E.; Sun, Q.; Ma, J.; Li, Q.; Cao, Z.; Wang, J.; Jia, Y.; Zhang, H.; Song, Z.; et al. The Prevalence of JAK2, MPL, and CALR Mutations in Chinese Patients with BCR-ABL1-Negative Myeloproliferative Neoplasms. Am. J. Clin. Pathol. 2015, 144, 165–171. [Google Scholar] [CrossRef]
- Magor, G.W.; Tallack, M.R.; Klose, N.M.; Taylor, D.; Korbie, D.; Mollee, P.; Trau, M.; Perkins, A.C. Rapid Molecular Profiling of Myeloproliferative Neoplasms Using Targeted Exon Resequencing of 86 Genes Involved in JAK-STAT Signaling and Epigenetic Regulation. J. Mol. Diagn. 2016, 18, 707–718. [Google Scholar] [CrossRef]
- Tashkandi, H.; Moore, E.M.; Tomlinson, B.; Goebel, T.; Sadri, N. Co-occurrence of type I CALR and two MPL mutations in patient with primary myelofibrosis. Ann. Hematol. 2017, 96, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: Document summary and in-depth discussion. Blood Cancer J. 2018, 8, 15. [Google Scholar] [CrossRef]
- Romano, C.; Di Gregorio, S.; Pennisi, M.S.; Tirro, E.; Broggi, G.; Caltabiano, R.; Manzella, L.; Ruggieri, M.; Vigneri, P.; Di Cataldo, A. Multiple primary malignances managed with surgical excision: A case report with next generation sequencing analysis. Mol. Biol. Rep. 2022, 49, 9059–9064. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.A.; Campbell, P.J.; Scott, L.M.; Bench, A.J.; Erber, W.N.; Bareford, D.; Wilkins, B.S.; Reilly, J.T.; Hasselbalch, H.C.; Bowman, R.; et al. MPL mutations in myeloproliferative disorders: Analysis of the PT-1 cohort. Blood 2008, 112, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chaligne, R.; James, C.; Tonetti, C.; Besancenot, R.; Le Couedic, J.P.; Fava, F.; Mazurier, F.; Godin, I.; Maloum, K.; Larbret, F.; et al. Evidence for MPL W515L/K mutations in hematopoietic stem cells in primitive myelofibrosis. Blood 2007, 110, 3735–3743. [Google Scholar] [CrossRef]
- Foster, R.; Byrnes, E.; Meldrum, C.; Griffith, R.; Ross, G.; Upjohn, E.; Braue, A.; Scott, R.; Varigos, G.; Ferrao, P.; et al. Association of paediatric mastocytosis with a polymorphism resulting in an amino acid substitution (M541L) in the transmembrane domain of c-KIT. Br. J. Dermatol. 2008, 159, 1160–1169. [Google Scholar] [CrossRef]
- Iurlo, A.; Gianelli, U.; Beghini, A.; Spinelli, O.; Orofino, N.; Lazzaroni, F.; Cambiaghi, S.; Intermesoli, T.; Rambaldi, A.; Cortelezzi, A. Identification of kit(M541L) somatic mutation in chronic eosinophilic leukemia, not otherwise specified and its implication in low-dose imatinib response. Oncotarget 2014, 5, 4665–4670. [Google Scholar] [CrossRef]
- Duminuco, A.; Nardo, A.; Giuffrida, G.; Leotta, S.; Markovic, U.; Giallongo, C.; Tibullo, D.; Romano, A.; Di Raimondo, F.; Palumbo, G.A. Myelofibrosis and Survival Prognostic Models: A Journey between Past and Future. J. Clin. Med. 2023, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Pardanani, A.D.; Levine, R.L.; Lasho, T.; Pikman, Y.; Mesa, R.A.; Wadleigh, M.; Steensma, D.P.; Elliott, M.A.; Wolanskyj, A.P.; Hogan, W.J.; et al. MPL515 mutations in myeloproliferative and other myeloid disorders: A study of 1182 patients. Blood 2006, 108, 3472–3476. [Google Scholar] [CrossRef] [PubMed]
- Lasho, T.L.; Pardanani, A.; McClure, R.F.; Mesa, R.A.; Levine, R.L.; Gilliland, D.G.; Tefferi, A. Concurrent MPL515 and JAK2V617F mutations in myelofibrosis: Chronology of clonal emergence and changes in mutant allele burden over time. Br. J. Haematol. 2006, 135, 683–687. [Google Scholar] [CrossRef]
- Serrati, S.; De Summa, S.; Pilato, B.; Petriella, D.; Lacalamita, R.; Tommasi, S.; Pinto, R. Next-generation sequencing: Advances and applications in cancer diagnosis. Onco Targets Ther. 2016, 9, 7355–7365. [Google Scholar] [CrossRef]
- Nielsen, C.; Bojesen, S.E.; Nordestgaard, B.G.; Kofoed, K.F.; Birgens, H.S. JAK2V617F somatic mutation in the general population: Myeloproliferative neoplasm development and progression rate. Haematologica 2014, 99, 1448–1455. [Google Scholar] [CrossRef]
- Langabeer, S.E.; Andrikovics, H.; Asp, J.; Bellosillo, B.; Carillo, S.; Haslam, K.; Kjaer, L.; Lippert, E.; Mansier, O.; Oppliger Leibundgut, E.; et al. Molecular diagnostics of myeloproliferative neoplasms. Eur. J. Haematol. 2015, 95, 270–279. [Google Scholar] [CrossRef]
- Bridgford, J.L.; Lee, S.M.; Lee, C.M.M.; Guglielmelli, P.; Rumi, E.; Pietra, D.; Wilcox, S.; Chhabra, Y.; Rubin, A.F.; Cazzola, M.; et al. Novel drivers and modifiers of MPL-dependent oncogenic transformation identified by deep mutational scanning. Blood 2020, 135, 287–292. [Google Scholar] [CrossRef]

| Clinical Data of Patient 1 | |
|---|---|
| Age | 57 |
| Gender | female |
| Platelet count | 807 × 109/L |
| Hemoglobin level | 12.8 g/dL |
| Red blood Cells (RBC) | 4.13 × 109/L |
| White blood Cells (WBC) | 6.58 × 109/L |
| Liver and spleen dimension | N.I. |
| Other diseases | ischemic colitis, splenic aneurysm, and retinal vascular occlusion |
| Gene | Coding | Protein | Cosmic ID | Type of Mutation | VAF% | Fathmm Prediction Score |
|---|---|---|---|---|---|---|
| DNA from peripheral blood | ||||||
| MPL | c.1502T>C | p.V501A | COSM86964 | Substitution-Missense | 27.36 | Neutral (0.40) |
| MPL | c.1543T>A | p.W515R | COSM29008 | Substitution-Missense | 14.36 | Pathogenic (0.54) |
| DNA from bone marrow | ||||||
| MPL | c.1502T>C | p.V501A | COSM86964 | Substitution-Missense | 100 | Neutral (0.40) |
| MPL | c.1543T>A | p.W515R | COSM29008 | Substitution-Missense | 100 | Pathogenic (0.54) |
| JAK2 | c.1849G>T | p.V617F | COSM12600 | Substitution-Missense | 39.77 | Pathogenic (0.94) |
| PTEN | c.51A>C | p.Q17H | Novel | Substitution –Missense | 27.88 | - |
| TP53 | c.532delC | p. H178Tfs*69 | COSM43978 | Deletion Frameshift | 11.5 | n/a |
| PIK3CA | c.3130A>G | p.N1044D | COSM27134 | Substitution-Missense | 6.02 | Pathogenic (0.96) |
| KIT | c.1697A>C | p.N566T | COSM9233350 | Substitution-Missense | 1.62 | Pathogenic (0.97) |
| Clinical Data of Patient 2 | |
|---|---|
| Age | 68 |
| Gender | male |
| Platelet count | 785 × 109/L |
| Hemoglobin level | 12.5 g/dL |
| Red blood Cells (RBC) | 4.25 × 109/L |
| White blood Cells (WBC) | 102 × 109/L |
| Liver and spleen dimension | mild splenomegaly |
| Other diseases | hypertension and diabetes mellitus |
| Gene | Coding | Protein | Cosmic ID | Type of Mutation | VAF% | Fathmm Prediction Score |
|---|---|---|---|---|---|---|
| MPL | c.1502T>C | p.V501A | COSM86964 | Substitution-Missense | 38.5 | Neutral (0.40) |
| MPL | c.1544G>T | p.W515L | COSM18918 | Substitution-Missense | 34.4 | Pathogenic (0.70) |
| KIT | c.1621A>C | p.M541L | COSM28026 | Substitution-Missense | 59.7 | Pathogenic (0.74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennisi, M.S.; Di Gregorio, S.; Tirrò, E.; Romano, C.; Duminuco, A.; Garibaldi, B.; Giuffrida, G.; Manzella, L.; Vigneri, P.; Palumbo, G.A. Additional Genetic Alterations and Clonal Evolution of MPNs with Double Mutations on the MPL Gene: Two Case Reports. Hematol. Rep. 2023, 15, 317-324. https://doi.org/10.3390/hematolrep15020033
Pennisi MS, Di Gregorio S, Tirrò E, Romano C, Duminuco A, Garibaldi B, Giuffrida G, Manzella L, Vigneri P, Palumbo GA. Additional Genetic Alterations and Clonal Evolution of MPNs with Double Mutations on the MPL Gene: Two Case Reports. Hematology Reports. 2023; 15(2):317-324. https://doi.org/10.3390/hematolrep15020033
Chicago/Turabian StylePennisi, Maria Stella, Sandra Di Gregorio, Elena Tirrò, Chiara Romano, Andrea Duminuco, Bruno Garibaldi, Gaetano Giuffrida, Livia Manzella, Paolo Vigneri, and Giuseppe A. Palumbo. 2023. "Additional Genetic Alterations and Clonal Evolution of MPNs with Double Mutations on the MPL Gene: Two Case Reports" Hematology Reports 15, no. 2: 317-324. https://doi.org/10.3390/hematolrep15020033
APA StylePennisi, M. S., Di Gregorio, S., Tirrò, E., Romano, C., Duminuco, A., Garibaldi, B., Giuffrida, G., Manzella, L., Vigneri, P., & Palumbo, G. A. (2023). Additional Genetic Alterations and Clonal Evolution of MPNs with Double Mutations on the MPL Gene: Two Case Reports. Hematology Reports, 15(2), 317-324. https://doi.org/10.3390/hematolrep15020033








