Comparison of Two Methods of Capillary Sampling in Blood Pre-Donation Anemia Screening in Brazil
Abstract
1. Introduction
2. Method
2.1. Study Design
2.2. Study Population
2.3. Laboratory Tests
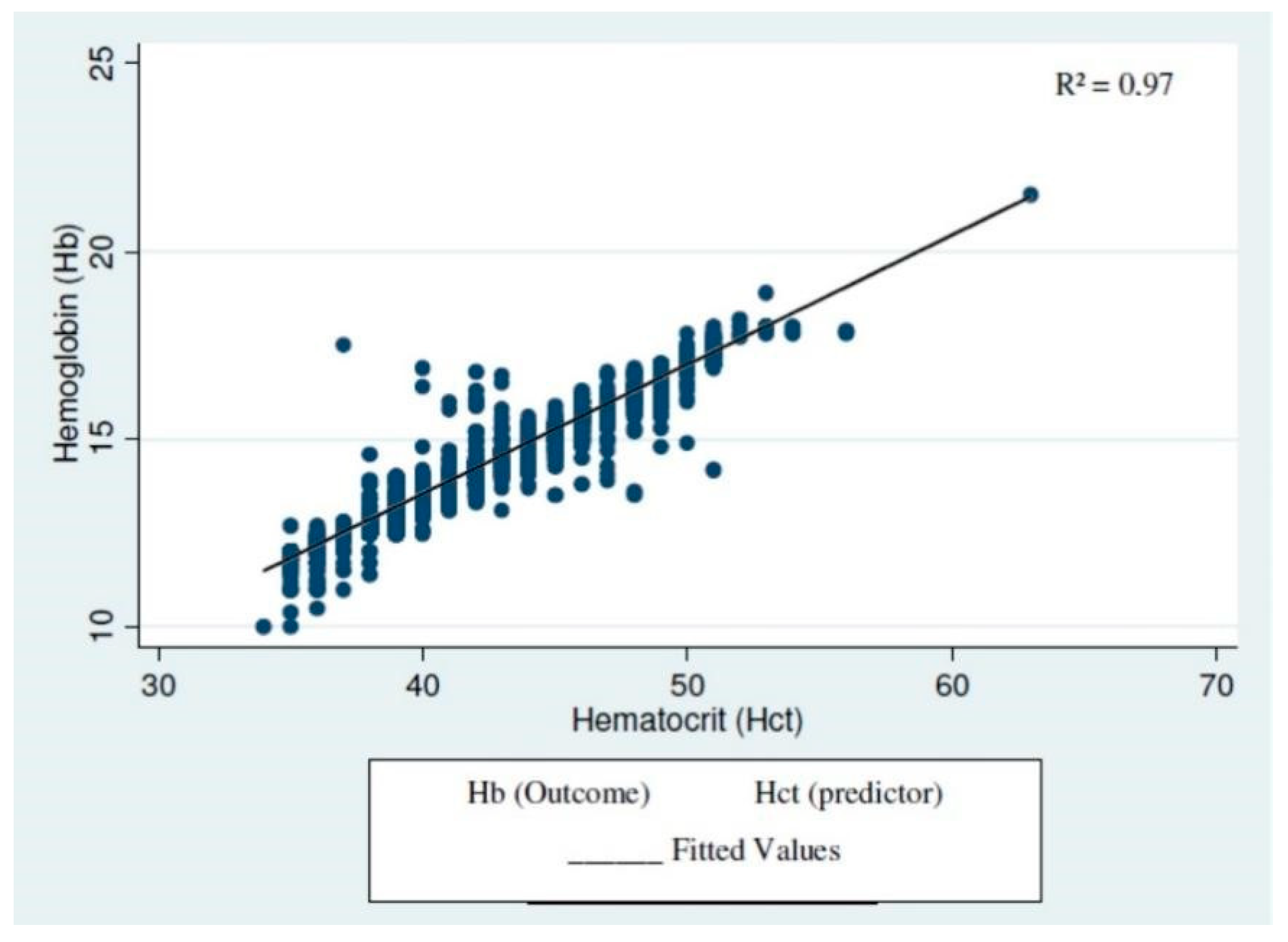
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moura, A.S.; de Moreira, C.T.; Machado, C.A.; Vasconcelos Neto, J.A.; Machado, M.D.F.A.S. Doador de sangue habitual e fidelizado: Fatores motivacionais de adesão ao programa. Rev. Bras. Promoção Saúde 2006, 19, 61–67. [Google Scholar] [CrossRef]
- Tobergte, D.R.; Curtis, S. O Uso Clínico do Sangue na Medicina, Obstetrícia, Pediatria e Neonatologia, Cirurgia e Anestesia, Traumas e Queimaduras. J. Chem. Inf. Model 2013, 53, 1689–1699. [Google Scholar] [CrossRef]
- Figueiredo, M.S. Anemia and the blood donor. Rev. Bras. Hematol. Hemoter. 2012, 34, 328–329. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.D.L.; De Almeida-Neto, C.; Liu, E.J.; Sabino, E.C.; Leão, S.C.; Loureiro, P.; Wright, D.; Custer, B.; Gonçalez, T.T.; Capuani, L.; et al. Temporal distribution of blood donations in three Brazilian blood centers and its repercussion on the blood supply. Rev. Bras. Hematol. Hemoter. 2013, 35, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.D.L.; Martins, G.; Custer, B.; Proietti, F.A.; Carneiro-Proietti, A.B.F.; César, C.C. Hierarchical analysis of anaemia deferral in blood donor candidates: The individual in the population perspective. Transfus. Med. 2011, 21, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.R.; Terra, F.D.; Santos, S.V.; Miranda, R.P. Perfil sociodemográfico e epidemiológico de candidatos a doação de sangue. Arq. Ciênc. Saúde 2015, 22, 87–91. [Google Scholar] [CrossRef]
- Murphy, E.L.; Wright, D.J.; Sacher, R.A.; Gottschall, J.L. Iron Deficiency in Blood Donors: The REDS-II Donor Iron Status Evaluation (RISE) Study. Transfusion 2012, 52, 702–711. [Google Scholar]
- Fillet, A.M.; Gross, S. Prévention de l’anémie chez les donneurs de sang. Transfus. Clin. Biol. 2017, 24, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef] [PubMed]
- da Saúde, B.M. Anemia por Deficiência de Ferro. Protocolos Clínicos e Diretrizes Terapêuticas Published Online 2014:27–46. Available online: https://www.gov.br/saude/pt-br/assuntos/protocolos-clinicos-e-diretrizes-terapeuticas-pcdt/arquivos/2014/pcdt_anemia_deficienciaferro_2014.pdf (accessed on 6 June 2022).
- Quinto, L.; Aponte, J.J.; Menendez, C.; Sacarlal, J.; Aide, P.; Espasa, M.; Mandomando, I.; Guinovart, C.; Macete, E.; Hirt, R.; et al. Relationship between haemoglobin and haematocrit in the definition of anaemia. Trop. Med. Int. Health 2006, 11, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Mendrone, A.; Sabino, E.C.; Sampaio, L.; Neto, C.A.; Schreiber, G.B.; Chamone, D.D.A.F.; Dorlhiac-Llacer, P.E. Anemia screening in potential female blood donors: Comparison of two different quantitative methods. Transfusion 2009, 49, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Pomeransky, A.A.; Khriplovich, I.B. Equations of motion of spinning relativistic particle in external fields. Surv. High Energy Phys. 1999, 14, 145–173. [Google Scholar] [CrossRef]
- Setia, M.S. Methodology series module 3: Cross-sectional studies. Indian J. Dermatol. 2016, 61, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Carneiro-Proietti, A.B.; Sabino, E.C.; Sampaio, D.; Proietti, F.A.; Gonçalez, T.T.; Oliveira, C.D.; Ferreira, J.E.; Liu, J.; Custer, B.; Schreiber, G.B.; et al. Demographic profile of blood donors at three major Brazilian blood centers: Results from the International REDS-II study, 2007 to 2008. Transfusion 2010, 50, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, P.; DE Almeida-Neto, C.; Proietti, A.B.C.; Capuani, L.; Gonçalez, T.T.; Oliveira, C.D.L.D.; Leão, S.C.; Lopes, M.I.; Sampaio, D.; Patavino, G.M.; et al. Contribution of the Retrovirus Epidemiology Donor Study (REDS) to research on blood transfusion safety in Brazil. Rev. Bras. Hematol. Hemoter. 2014, 36, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Brunken, G.S.; De França, G.V.A.; Luiz, R.R.; Szarfarc, S.C. Agreement assessment between hemoglobin and hematocrit to detect anemia prevalence in children less than 5 years old. Cad. Saúde Coletiva 2016, 24, 118–123. [Google Scholar] [CrossRef]
- Cable, R.G.; Steele, W.R.; Melmed, R.S.; Johnson, B.; Mast, A.E.; Carey, P.; Kiss, J.E.; Kleinman, S.H.; Wright, D.J.; NHLBI Retrovirus Epidemiology Donor Study‐II (REDS‐II). The difference between fingerstick and venous hemoglobin and hematocrit varies by sex and iron stores. Transfusion 2011, 52, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, I.A.; Drakeley, C.J.; Owusu-Agyei, S.; Mmbando, B.; Chandramohan, D. Haemoglobin and haematocrit: Is the threefold conversion valid for assessing anaemia in malaria-endemic settings? Malar. J. 2007, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Insiripong, S.; Supattarobol, T.; Jetsrisuparb, A. Comparison of hematocrit/hemoglobin ratios in subjects with alpha-thalassemia, with subjects having chronic kidney disease and normal subjects. Southeast Asian J. Trop. Med. Public Health 2013, 44, 707–711. [Google Scholar] [PubMed]
- Weatherall, M.S.; Sherry, K.M. An evaluation of the SpuncritTM infra-red analyser for measurement of haematocrit. Clin. Lab. Haematol. 1997, 19, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde. Portaria MS no 1.353, de 13.06.2011. Portaria MS no 1.353, de 13.06.2011. 2011. Available online: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt1353_13_06_2011.html (accessed on 4 April 2022).
| Variable | Frequency (%) |
|---|---|
| Gender | |
| Female | 4598 (29.6%) |
| Male | 10,923 (70.4%) |
| Age (years) | 36.51 ± 9.13 |
| 18 to 24 years | 2526 (16.3%) |
| 25 to 34 years | 4839 (31.2%) |
| 35 to 44 years | 3824 (24.6%) |
| 45 to 54 years | 2633 (17.0%) |
| 55 to 65 years | 985 (6.3%) |
| No information | 714 (4.6%) |
| Self-declared skin color | |
| Mixed | 6306 (40.6%) |
| White | 6985 (45.0%) |
| Black | 1261 (8.1%) |
| Other | 72 (0.5%) |
| No response | 897 (5.8%) |
| Education | |
| Less than 8 years of schooling | 2470 (16.0%) |
| 8 to 10 years of schooling | 1806 (11.6%) |
| 11 years of schooling | 7750 (49.9%) |
| Graduation or above | 3495 (22.5%) |
| Gender | ||||
|---|---|---|---|---|
| Female (N = 4598) | Male (N = 10,923) | |||
| Hb (g/dL) | Hct (%) | Hb (g/dL) | Hct (%) | |
| Mean ± SD | 13.5 ± 0.8 | 39.9 ± 2.3 | 14.9 ± 1.1 | 43.8 ± 3.2 |
| Median | 13.4 | 39.0 | 14.8 | 44.0 |
| Range | 10.0–21.5 | 34–63 | 11.0–18.9 | 35–56 |
| Hematological Variables | Gender | |||
|---|---|---|---|---|
| Female * (N = 4598) | Male ** (N = 10,923) | |||
| Anemia | Non Anemia | Anemia | Non Anemia | |
| Hemoglobin *** | 246 (5.4%) | 4352 (94.6%) | 126 (1.2%) | 10,797 (98.8%) |
| Hematocrit **** | 282 (6.1%) | 4316 (93.9%) | 128 (1.2%) | 10,795 (98.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flor, C.R.; Baldoni, A.d.O.; Garcia Mateos, S.d.O.; Sabino, E.C.; Oliveira, C.D.L. Comparison of Two Methods of Capillary Sampling in Blood Pre-Donation Anemia Screening in Brazil. Hematol. Rep. 2023, 15, 298-304. https://doi.org/10.3390/hematolrep15020030
Flor CR, Baldoni AdO, Garcia Mateos SdO, Sabino EC, Oliveira CDL. Comparison of Two Methods of Capillary Sampling in Blood Pre-Donation Anemia Screening in Brazil. Hematology Reports. 2023; 15(2):298-304. https://doi.org/10.3390/hematolrep15020030
Chicago/Turabian StyleFlor, Cristina Rabelo, André de Oliveira Baldoni, Sheila de Oliveira Garcia Mateos, Ester Cerdeira Sabino, and Cláudia Di Lorenzo Oliveira. 2023. "Comparison of Two Methods of Capillary Sampling in Blood Pre-Donation Anemia Screening in Brazil" Hematology Reports 15, no. 2: 298-304. https://doi.org/10.3390/hematolrep15020030
APA StyleFlor, C. R., Baldoni, A. d. O., Garcia Mateos, S. d. O., Sabino, E. C., & Oliveira, C. D. L. (2023). Comparison of Two Methods of Capillary Sampling in Blood Pre-Donation Anemia Screening in Brazil. Hematology Reports, 15(2), 298-304. https://doi.org/10.3390/hematolrep15020030







