Comparative Analysis of Endovascular Intervention and Endarterectomy in Patients with Femoral Artery Disease: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Information Source and Search
2.2. Eligibility Criteria
2.3. Outcomes Assessed
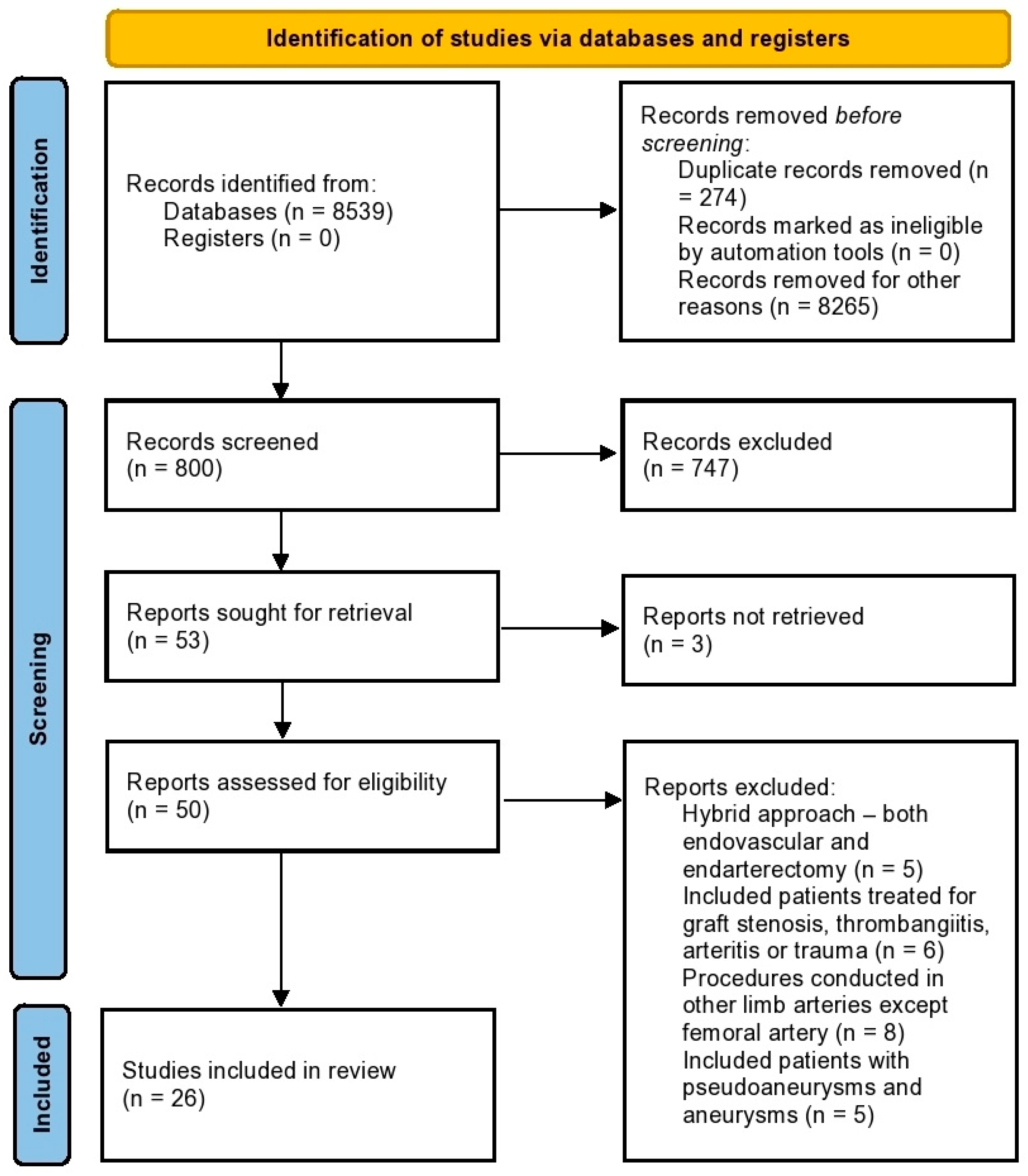
2.4. Study Selection and Data Collection
2.5. Risk of Bias in Individual Studies
2.6. Summary Measure and Synthesis of Results
2.7. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Risk of Bias
3.3. Patient Characteristics
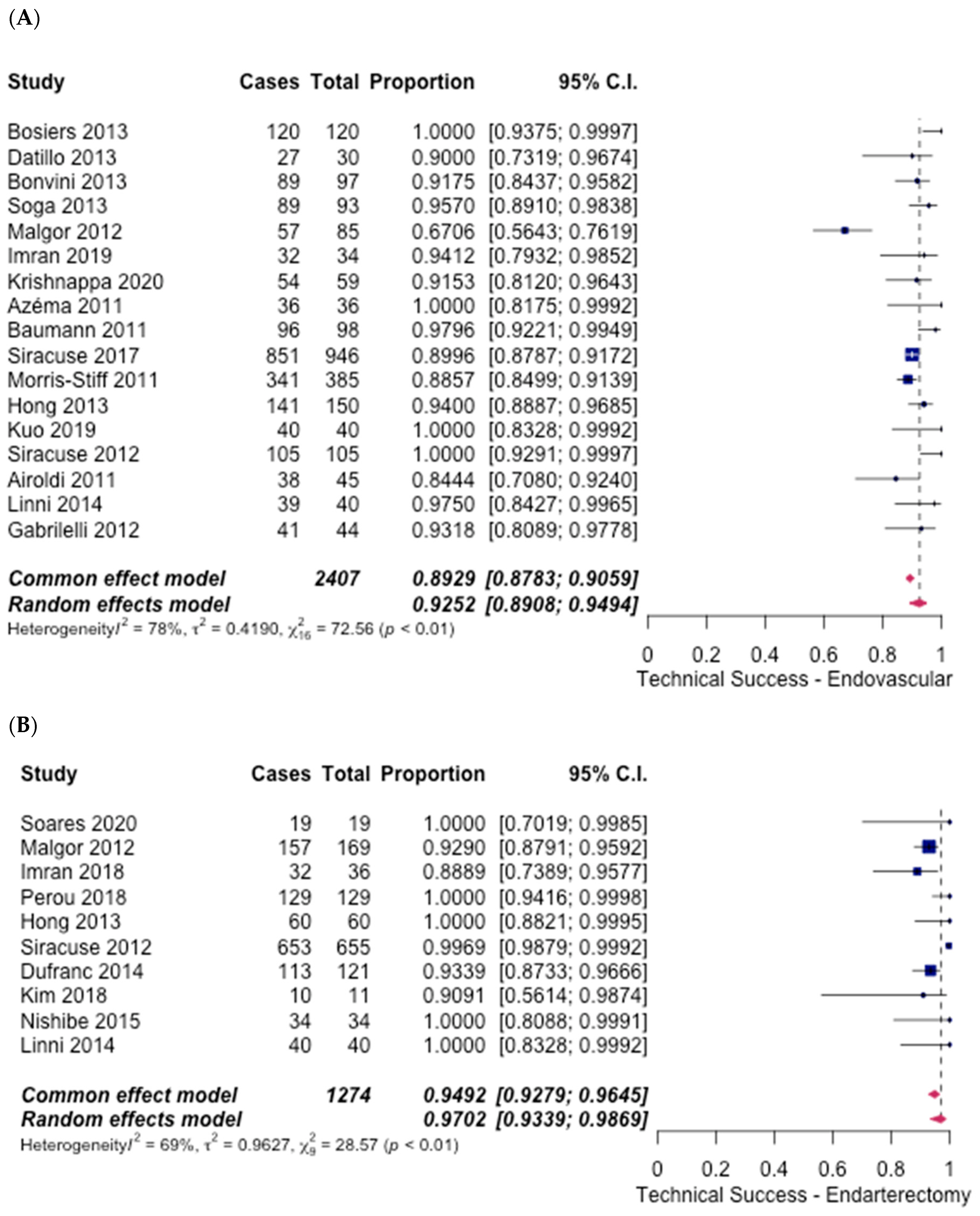
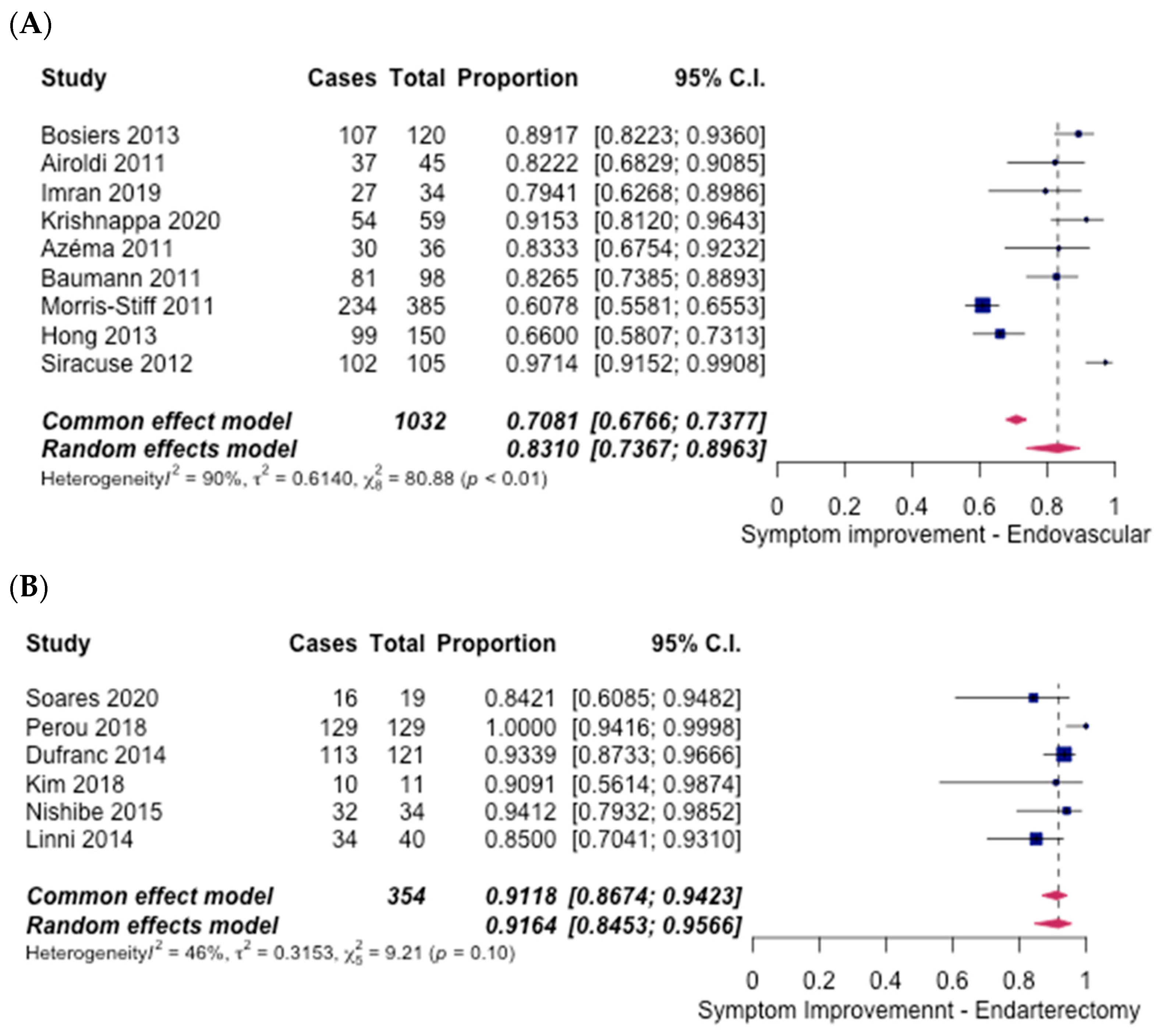
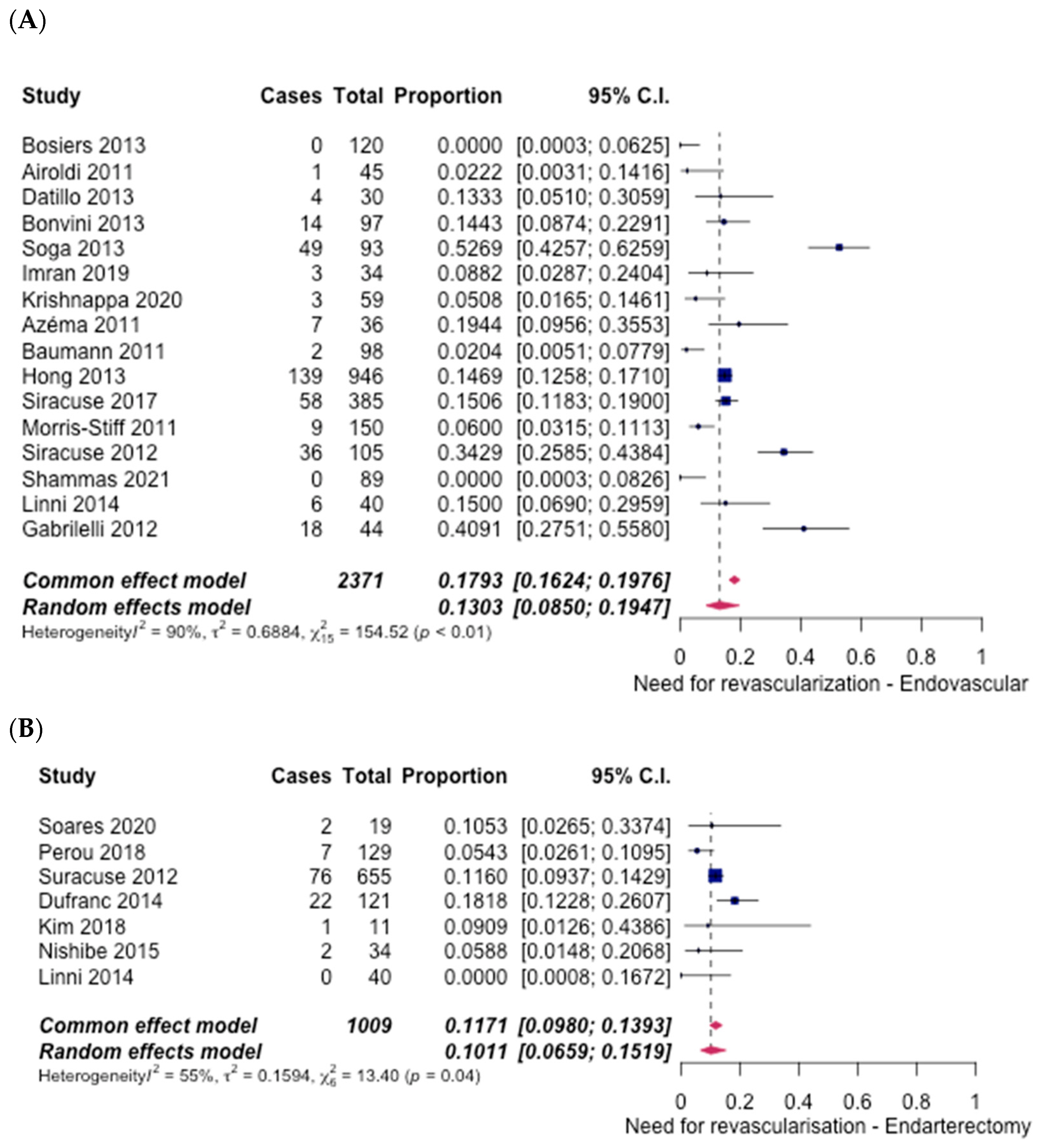
3.4. Technical Success
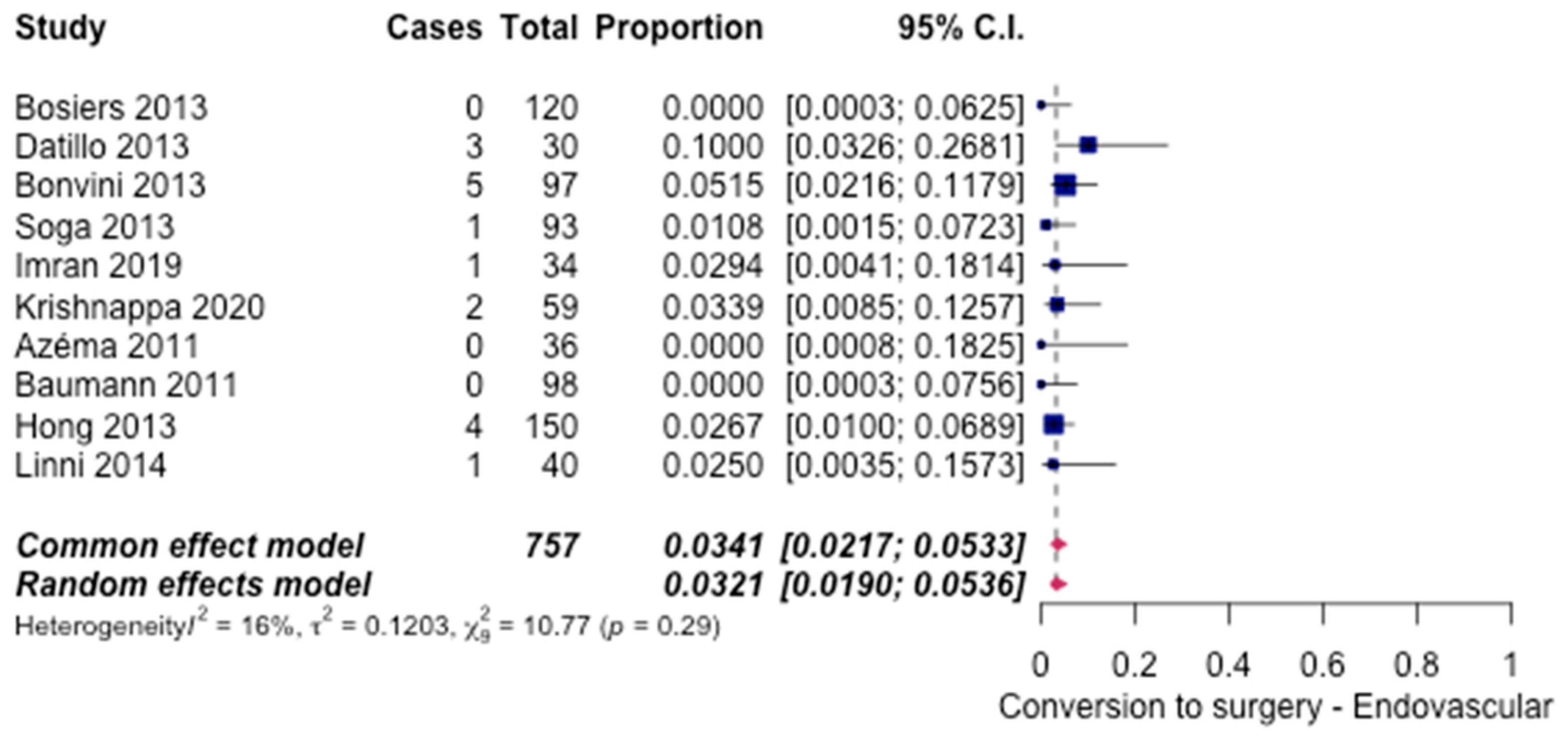
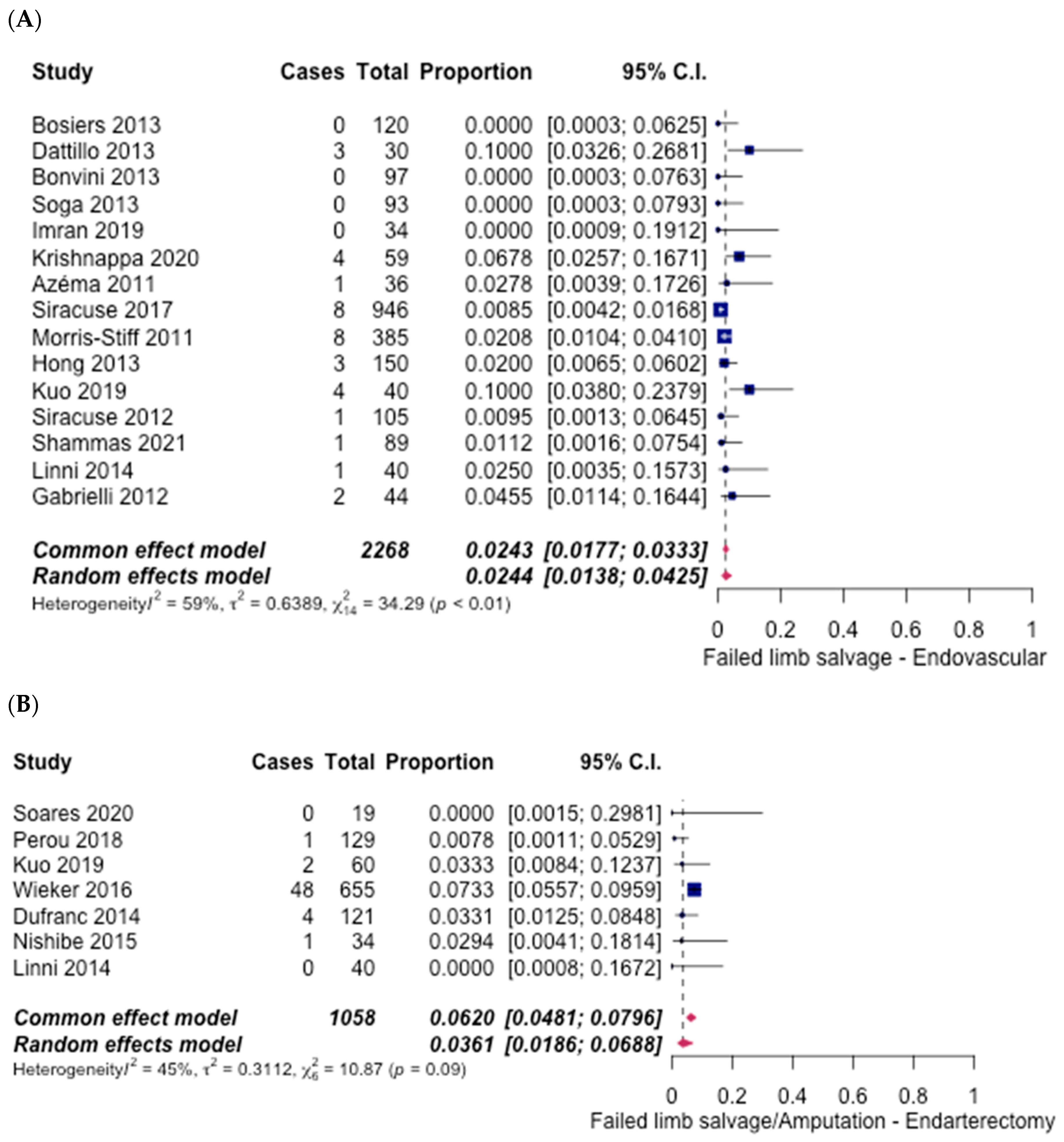
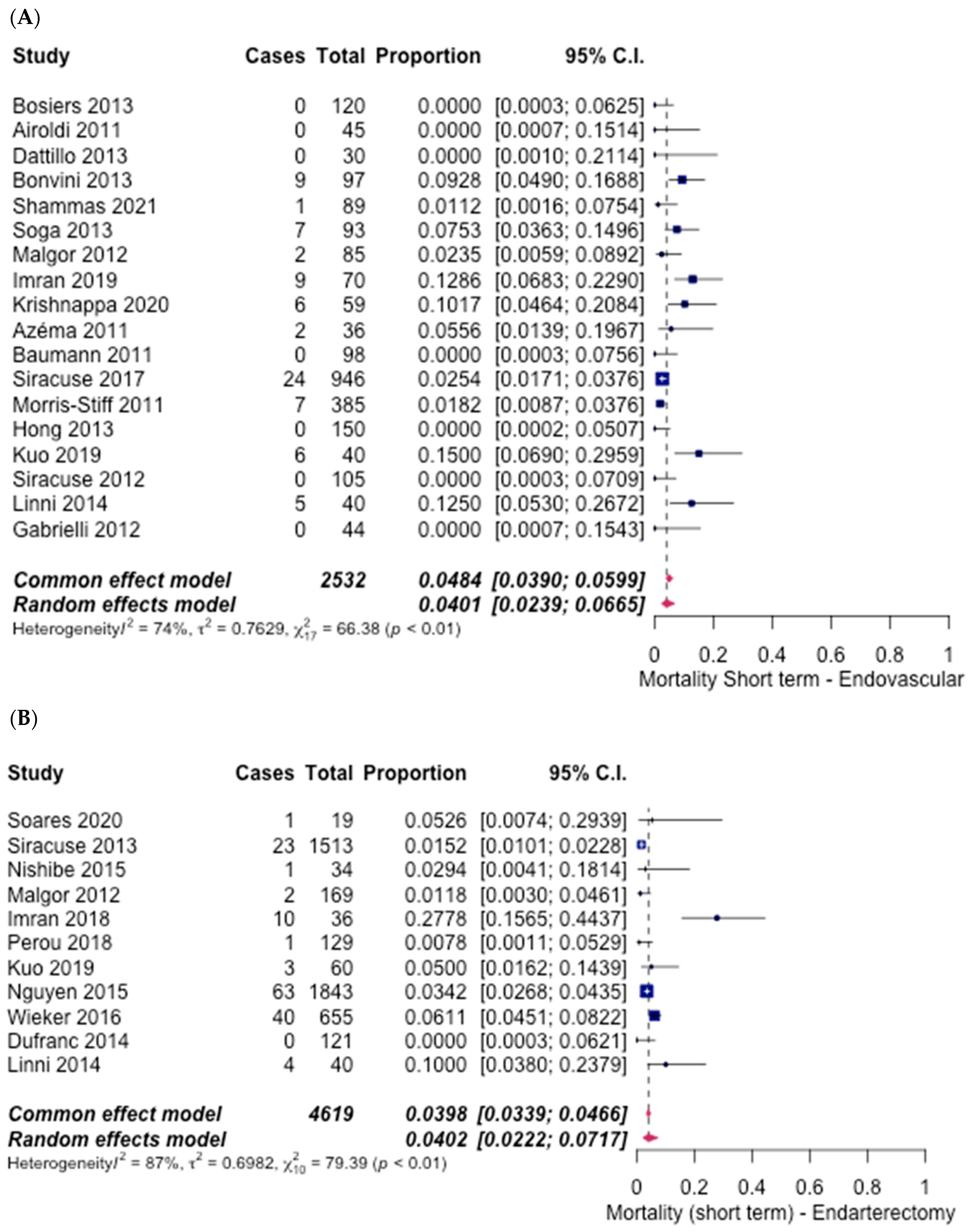
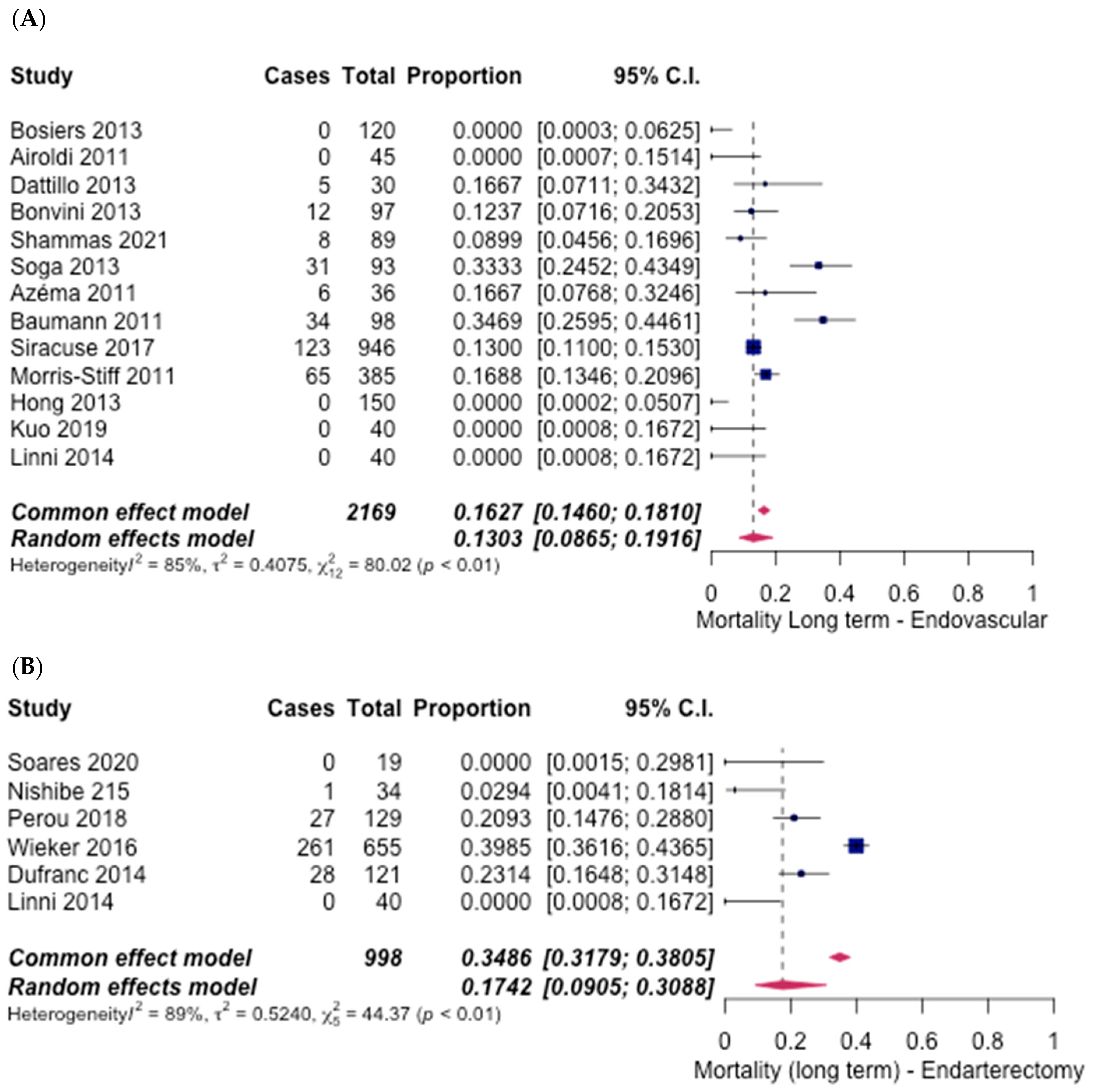
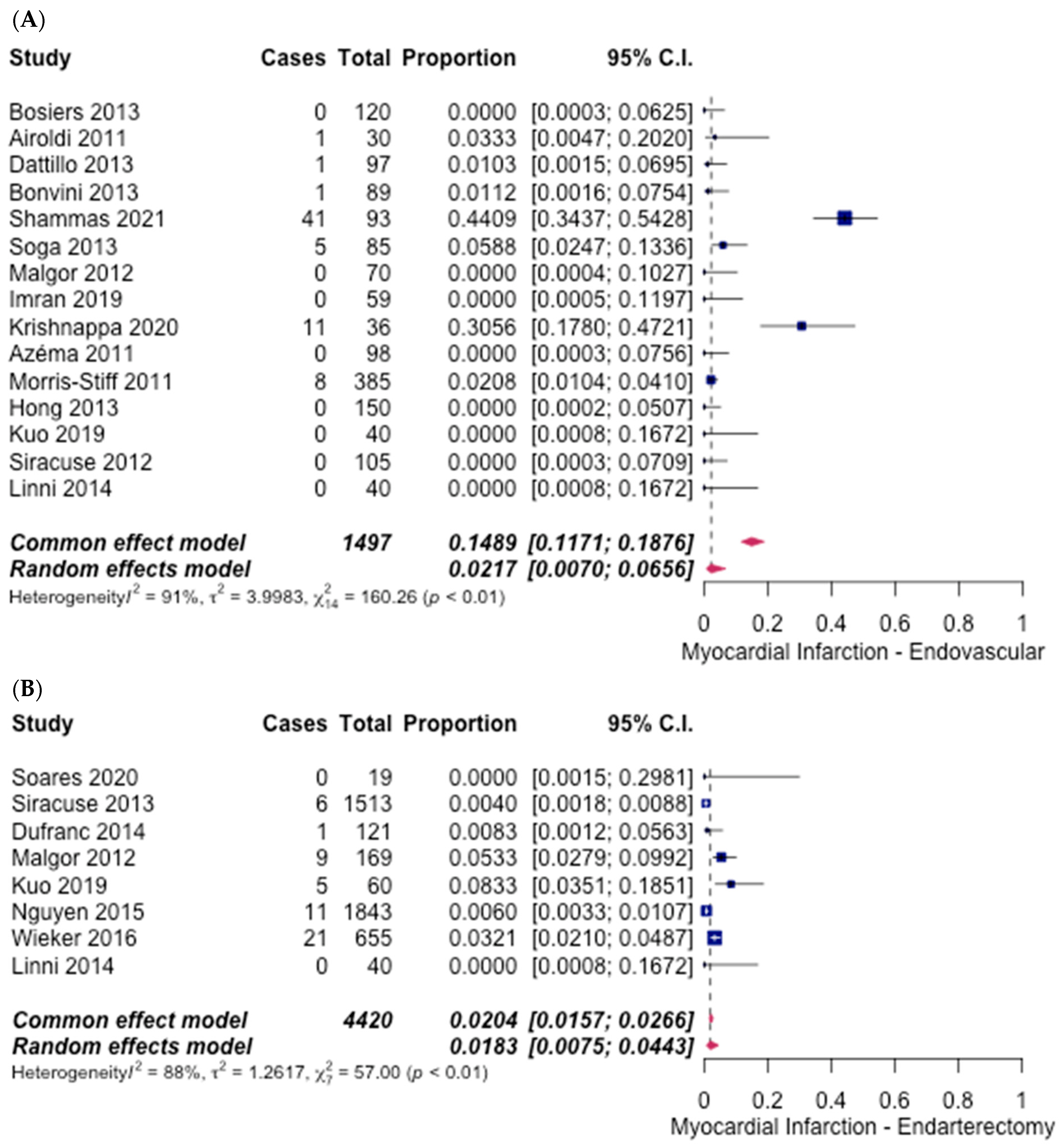
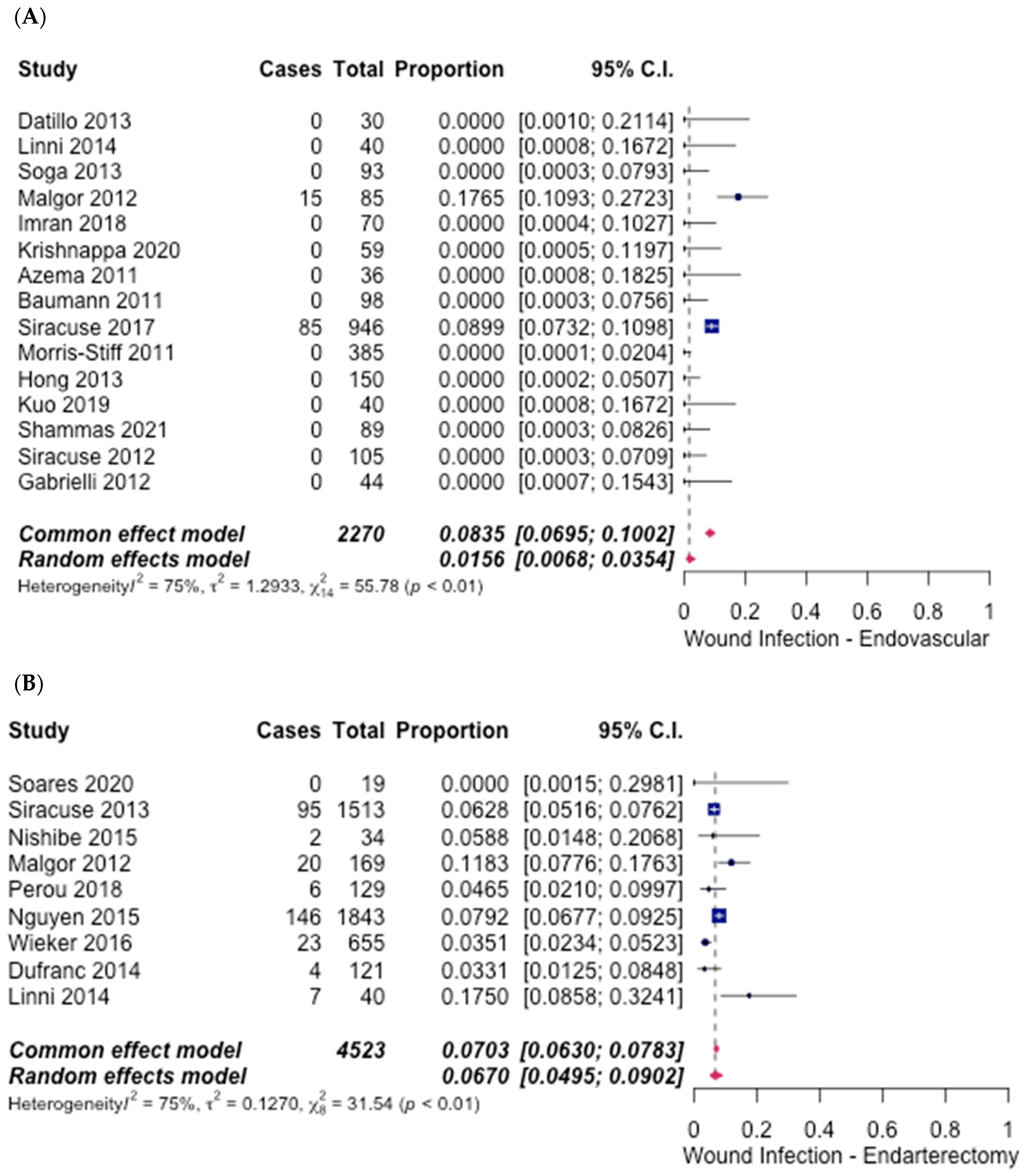
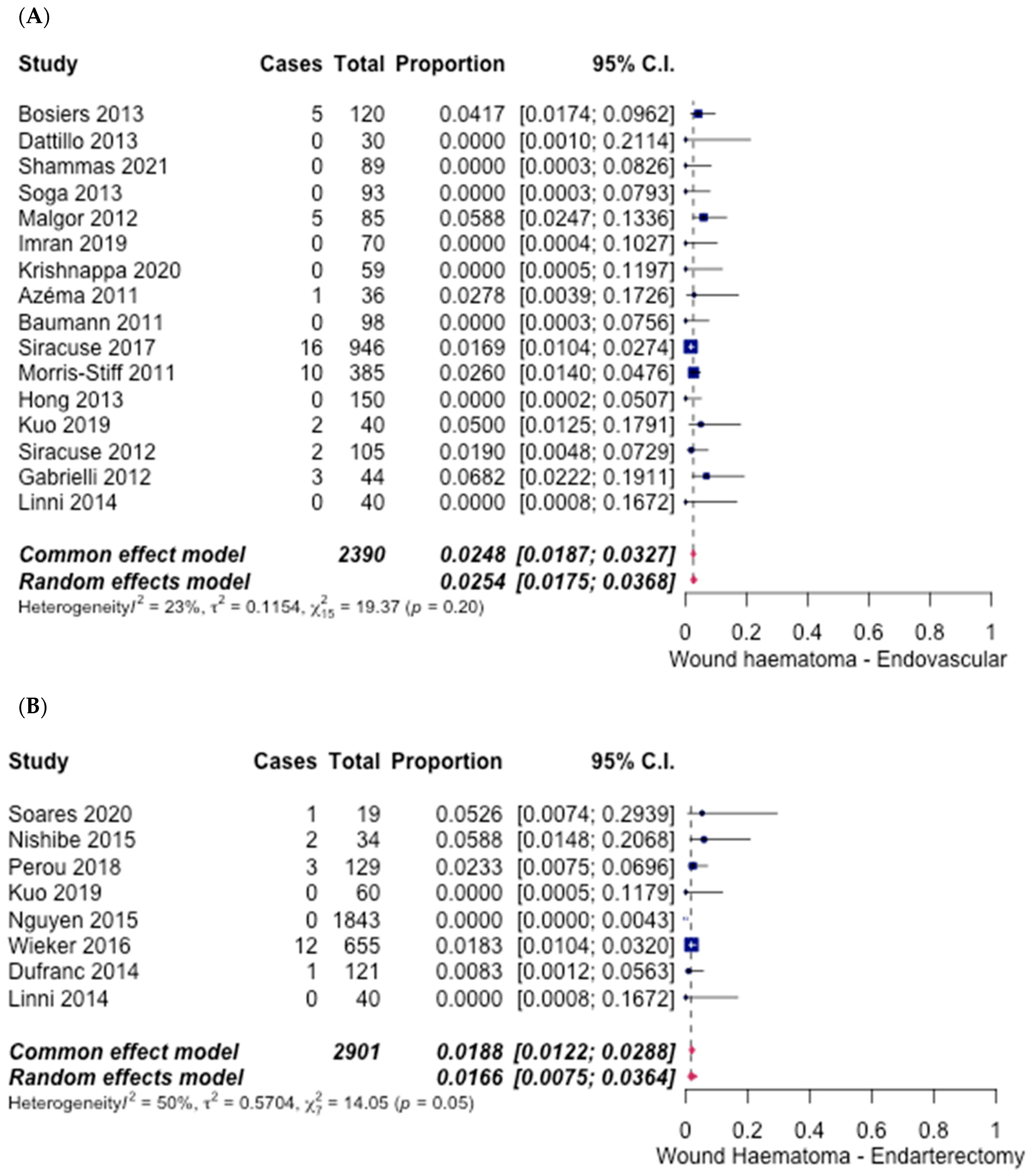
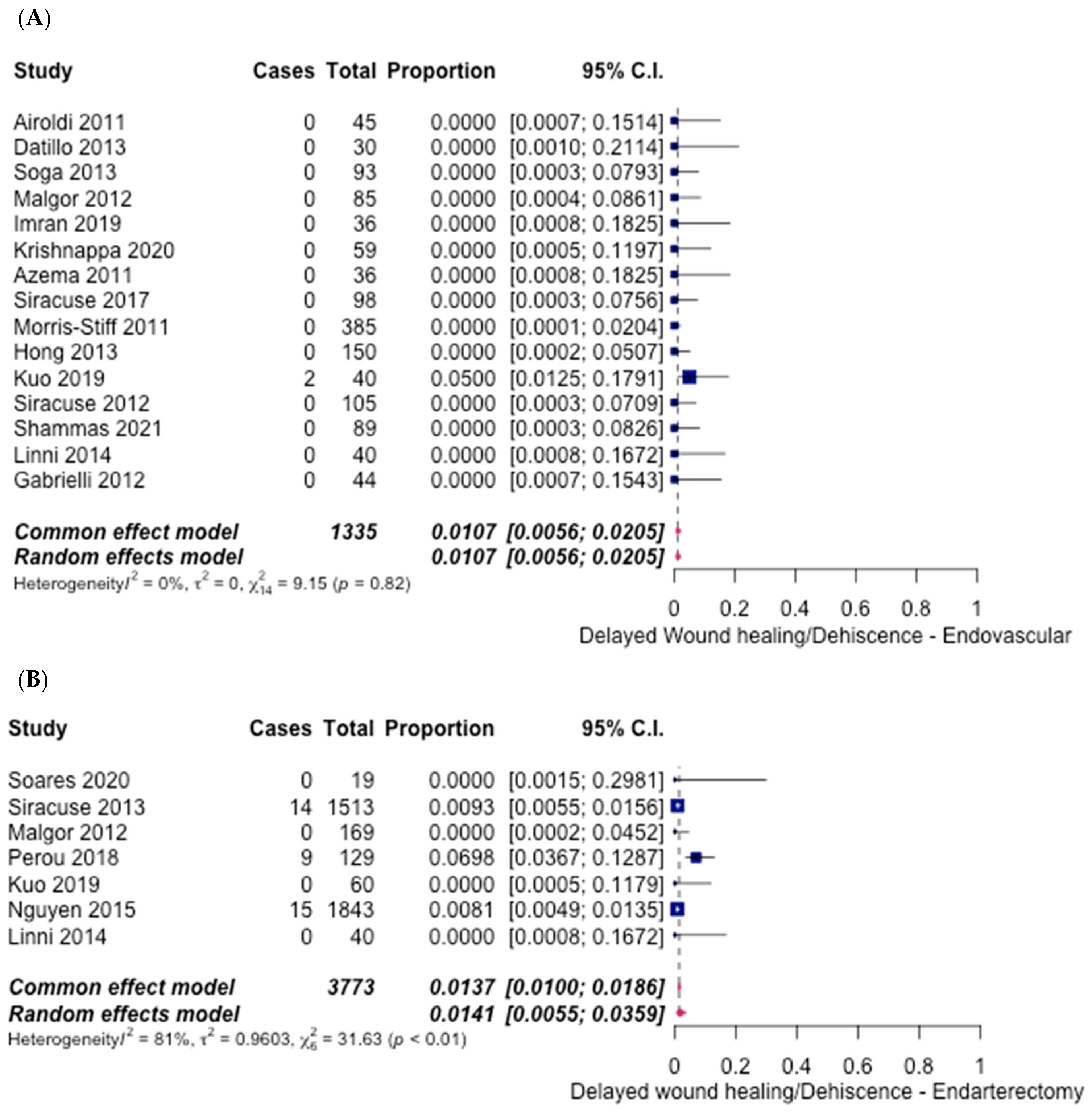
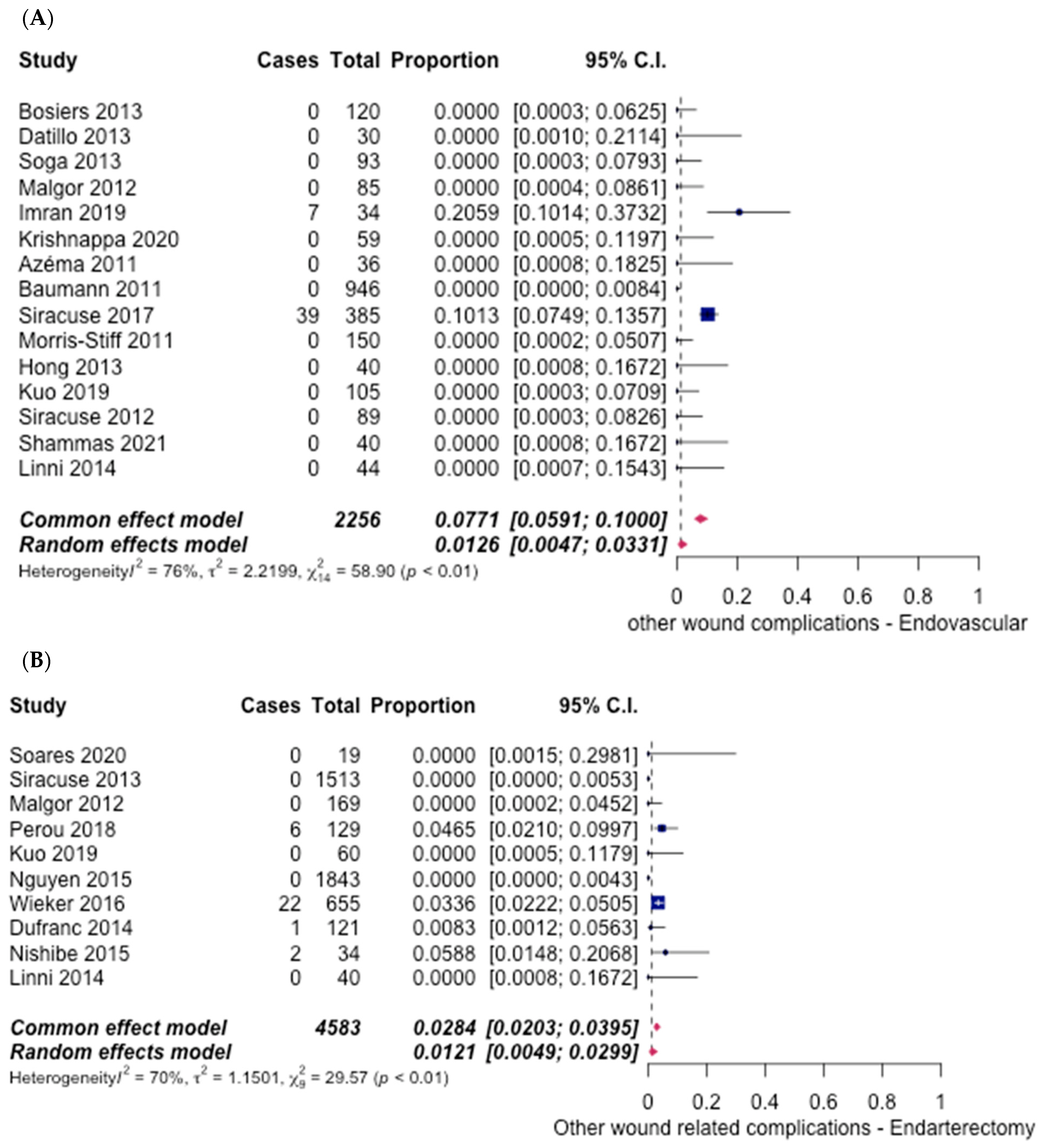
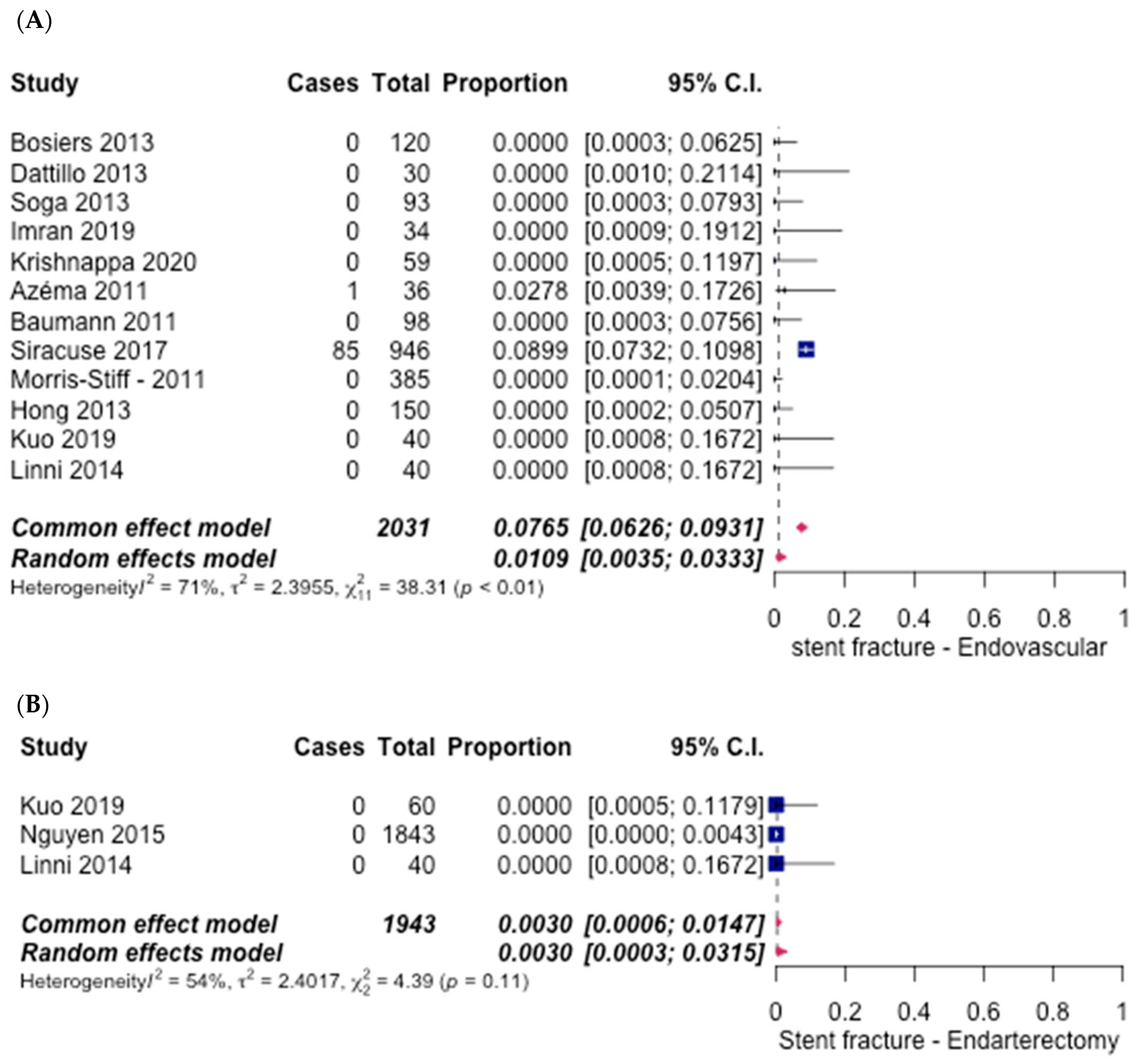
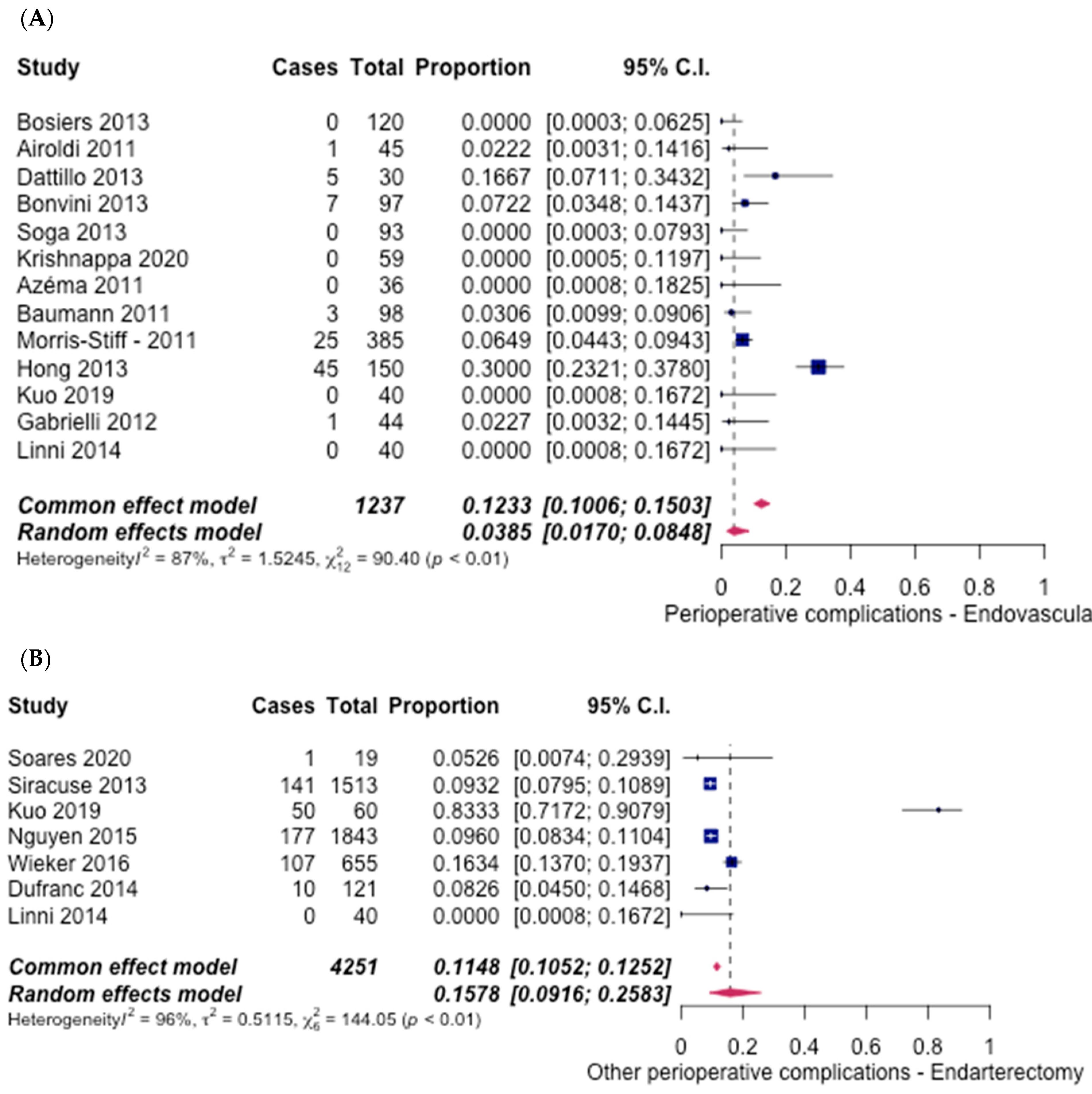
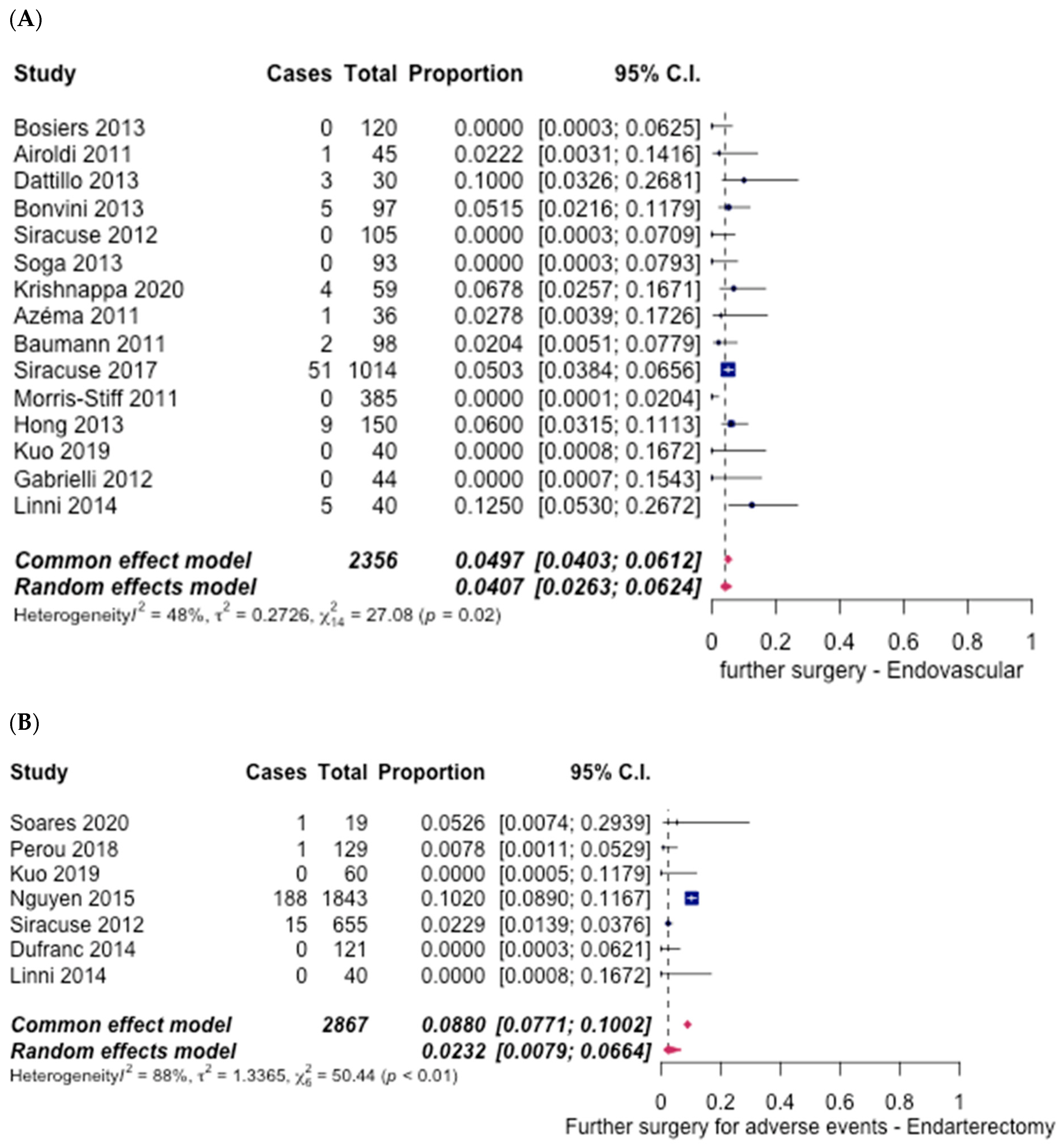
3.5. Safety Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhaliwal, G.; Mukherjee, D. Peripheral arterial disease: Epidemiology, natural history, diagnosis and treatment. Int. J. Angiol. 2007, 16, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.; Craig, L. Acute Arterial Occlusion; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ballotta, E.; Gruppo, M.; Mazzalai, F.; Da Giau, G. Common femoral artery endarterectomy for occlusive disease: An 8-year single-center prospective study. J. Surg. 2010, 147, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Serruys, P. Coronary Stents: Current Status. J. Am. Coll. Cardiol. 2010, 56, S1–S42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Byung, J.S.; Seung, J.B.; Kyung, Y.K. The Advantage of Common Femoral Endarterectomy Alone or Combined with Endovascular Treatment. Vasc. Spec. Int. 2018, 34, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Linni, K.; Ugurluoglu, A.; Hitzl, W.; Aspalter, M.; Hölzenbein, T. Bioabsorbable stent implantation vs. common femoral artery endarterectomy: Early Results A Randomized Trial. J. Endovasc. Ther. 2014, 21, 493–502. [Google Scholar]
- Gabrielli, R.; Rosati, M.S.; Vitale, S.; Baciarello, G.; Siani, A.; Chiappa, R.; Caselli, G.; Irace, L. Randomized controlled trial of remote endarterectomy versus endovascular intervention for TransAtlantic Inter-Society Consensus II D femoropopliteal lesions. J. Vasc. Surg. 2012, 56, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Bosiers, M.; Deloose, K.; Callaert, J.; Keirse, K.; Verbist, J.; Hendriks, J.; Lauwers, P.; D’Archambeau, O.; Scheinert, D.; Torsello, G.; et al. 4-French-compatible endovascular material is safe and effective in the treatment of femoropopliteal occlusive disease: Results of the 4-EVER trial. J. Endovasc. Ther. 2013, 20, 746–756. [Google Scholar] [CrossRef]
- Airoldi, F.; Faglia, E.; Losa, S.; Tavano, D.; Latib, A.; Mantero, M.; Lanza, G.; Clerici, G. A novel device for true lumen re-entry after subintimal recanalization of superficial femoral arteries: First-in-man experience and technical description. Cardiovasc. Interv. Radiol. 2011, 34, 166–169. [Google Scholar] [CrossRef]
- Dattilo, P.B.; Tsai, T.T.; Kevin Rogers, R.; Casserly, I.P. Acute and medium-term outcomes of endovascular therapy of obstructive disease of diverse etiology of the common femoral artery. Catheter. Cardiovasc. Interv. 2013, 81, 1013–1022. [Google Scholar] [CrossRef]
- Bonvini, R.F.; Rastan, A.; Sixt, S.; Beschorner, U.; Noory, E.; Schwarz, T.; Roffi, M.; Dorsaz, P.A.; Schwarzwälder, U.; Bürgelin, K.; et al. Angioplasty and provisional stent treatment of common femoral artery lesions. J. Vasc. Interv. Radiol. 2013, 24, 175–183. [Google Scholar] [CrossRef]
- Soga, Y.; Tomoi, Y.; Sato, K.; Iida, O.; Yokoi, H. Clinical outcome after endovascular treatment for isolated common femoral and popliteal artery disease. Cardiovasc. Interv. Ther. 2013, 28, 250–257. [Google Scholar] [CrossRef]
- Malgor, R.D.; Ricotta, J.J.; Bower, T.C.; Oderich, G.S.; Kalra, M.; Duncan, A.A.; Gloviczki, P. Common femoral artery endarterectomy for lower-extremity ischemia: Evaluating the need for additional distal limb revascularization. Ann. Vasc. Surg. 2012, 26, 946–956. [Google Scholar] [CrossRef]
- Imran, H.M.; Hyder, O.N.; Soukas, P.A. Efficacy and safety of adjunctive drug-coated balloon therapy in endovascular treatment of common femoral artery disease. Cardiovasc. Revasc. Med. 2019, 20, 210–214. [Google Scholar] [CrossRef]
- Krishnappa, S.; Rachaiah, J.M.; Mariappa, H.M.; Doddamadaiah, C.; Nanjappa, M.C. Endovascular Interventions to Superficial Femoral Artery Occlusion: Different Approaches, Technique, and Follow-up. Heart Views 2020, 21, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Azéma, L.; Davaine, J.M.; Guyomarch, B.; Chaillou, P.; Costargent, A.; Patra, P.; Gouëffic, Y. Endovascular repair of common femoral artery and concomitant arterial lesions. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, F.; Ruch, M.; Willenberg, T.; Dick, F.; Do, D.-D.; Keo, H.-H.; Baumgartner, I.; Diehm, N. Endovascular treatment of common femoral artery obstructions. J. Vasc. Surg. 2011, 53, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Siracuse, J.J.; Van Orden, K.; Kalish, J.A.; Eslami, M.; Schermerhorn, M.L.; Patel, V.I.; Rybin, D.; Farber, A. Endovascular treatment of the common femoral artery in the Vascular Quality Initiative. J. Vasc. Surg. 2017, 65, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Morris-Stiff, G.; Moawad, M.; Appleton, N.; Davies, G.; Hicks, E.; Davies, C.; Lewis, M.H. Long-term clinical outcome following lower limb arterial angioplasty. Ann. R Coll. Surg. Engl. 2011, 93, 250–254. [Google Scholar] [CrossRef]
- Hong, S.-J.; Ko, Y.-G.; Kim, J.-S.; Hong, M.-K.; Jang, Y.; Choi, D. Midterm outcomes of subintimal angioplasty supported by primary proximal stenting for chronic total occlusion of the superficial femoral artery. J. Endovasc. Ther. 2013, 20, 782–791. [Google Scholar] [CrossRef]
- Kuo, T.-T.; Chen, P.-L.; Huang, C.-Y.; Lee, C.-Y.; Shih, C.-C.; Chen, I.-M. Outcome of drug-eluting balloon angioplasty versus endarterectomy in common femoral artery occlusive disease. J. Vasc. Surg. 2019, 69, 141–147. [Google Scholar] [CrossRef]
- Siracuse, J.J.; Giles, K.A.; Pomposelli, F.B.; Hamdan, A.D.; Wyers, M.C.; Chaikof, E.L.; Nedeau, A.E.; Schermerhorn, M.L. Results for primary bypass versus primary angioplasty/stent for intermittent claudication due to superficial femoral artery occlusive disease. J. Vasc. Surg. 2012, 55, 1001–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, T.R.; Amorim, P.; Manuel, V.; Lopes, A.; E Fernandes, R.F.; Martins, C.; Pedro, L. A single-center experience in the eversion femoral endarterectomy. Vascular 2020, 28, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Perou, S.; Pirvu, A.; Morel, J.; Magne, J.-L.; Elie, A.; Spear, R. Femoral Bifurcation Endarterectomy with Transection-Eversion of the Superficial Femoral Artery: Technique and Results. Ann. Vasc. Surg. 2018, 53, 177–183. [Google Scholar] [CrossRef]
- Dufranc, J.; Palcau, L.; Heyndrickx, M.; Gouicem, D.; Coffin, O.; Felisaz, A.; Berger, L. Technique and results of femoral bifurcation endarterectomy by eversion. J. Vasc. Surg. 2015, 61, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Nishibe, T.; Maruno, K.; Iwahori, A.; Fujiyoshi, T.; Suzuki, S.; Takahashi, S.; Ogino, H.; Nishibe, M. The Role of Common Femoral Artery Endarterectomy in the Endovascular Era. Ann. Vasc. Surg. 2015, 29, 1501–1507. [Google Scholar] [CrossRef]
- Shammas, N.W.; Shammas, G.A.; Karia, R.; Khalafallah, R.; Jones-Miller, S.; Shammas, A.N. Two-Year Outcomes of Endovascular Interventions of the Common Femoral Artery: A Retrospective Analysis from Two Medical Centers. Cardiovasc. Revasc. Med. 2021, 24, 72–76. [Google Scholar] [CrossRef]
- Wieker, C.M.; Schönefeld, E.; Osada, N.; Lührs, C.; Beneking, R.; Torsello, G.; Böckler, D. Results of common femoral artery thromboendarterectomy evaluation of a traditional surgical management in the endovascular era. J. Vasc. Surg. 2016, 64, 995–1001. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, B.-N.; Amdur, R.L.; Abugideiri, M.; Rahbar, R.; Neville, R.F.; Sidawy, A.N. Postoperative complications after common femoral endarterectomy. J. Vasc. Surg. 2015, 61, 1489–1494.e1. [Google Scholar] [CrossRef] [Green Version]
- Siracuse, J.J.; Gill, H.L.; Schneider, D.B.; Graham, A.R.; Connolly, P.H.; Jones, D.W.; Meltzer, A.J. Assessing the perioperative safety of common femoral endarterectomy in the endovascular era. Vasc. Endovasc. Surg. 2014, 48, 27–33. [Google Scholar] [CrossRef]
- Kang, J.L.; Patel, V.I.; Conrad, M.F.; Lamuraglia, G.M.; Chung, T.K.; Cambria, R.P. Common femoral artery occlusive disease: Contemporary results following surgical endarterectomy. J. Vasc. Surg. 2008, 48, 872–877. [Google Scholar] [CrossRef] [Green Version]
- Marmagkiolis, K.; Hakeem, A.; Choksi, N.; Al-Hawwas, M.; Edupuganti, M.M.; Leesar, M.A.; Cilingiroglu, M. 12-month primary patency rates of contemporary endovascular device therapy for femoro-popliteal occlusive disease in 6024 patients: Beyond balloon angioplasty. Catheter. Cardiovasc. Interv. 2014, 84, 555–564. [Google Scholar] [CrossRef]
- Sajid, M.S.; Desai, M.; Rimpel, J.; Baker, D.M.; Hamilton, G. Functional outcome after femoral endarterectomy: A single-centre experience. Int. J. Angiol. 2008, 17, 33–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litsky, J.; Chanda, A.; Stilp, E.; Lansky, A.; Mena, C. Critical evaluation of stents in the peripheral arterial disease of the superficial femoral artery—Focus on paclitaxel eluting stent. Med. Devices Auckl. 2014, 7, 149–156. [Google Scholar]
- Changal, K.H.; Syed, M.A.; Dar, T.; Mangi, M.A.; Sheikh, M.A. Systematic Review and Proportional Meta-Analysis of Endarterectomy and Endovascular Therapy with Routine or Selective Stenting for Common Femoral Artery Atherosclerotic Disease. J. Interven. Cardiol. 2019, 2019, 1593401. [Google Scholar] [CrossRef]
- Topfer, L.A.; Spry, C. New Technologies for the Treatment of Peripheral Artery Disease. In CADTH Issues in Emerging Health Technologies; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2016; p. 172. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519606/ (accessed on 10 May 2022).
- Brodmann, M.; Werner, M.; Brinton, T.J.; Illindala, U.; Lansky, A.; Jaff, M.R.; Holden, A. Safety and Performance of Lithoplasty for Treatment of Calcified Peripheral Artery Lesions. J. Am. Coll. Cardiol. 2017, 70, 908–910. [Google Scholar] [CrossRef]
- Fukuda, K.; Yokoi, Y. Application of rotational atherectomy for a calcified superficial femoral artery lesion. Cardiovasc. Interv. Ther. 2015, 30, 351–355. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, P.; Sun, T.; Han, M.; Wang, Y.; Wu, W.; Li, X.; Wang, D. Hybrid Recanalization for the Treatment of Carotid/Vertebral In-stent Restenosis or Occlusion: Pilot Surgery Experiences from One Single Center. Front. Neurol. 2020, 11, 604672. [Google Scholar] [CrossRef]
- Hiramoto, J.S.; Katz, R.; Weisman, S.; Conte, M. Gender-specific risk factors for peripheral artery disease in a voluntary screening population. J. Am. Heart Assoc. 2014, 3, e000651. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Park, T.K.; Kim, J.; Ko, Y.G.; Yu, C.W.; Yoon, C.H.; Lee, J.H.; Min, P.K.; Koh, Y.S.; Chae, I.H.; et al. K-VIS Investigators. Sex Differences in Outcomes Following Endovascular Treatment for Symptomatic Peripheral Artery Disease: An Analysis From the K- VIS ELLA Registry. J. Am. Heart Assoc. 2019, 8, e010849. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Paravastu, S.C.V.; Thomas, S.D.; Tan, E.; Farmer, E.; Varcoe, R.L. Cost Analysis of Initial Treatment with Endovascular Revascularization, Open Surgery, or Primary Major Amputation in Patients with Peripheral Artery Disease. J. Endovasc. Ther. 2018, 25, 504–511. [Google Scholar] [CrossRef]
| Endovascular Intervention | Endarterectomy | |
|---|---|---|
| No. of Studies Selected | n = 14 | n = 12 |
| No. of RCT Studies Selected | n = 1 | n = 1 |
| No. of Retrospective Studies Selected | n = 10 | n = 11 |
| No. of Prospective Studies Selected | n = 3 | n = 0 |
| No. of Patients | n = 2496 | n = 4630 |
| No. of Males | n = 1763 | n = 3098 |
| No. of Females | n = 978 | n = 1711 |
| Mean Age of Patients | 68.24 | 69.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravikumar, N.; Sreejith, G.; Law, S.H.C.; Anand, P.; Varghese, N.; Kagdi, S.; Kang, N.; Nashnoush, M.; Salam, S.; Ongidi, I. Comparative Analysis of Endovascular Intervention and Endarterectomy in Patients with Femoral Artery Disease: A Systematic Review and Meta-Analysis. Hematol. Rep. 2022, 14, 179-202. https://doi.org/10.3390/hematolrep14020026
Ravikumar N, Sreejith G, Law SHC, Anand P, Varghese N, Kagdi S, Kang N, Nashnoush M, Salam S, Ongidi I. Comparative Analysis of Endovascular Intervention and Endarterectomy in Patients with Femoral Artery Disease: A Systematic Review and Meta-Analysis. Hematology Reports. 2022; 14(2):179-202. https://doi.org/10.3390/hematolrep14020026
Chicago/Turabian StyleRavikumar, Nidhruv, Gopika Sreejith, Sharon Hiu Ching Law, Prakhar Anand, Noah Varghese, Samrin Kagdi, Navneet Kang, Mohamed Nashnoush, Sihat Salam, and Ibsen Ongidi. 2022. "Comparative Analysis of Endovascular Intervention and Endarterectomy in Patients with Femoral Artery Disease: A Systematic Review and Meta-Analysis" Hematology Reports 14, no. 2: 179-202. https://doi.org/10.3390/hematolrep14020026
APA StyleRavikumar, N., Sreejith, G., Law, S. H. C., Anand, P., Varghese, N., Kagdi, S., Kang, N., Nashnoush, M., Salam, S., & Ongidi, I. (2022). Comparative Analysis of Endovascular Intervention and Endarterectomy in Patients with Femoral Artery Disease: A Systematic Review and Meta-Analysis. Hematology Reports, 14(2), 179-202. https://doi.org/10.3390/hematolrep14020026






