Formation and Evaluation of Complete Blood Count Proficiency Testing Program
Abstract
:1. Introduction
2. Materials and Methods
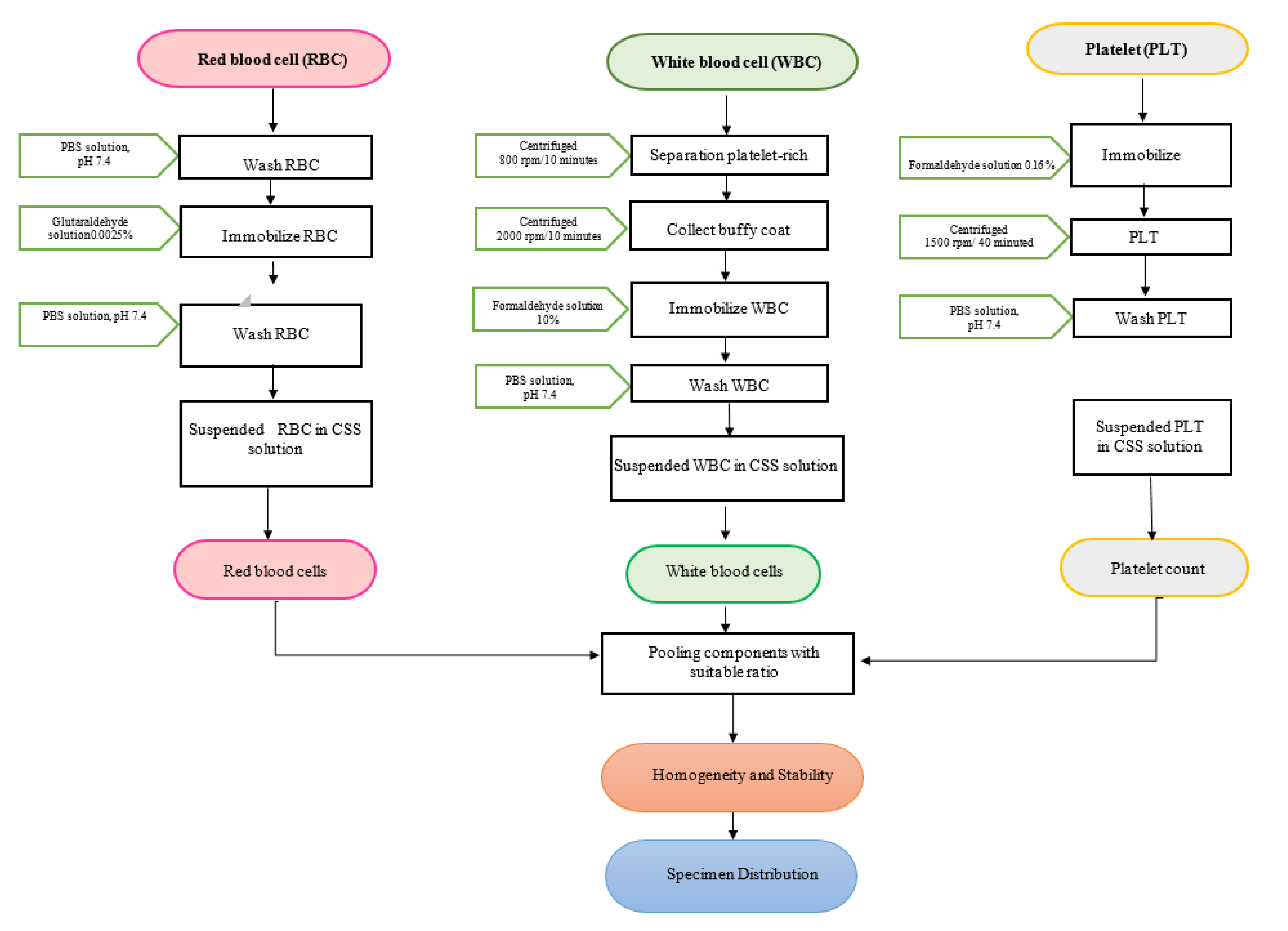
2.1. Material Preparation
- Level 1: RBC (1.5–3.0 1012/L), WBC (2–4 109/L), and PLT (40–150 109/L)
- Level 2: RBC (3.5–4.5 × 1012/L), WBC (5–9 × 109/L), and PLT (150–350 × 109/L)
- Level 3: RBC (4.5–6.0 × 1012/L), WBC (9–30 × 109/L), and PLT (400–700 × 109/L)
2.2. Homogeneity and Stability
2.3. Specimen Distribution
2.4. Instrument Grouping
2.5. Statistical Analysis
- Assigned value ( or mean): determined by the consensus of the participating laboratory; the mean when the data are normally distributed or median when the data are not properly distributed.
- SD: standard deviation
3. Results
3.1. Homogeneity
3.2. Stability
3.3. Peer-Group Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ISO 15189:2012; Medical Laboratories-Requirements for Quality and Competence. International Organization for Standardization: Geneva, Switzerland, 2012.
- Aslan, D. Which Skills are Needed and How They Should be Gained by Laboratory Medicine Professionals for Successful ISO 15189 Accreditation. Electr. J. Int. FCC 2018, 29, 264–273. [Google Scholar]
- Sciacovelli, L.; Secchiero, S.; Padoan, A.; Plebani, M. External quality assessment programs in the context of ISO 15189 accreditation. Clin. Chem. Lab. Med. 2018, 56, 1644–1654. [Google Scholar] [PubMed]
- WHO. Requirements and Guidance for External Quality Assessment Schemes for Health Laboratories, 2nd ed.; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- Lewis, S.M.; Health Laboratory Technology Blood Safety Unit. Quality Assurance in Haematology; World Health Organization: Geneva, Switzerland, 1998; pp. 104–109. [Google Scholar]
- Common Buffers, Media, and Stock Solutions. In Current Protocols in Human Genetics; Willey: Hoboken, NJ, USA, 2000; pp. A.2D.1–A.2D.13. [CrossRef]
- Hunsley, B.; Scholl, J.W. Suspension Composition for Hematology Analysis Control. U.S. Patent Application PCT/US17/044368, 13 June 2019. [Google Scholar]
- De la Salle, B. Survey material choices in haematology EQA: A confounding factor in automated counting performance assessment. Biochem. Med. 2017, 27, 63–72. [Google Scholar]
- Westgard, Q.C. Consolidated Comparison of Hematology Performance Specifications. 2019. Available online: https://www.westgard.com/hematology-goals.htm (accessed on 30 April 2021).
- ISO/IEC 17043; Conformity Assessment-General Requirements for Proficiency Testing. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO Guide 13528; Statistical Methods for Use in Proficiency Testing by Interlaboratory Comparisons. International Organization for Standardization: Geneva, Switzerland, 2015.
- Miller, W.G. Specimen materials, target values and commutability for external quality assessment (proficiency testing) schemes. Clin. Chim. Acta 2003, 327, 25–37. [Google Scholar] [PubMed]
- Vidali, M.; Carobene, A.; Apassiti Esposito, S.; Napolitano, G.; Caracciolo, A.; Seghezzi, M.; Previtali, G.; Lippi, G.; Buoro, S. Standardization and harmonization in hematology: Instrument alignment, quality control materials, and commutability issue. Int. J. Lab. Hematol. 2021, 43, 364–371. [Google Scholar]
- Ebrahim, A.; James Barry, K.D. Stable reference materials for automated hematology testing platforms. U.S. Patent Application PCT/US2021/015351, 28 January 2021. [Google Scholar]
- Kim, J.; Liu, O.; Agresti, J.; Nguyen, A.T. Hydrogel Particles with Tunable Optical Properties and Methods for Using the Same. U.S. Patent Application US-2018275040-A1, 9 February 2015. [Google Scholar]
- Kristensen, G.B.; Meijer, P. Interpretation of EQA results and EQA-based trouble shooting. Biochem. Med. 2017, 27, 49–62. [Google Scholar]
- Zeng, J.; Qi, T.; Wang, S.; Zhang, T.; Zhou, W.; Zhao, H.; Ma, R.; Zhang, J.; Yan, Y.; Dong, J. Commutability of control materials for external quality assessment of serum apolipoprotein A-I measurement. Clin. Chem. Lab. Med. 2018, 56, 789–795. [Google Scholar] [PubMed]
- Miller, W.G.; Jones, G.R.D.; Horowitz, G.L.; Weykamp, C. Proficiency testing/external quality assessment: Current challenges and future directions. Clin. Chem. 2011, 57, 1670–1680. [Google Scholar] [PubMed]
- Miller, W.G.; Myers, G.L. Commutability still matters. Clin. Chem. 2013, 59, 1291–1293. [Google Scholar] [PubMed]
- Miller, W.G.; Myers, G.L.; Rej, R. Why commutability matters. Clin. Chem. 2006, 52, 553–554. [Google Scholar] [PubMed]
- Badrick, T.; Punyalack, W.; Graham, P. Commutability and traceability in EQA programs. Clin. Biochem. 2018, 56, 102–104. [Google Scholar] [PubMed]
- CLSI. Evaluation of Commutability of Processed Samples; Approved Guideline. Document EP14-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Warner, B.; Reardon, D. External quality assessment of the full blood count, and problems associated with the use of fixed blood preparations. Br. J. Biomed. Sci. 1993, 50, 96–102. [Google Scholar] [PubMed]
- The Joint Committee for Traceability in Laboratory Medicine. Database of Reference Materials and Measurement Procedures. Available online: https://www.bipm.org/jctlm/ (accessed on 6 April 2021).
- Deutsches Institut für Normung e. V. (DIN). DIN 58932-5:2007–10; Haematology-Determination of the Concentration of Blood Corpuscles in Blood–Part 5: Reference Method for the Determination of the Concentration of Thrombocytes. DIN: Berlin, Germany, 2007.
- De la Salle, B.J.; McTaggart, P.N.; Briggs, C.; Harrison, P.; Doré, C.J.; Longair, I.; Machin, S.J.; Hyde, K. The accuracy of platelet counting in thrombocytopenic blood samples distributed by the UK National External Quality Assessment Scheme for General Haematology. Am. J. Clin. Pathol. 2012, 137, 65–74. [Google Scholar] [PubMed]
- Hervig, T.; Haugen, T.; Liseth, K.; Kjeldsen-Kragh, J.; Scott, C.S.; Johannessen, B. The platelet count accuracy of platelet concentrates obtained by using automated analysis is influenced by instrument bias and activated platelet components. Vox Sang. 2004, 87, 196–203. [Google Scholar] [PubMed]
| Parameter | Level 1 (n = 10) | Level 2 (n = 10) | Level 3 (n = 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | ss | 0.3 σpt | Mean | ss | 0.3 σpt | Mean | ss | 0.3 σpt | |
| RBC (1012/L) | 1.78 | 0.00 | 0.03 | 4.07 | 0.00 | 0.07 | 5.48 | 0.02 | 0.10 |
| Hb (g/L) | 48.00 | 0.00 | 1.01 | 111.00 | 0.00 | 2.33 | 151.5 | 0.42 | 3.18 |
| Hct (%) | 13.90 | 0.02 | 0.25 | 32.20 | 0.05 | 0.58 | 44.37 | 0.19 | 0.80 |
| MCH (pg) | 27.00 | 0.03 | 0.73 | 27.15 | 0.07 | 0.73 | 27.65 | 0.11 | 0.75 |
| MCV (fL) | 78.10 | 0.03 | 2.34 | 78.70 | 0.15 | 2.36 | 80.97 | 0.09 | 2.43 |
| MCHC (g/L) | 345.50 | 0.46 | 7.26 | 344.50 | 0.75 | 7.23 | 341.40 | 0.64 | 7.17 |
| RDW-CV (%) | 14.75 | 0.03 | 0.04 | 14.95 | 0.02 | 0.04 | 14.90 | 0.03 | 0.04 |
| WBC (109/L) | 2.83 | 0.01 | 0.13 | 6.24 | 0.09 | 0.28 | 29.03 | 0.09 | 1.31 |
| PLT (109/L) | 43.00 | 1.13 | 3.23 | 207.50 | 1.25 | 15.56 | 698.00 | 0.17 | 52.35 |
| MPV (fL) | 13.40 | 0.07 | 0.52 | 13.00 | 0.15 | 0.51 | 12.00 | 0.18 | 0.47 |
| Level | RBC (T/L) | Hb (g/L) | Hct % | MCH (pg) | MCV (fL) | MCHC (g/L) | RD % | WBC (G/L) | PLT (G/L) | MPV (fL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (n = 30) | 1 | 1.78 | 48.00 | 13.90 | 27.00 | 78.10 | 345.50 | 14.75 | 2.83 | 43.00 | 13.40 |
| 0.00 | 0.00 | 0.20 | 0.10 | 0.89 | 5.10 | 0.35 | 0.10 | 1.00 | 0.00 | ||
| 0.00 | 0.00 | 0.22 | 0.10 | 1.00 | 5.60 | 0.45 | 0.12 | 2.00 | 0.10 | ||
| 0.02 | 0.00 | 0.30 | 0.35 | 0.79 | 7.50 | 0.65 | 0.16 | 2.50 | 0.10 | ||
| 0.03 | 0.00 | 0.35 | 0.45 | 0.63 | 8.70 | 0.85 | 0.20 | 3.50 | 0.00 | ||
| 0.01 | 0.25 | 0.40 | 0.10 | 1.57 | 8.10 | 1.40 | 0.26 | 7.00 | 0.10 | ||
| 0.3 σpt | 0.03 | 1.01 | 0.25 | 0.73 | 2.34 | 7.26 | 0.44 | 0.13 | 3.23 | 0.52 | |
| 2 (n = 30) | 1 | 4.07 | 111.00 | 32.20 | 27.15 | 78.70 | 344.50 | 14.95 | 6.24 | 207.50 | 13.00 |
| 0.02 | 0.00 | 0.24 | 0.26 | 1.40 | 2.30 | 0.40 | 0.09 | 12.50 | 0.15 | ||
| 0.01 | 0.00 | 0.40 | 0.19 | 1.60 | 4.00 | 0.70 | 0.21 | 11.50 | 0.30 | ||
| 0.01 | 0.00 | 0.61 | 0.22 | 2.20 | 6.20 | 0.75 | 0.29 | 10.00 | 0.40 | ||
| 0.02 | 0.00 | 0.56 | 0.29 | 2.30 | 5.70 | 0.85 | 0.35 | 17.50 | 0.50 | ||
| 0.02 | 0.00 | 0.63 | 0.26 | 2.35 | 6.40 | 0.85 | 0.35 | 19.50 | 0.40 | ||
| 0.3 σpt | 0.07 | 2.33 | 0.58 | 0.73 | 2.36 | 7.23 | 0.45 | 0.28 | 15.56 | 0.51 | |
| 3 (n = 30) | 1 | 5.48 | 151.50 | 44.37 | 27.65 | 80.97 | 341.40 | 14.90 | 29.03 | 698.00 | 12.00 |
| 0.03 | 3.00 | 0.78 | 0.70 | 1.87 | 0.80 | 0.40 | 0.43 | 18.00 | 0.20 | ||
| 0.06 | 2.50 | 0.98 | 0.10 | 0.89 | 1.80 | 0.50 | 0.48 | 23.00 | 0.40 | ||
| 0.06 | 2.50 | 1.13 | 0.10 | 1.16 | 2.90 | 0.60 | 1.33 | 35.00 | 0.40 | ||
| 0.07 | 2.50 | 1.08 | 0.10 | 0.92 | 2.60 | 0.80 | 1.48 | 53.00 | 0.20 | ||
| 0.05 | 3.00 | 1.23 | 0.28 | 1.51 | 2.65 | 0.95 | 1.44 | 64.75 | 0.23 | ||
| 0.3 σpt | 0.10 | 3.18 | 0.80 | 0.75 | 2.43 | 7.17 | 0.45 | 1.31 | 52.35 | 0.47 |
| Level | Level 1 (N = 78) | Level 3 (N = 75) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | RBC (1012/L) | PLT (109/L) | WBC (109/L) | RBC (1012/L) | PLT (109/L) | WBC (109/L) | ||||||||||||
| Method: Impedance count | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt |
| Sysmex XN Series (n = 15) | 2.7 | 0.01 | 0.05 | 103.0 | 1.56 | 7.73 | 4.9 | 0.03 | 0.09 | 559.1 | 4.26 | 41.94 | ||||||
| Beckman Coulter DxH 600/800/900 series (n = 15) | 2.7 | 0.01 | 0.05 | 111.1 | 2.08 | 8.33 | 2.4 | 0.02 | 0.11 | 4.8 | 0.02 | 0.09 | 569.0 | 5.55 | 42.68 | 10.3 | 0.07 | 0.46 |
| Mindray BC 1000/2000/3000 series (n = 12) | 2.7 | 0.03 | 0.05 | 114.4 | 1.59 | 8.58 | 2.6 | 0.02 | 0.12 | 4.9 | 0.02 | 0.09 | 536.8 | 3.15 | 40.26 | 11.0 | 0.08 | 0.49 |
| Nihon Kohden Celtac Alpha (n = 13) | 2.7 | 0.02 | 0.05 | 117.9 | 3.61 | 8.84 | 2.6 | 0.04 | 0.12 | 4.8 | 0.05 | 0.09 | 563.7 | 4.37 | 42.28 | 10.0 | 0.13 | 0.45 |
| Method: Optical count | ||||||||||||||||||
| Siemens/Bayer Advia 120/2120 (n = 13) | 2.5 | 0.02 | 0.04 | 94.6 | 2.02 | 7.10 | 2.2 | 0.03 | 0.10 | 4.7 | 0.04 | 0.08 | 526.7 | 3.35 | 39.50 | 9.5 | 0.10 | 0.43 |
| Abbott Cell-Dyn Ruby (n = 15) | 2.7 | 0.02 | 0.05 | 122.4 | 2.58 | 9.18 | 2.3 | 0.03 | 0.10 | 5.0 | 0.02 | 0.09 | 550.1 | 4.55 | 41.26 | 10.0 | 0.11 | 0.45 |
| Sysmex XN Series (n = 15) | 2.3 | 0.02 | 0.10 | 10.0 | 0.08 | 0.45 | ||||||||||||
| Level | Level 1 (N = 78) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Hb (g/L) | Hct (%) | MCH (pg) | MCHC (g/L) | MCV (fL) | RDW-CV (%) | MPV (fL) | ||||||||||||||
| Manufacturer/Model | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt |
| Sysmex XN Series (n = 15) | 77.1 | 0.28 | 1.62 | 21.2 | 0.15 | 0.38 | 28.7 | 0.13 | 0.77 | 363.7 | 2.30 | 7.64 | 78.9 | 0.30 | 2.37 | 13.2 | 0.05 | 0.04 | 11.1 | 0.05 | 0.43 |
| Abbott Cell-Dyn Ruby (n = 15) | 76.6 | 0.26 | 1.61 | 18.1 | 0.16 | 0.33 | 28.5 | 0.19 | 0.77 | 422.4 | 2.97 | 8.87 | 67.5 | 0.50 | 2.03 | 6.9 | 0.06 | 0.02 | 6.4 | 0.15 | 0.25 |
| Siemens/Bayer Advia 120/2120 (n = 13) | 80.0 | 0.32 | 1.68 | 18.0 | 0.12 | 0.32 | 32.6 | 0.22 | 0.88 | 446.6 | 3.27 | 9.38 | 73.1 | 0.41 | 2.19 | 14.2 | 0.06 | 0.04 | 10.2 | 0.11 | 0.40 |
| Beckman Coulter DxH 600/800/900 series (n = 15) | 76.5 | 0.51 | 1.61 | 21.7 | 0.10 | 0.39 | 28.6 | 0.18 | 0.77 | 352.9 | 2.47 | 7.41 | 80.9 | 0.29 | 2.43 | 14.5 | 0.08 | 0.04 | 8.5 | 0.11 | 0.33 |
| Mindray BC 1000/2000/3000 series (n = 12) | 76.6 | 0.42 | 1.61 | 21.7 | 0.23 | 0.39 | 28.2 | 0.28 | 0.76 | 350.6 | 5.01 | 7.36 | 79.8 | 0.85 | 2.39 | 13.4 | 0.11 | 0.04 | 8.9 | 0.12 | 0.35 |
| Nihon Kohden Celtac Alpha (n = 13) | 78.9 | 0.93 | 1.66 | 21.9 | 0.27 | 0.39 | 29.3 | 0.21 | 0.79 | 359.6 | 3.01 | 7.55 | 81.6 | 0.68 | 2.45 | 14.3 | 0.27 | 0.04 | 8.1 | 0.24 | 0.32 |
| Level | Level 3 (N = 75) | ||||||||||||||||||||
| Parameter | Hb (g/L) | Hct (%) | MCH (pg) | MCHC (g/L) | MCV (fL) | RDW-CV (%) | MPV (fL) | ||||||||||||||
| Manufacturer/Model | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt | Mean | Um | 0.3σ _pt |
| Sysmex XN Series (n = 15) | 139.9 | 0.35 | 2.94 | 39.1 | 0.21 | 0.70 | 28.4 | 0.17 | 0.77 | 358.3 | 1.77 | 7.53 | 79.1 | 0.27 | 2.37 | 13.4 | 0.09 | 0.04 | 11.2 | 0.07 | 0.44 |
| Abbott Cell-Dyn Ruby (n = 15) | 138.5 | 0.39 | 2.91 | 33.9 | 0.16 | 0.61 | 27.8 | 0.15 | 0.75 | 408.1 | 1.62 | 8.57 | 68.1 | 0.24 | 2.04 | 7.2 | 0.06 | 0.02 | 5.8 | 0.15 | 0.23 |
| Siemens/Bayer Advia 120/2120 (n = 13) | 141.5 | 0.34 | 2.97 | 33.7 | 0.33 | 0.61 | 30.3 | 0.23 | 0.82 | 419.1 | 3.78 | 8.80 | 72.6 | 0.60 | 2.18 | 12.7 | 0.11 | 0.04 | 10.5 | 0.09 | 0.41 |
| Beckman Coulter D × H 600/800/900 series (n = 15) | 137.4 | 0.43 | 2.89 | 39.3 | 0.15 | 0.71 | 28.4 | 0.13 | 0.77 | 349.6 | 1.43 | 7.34 | 81.4 | 0.12 | 2.44 | 14.7 | 0.06 | 0.04 | 9.1 | 0.12 | 0.36 |
| Mindray BC 1000/2000/3000 series (n = 12) | 141.3 | 0.44 | 2.97 | 39.9 | 0.14 | 0.72 | 28.6 | 0.08 | 0.77 | 354.2 | 1.02 | 7.44 | 80.8 | 0.09 | 2.42 | 13.5 | 0.05 | 0.04 | 9.0 | 0.07 | 0.35 |
| Nihon Kohden Celtac Alpha (n = 13) | 140.8 | 0.25 | 2.96 | 39.0 | 0.41 | 0.70 | 29.2 | 0.30 | 0.79 | 361.0 | 3.65 | 7.58 | 80.9 | 0.07 | 2.43 | 13.8 | 0.09 | 0.04 | 8.4 | 0.27 | 0.33 |
| Level | Level 1 (N = 78) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | RBC (1012/L) | Hb (g/L) | Hct (%) | MCH (pg) | MCHC (g/L) | MCV (fL) | RDW-CV (%) | WBC (109/L) | PLT (109/L) | MPV (fL) |
| All methods CV (%) | 1.7 | 1.5 | 2.2 | 1.8 | 2.2 | 1.7 | 2.1 | 2.8 | 5f.2 | 4.1 |
| Mean CV(%) by instrument | ||||||||||
| Sysmex XN Series (n = 15) | 1.5 | 1.1 | 2.1 | 1.4 | 1.9 | 1.1 | 1.1 | 3.0 | 4.5 | 1.5 |
| Abbott Cell-Dyn Ruby (n = 15) | 2.0 | 0.9 | 2.3 | 1.7 | 1.8 | 1.9 | 2.3 | 2.9 | 5.3 | 6.1 |
| Siemens/Bayer Advia 120/2120 (n = 13) | 1.6 | 1.0 | 1.7 | 1.7 | 1.9 | 1.4 | 1.1 | 2.9 | 5.4 | 2.8 |
| Beckman Coulter DxH 600/800/900 series (n = 15) | 1.0 | 1.7 | 1.2 | 1.6 | 1.8 | 0.9 | 1.4 | 2.0 | 4.7 | 3.2 |
| Mindray BC 1000/2000/3000 series (n = 12) | 2.4 | 1.4 | 2.7 | 2.5 | 3.6 | 2.7 | 2.1 | 2.0 | 3.5 | 3.3 |
| Nihon Kohden Celtac Alpha (n = 13) | 1.9 | 3.0 | 3.1 | 1.9 | 2.1 | 2.1 | 4.8 | 4.2 | 7.7 | 7.6 |
| Level | Level 3 (N = 75) | |||||||||
| Parameter | RBC (1012/L) | Hb (g/L) | Hct (%) | MCH (pg) | MCHC (g/L) | MCV (fL) | RDW-CV (%) | WBC (109/L) | PLT (109/L) | MPV (fL) |
| All methods CV (%) | 1.6 | 0.7 | 1.7 | 1.6 | 1.5 | 0.8 | 1.7 | 2.5 | 2.0 | 4.2 |
| Mean CV (%) by instrument | ||||||||||
| Sysmex XN Series (n = 15) | 1.7 | 0.8 | 1.7 | 1.9 | 1.5 | 1.0 | 2.1 | 2.4 | 2.4 | 2.0 |
| Abbott Cell-Dyn Ruby (n = 15) | 1.1 | 0.7 | 1.2 | 1.4 | 1.1 | 0.9 | 2.3 | 3.0 | 2.2 | 6.9 |
| Siemens/Bayer Advia 120/2120 (n = 13) | 2.2 | 0.6 | 2.4 | 1.9 | 2.3 | 2.1 | 2.2 | 2.5 | 1.6 | 2.2 |
| Beckman Coulter DxH 600/800/900 series (n = 15) | 0.8 | 0.8 | 1.0 | 1.1 | 1.0 | 0.4 | 1.1 | 1.8 | 2.5 | 3.4 |
| Mindray BC 1000/2000/3000 series (n = 12) | 1.1 | 0.9 | 1.0 | 0.8 | 0.8 | 0.3 | 0.7 | 2.1 | 1.6 | 2.3 |
| Nihon Kohden Celtac Alpha (n = 13) | 2.6 | 0.4 | 2.6 | 2.6 | 2.6 | 0.2 | 1.7 | 3.3 | 2.0 | 8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, H.Q.; Le, O.H.; Truong, D.C.; Nguyen, D.N.; Van, T.H.; Le, V.T.K.; Vang, L.T.T. Formation and Evaluation of Complete Blood Count Proficiency Testing Program. Hematol. Rep. 2022, 14, 73-84. https://doi.org/10.3390/hematolrep14020012
Vu HQ, Le OH, Truong DC, Nguyen DN, Van TH, Le VTK, Vang LTT. Formation and Evaluation of Complete Blood Count Proficiency Testing Program. Hematology Reports. 2022; 14(2):73-84. https://doi.org/10.3390/hematolrep14020012
Chicago/Turabian StyleVu, Huy Quang, Oanh Hoang Le, Duan Cong Truong, Dung Ngoc Nguyen, Triet Hy Van, Van Thi Kieu Le, and Linh Thi Truc Vang. 2022. "Formation and Evaluation of Complete Blood Count Proficiency Testing Program" Hematology Reports 14, no. 2: 73-84. https://doi.org/10.3390/hematolrep14020012
APA StyleVu, H. Q., Le, O. H., Truong, D. C., Nguyen, D. N., Van, T. H., Le, V. T. K., & Vang, L. T. T. (2022). Formation and Evaluation of Complete Blood Count Proficiency Testing Program. Hematology Reports, 14(2), 73-84. https://doi.org/10.3390/hematolrep14020012






