Impact of Interstock and Rootstock on the Growth and Productivity of Mango (Mangifera indica L.) Cultivar Kent in the San Lorenzo Valley, Peru
Abstract
1. Introduction
2. Materials and Methods
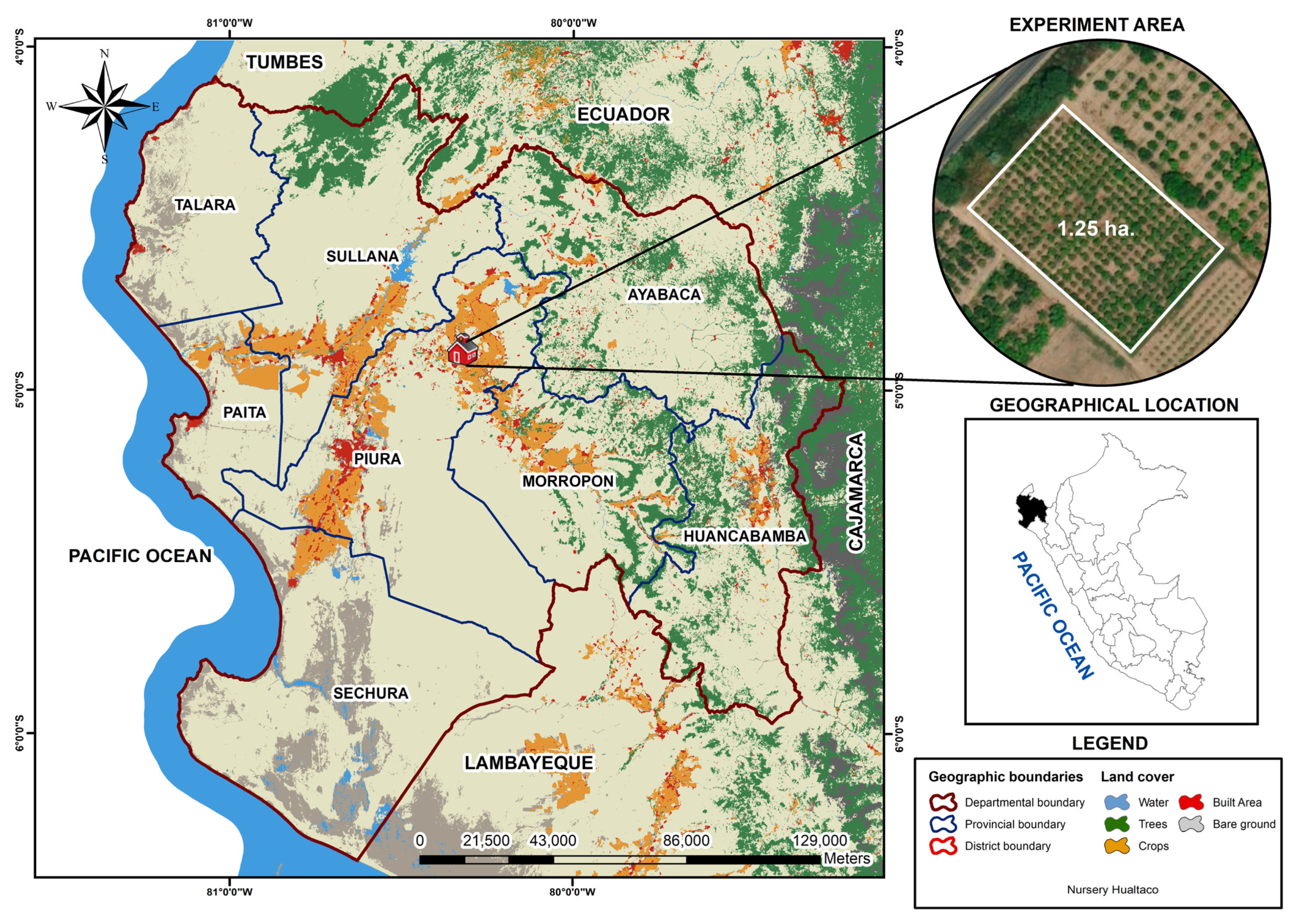
2.1. Study Area
2.2. Plant Material
2.3. Agronomic Management
2.4. Agronomic and Biometric Characterization of the Tree
2.5. Experimental Design
2.6. Statistical Analysis
3. Results
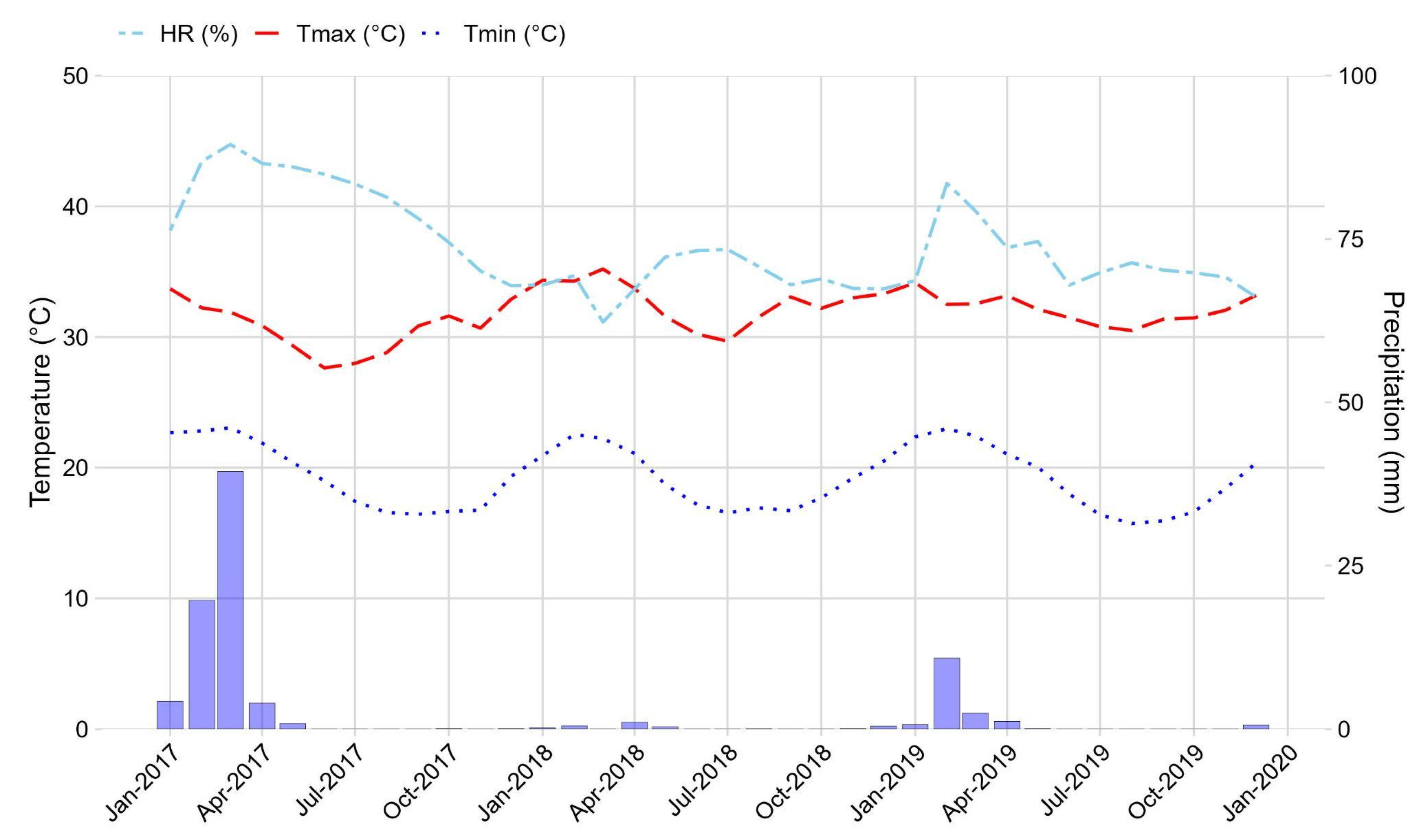
3.1. Weather Conditions
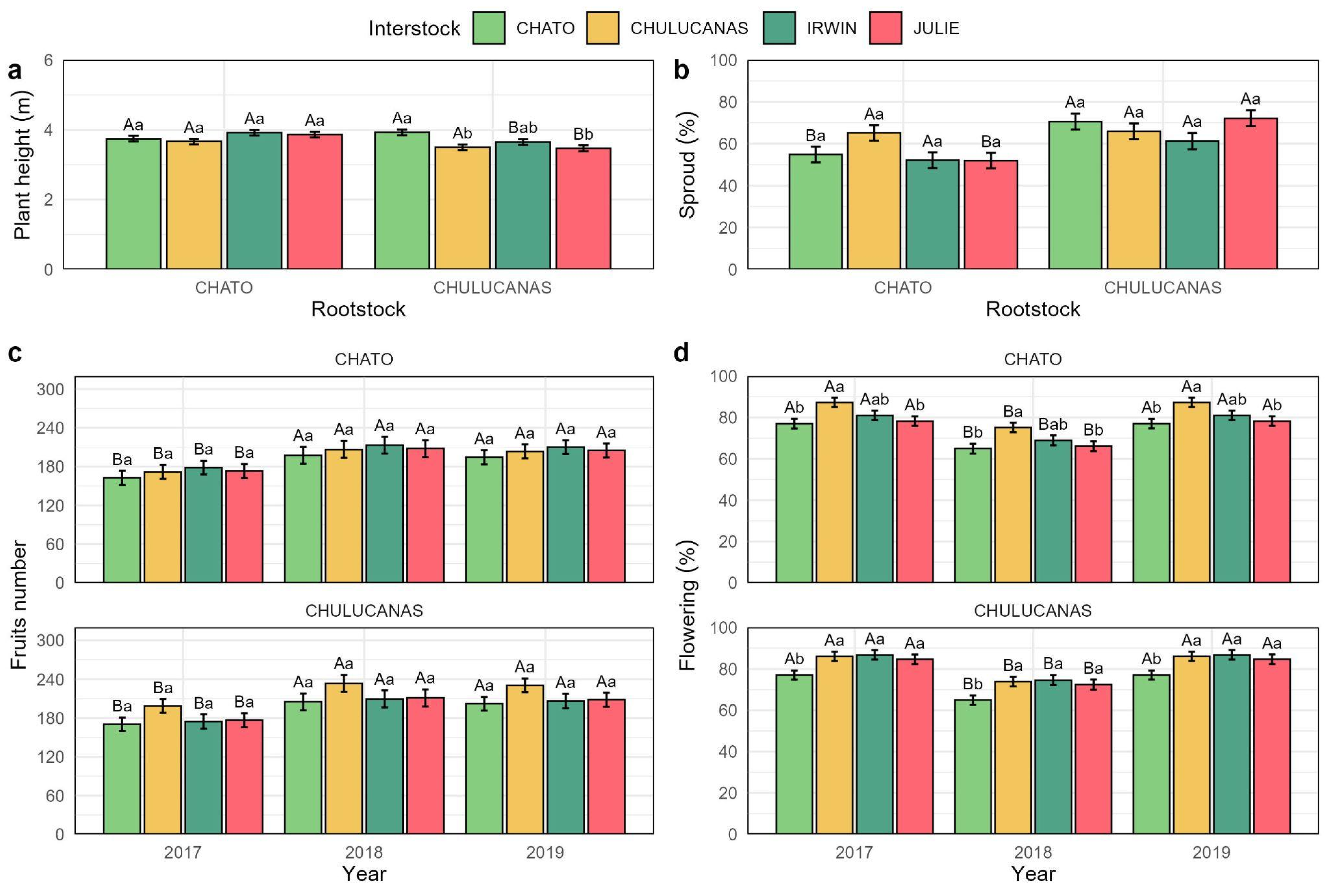
3.2. Agronomic Characterization
3.3. Biometric Characterization of the Fruit
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honja, T. Review of Mango Value Chain in Ethiopia. J. Biol. Agric. Healthc. 2014, 4, 230–239. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 5 November 2025).
- Ministerio de Desarrollo Agrario; Riego, M. Sistema Integrado de Estadísticas Agrarias. Available online: https://siea.midagri.gob.pe/portal/ (accessed on 10 April 2025).
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Ramírez, F.; Davenport, T.L. Mango (Mangifera indica L.) Pollination: A Review. Sci. Hortic. 2016, 203, 158–168. [Google Scholar] [CrossRef]
- Matheyambath, A.C.; Subramanian, J.; Paliyath, G. Mangoes. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 641–645. ISBN 978-0-12-384953-3. [Google Scholar]
- Galán Saúco, V.; de Queiroz Pinto, A.C.; Mitra, S.K.; Faleiro, F.G.; Ferreira, F.R. Mango Propagation. In The Mango Genome; Kole, C., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 31–44. ISBN 978-3-030-47829-2. [Google Scholar]
- Johnson, P.R.; Robinson, D.M. The Tatura Trellis System for High Density Mangoes. Acta Hortic. 2000, 509, 359–364. [Google Scholar] [CrossRef]
- Menzel, C.M.; Le Lagadec, M.D. Can the Productivity of Mango Orchards Be Increased by Using High-Density Plantings? Sci. Hortic. 2017, 219, 222–263. [Google Scholar] [CrossRef]
- Souza, M.P.; Queiroz, M.A.; Possídio, E.L.; Pereira, F.A.; Nunes, R.F.M. Study of Flowering and Alternate Bearing of Mango Varieties in the São Francisco Valley. Acta Hortic. 2004, 645, 353–358. [Google Scholar] [CrossRef]
- Beshir, W.; Alemayehu, M.; Dessalegn, Y. Effect of Grafting Time and Technique on the Success Rate of Grafted Mango (Mangifera indica L.) in Kalu District of Amhara Region, North Eastern Ethiopia. Cogent Food Agric. 2019, 5, 1577023. [Google Scholar] [CrossRef]
- Ibell, P.T.; Normand, F.; Wright, C.L.; Mahmud, K.; Bally, I.S.E. The Effects of Planting Density, Training System and Cultivar on Vegetative Growth and Fruit Production in Young Mango (Mangifera indica L.) Trees. Horticulturae 2024, 10, 937. [Google Scholar] [CrossRef]
- Coral, L.L.T.; Escobar-Garcia, H.A. Characterization of Fruits of Varieties of Mango (Mangifera indica) Conserved in Peru. Rev. Bras. Frutic. 2021, 43, 8. [Google Scholar] [CrossRef]
- Mahmud, K.P.; Ibell, P.T.; Wright, C.L.; Monks, D.; Bally, I. High-Density Espalier Trained Mangoes Make Better Use of Light. Agronomy 2023, 13, 2557. [Google Scholar] [CrossRef]
- Feng, M.; Augstein, F.; Kareem, A.; Melnyk, C.W. Plant Grafting: Molecular Mechanisms and Applications. Mol. Plant 2024, 17, 75–91. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Alcaraz-López, C.; Muries, B.; Mota-Cadenas, C.; Carvajal, M. Physiological Aspects of Rootstock–Scion Interactions. Sci. Hortic. 2010, 127, 112–118. [Google Scholar] [CrossRef]
- Castle, W.S.; Bowman, K.D.; Baldwin, J.C.; Grosser, J.W.; Gmitter, F.G. Rootstocks Affect Tree Growth, Yield, and Juice Quality of ‘Marsh’ Grapefruit. HortScience 2011, 46, 841–848. [Google Scholar] [CrossRef]
- Khurshid, T.; Creek, A.; Sanderson, G.; Zhao, X. Tree Performance, Yield, and Fruit Quality of ‘Valencia’ Sweet Orange (Citrus sinensis L. Osbeck) Selections on New Poncirus Trifoliata Rootstocks. Horticulturae 2024, 10, 393. [Google Scholar] [CrossRef]
- Lazare, S.; Haberman, A.; Yermiyahu, U.; Erel, R.; Simenski, E.; Dag, A. Avocado Rootstock Influences Scion Leaf Mineral Content. Arch. Agron. Soil. Sci. 2019, 66, 1399–1409. [Google Scholar] [CrossRef]
- Tietel, Z.; Srivastava, S.; Fait, A.; Tel-Zur, N.; Carmi, N.; Raveh, E. Impact of Scion/Rootstock Reciprocal Effects on Metabolomics of Fruit Juice and Phloem Sap in Grafted Citrus reticulata. PLoS ONE 2020, 15, e0227192. [Google Scholar] [CrossRef]
- Baron, D.; Esteves Amaro, A.C.; Pina, A.; Ferreira, G. An Overview of Grafting Re-Establishment in Woody Fruit Species. Sci. Hortic. 2019, 243, 84–91. [Google Scholar] [CrossRef]
- Lu, P.; Chacko, E.K.; Bithell, S.L.; Schaper, H.; Wiebel, J.; Cole, S.; Müller, W.J. Productivity Is Negatively Related to Shoot Growth across Five Mango Cultivars in the Seasonally Wet-Dry Tropics of Northern Australia. Fruits 2013, 68, 279–289. [Google Scholar] [CrossRef]
- Bally, I.S.E.; Johnson, P.R.; Kulkarni, V.J. Mango Production in Australia. Acta Hortic. 2000, 509, 59–68. [Google Scholar] [CrossRef]
- Silva, A.C.d.; Souza, A.P.d.; Leonel, S.; Souza, M.E.d.; Ramos, D.P.; Tanaka, A.A. Growth and Flowering of Five Mango Cultivar under Subtropics Conditions of Brazil. Am. J. Plant Sci. 2014, 5, 393–402. [Google Scholar] [CrossRef]
- Smith, M.W.; Bright, J.D.; Hoult, M.D.; Renfree, R.A.; Maddern, T.; Coombes, N. Field Evaluation of 64 Rootstocks for Growth and Yield of ‘Kensington Pride’ Mango. HortScience 2008, 43, 1720–1725. [Google Scholar] [CrossRef]
- Dayal, V.; Dubey, A.K.; Singh, S.K.; Sharma, R.M.; Dahuja, A.; Kaur, C. Growth, Yield and Physiology of Mango (Mangifera indica L.) Cultivars as Affected by Polyembryonic Rootstocks. Sci. Hortic. 2016, 199, 186–197. [Google Scholar] [CrossRef]
- Jukić Špika, M.; Dumičić, G.; Brkić Bubola, K.; Soldo, B.; Goreta Ban, S.; Vuletin Selak, G.; Ljubenkov, I.; Mandušić, M.; Žanić, K. Modification of the Sensory Profile and Volatile Aroma Compounds of Tomato Fruits by the Scion × Rootstock Interactive Effect. Front. Plant Sci. 2021, 11, 616431. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, E.; Sôcola, Z.; Choque, T.; Hernandez, M. Evaluation of the Color of Mango Puree (Mangifera indica) Creole Variety of Chulucanas Influenced by the Size of the Refined Pulp and Interaction Time to Environmental Air. J. Phys. Conf. Ser. 2021, 1828, 012128. [Google Scholar] [CrossRef]
- Knight, R.J., Jr.; Campbell, R.J.; Maguire, I. Important Mango Cultivars and Their Descriptors. In The Mango: Botany, Production and Uses; Botany, Production and Uses; CAB International: Wallingford, UK, 2009; pp. 42–66. ISBN 978-1-84593-489-7. [Google Scholar]
- Wijethunga, W.M.U.D.; Shin, M.H.; Jayasooriya, L.S.H.; Kim, G.H.; Park, K.M.; Cheon, M.G.; Choi, S.W.; Kim, H.L.; Kim, J.G. Evaluation of Fruit Quality Characteristics in ‘Irwin’ Mango Grown via Forcing Cultivation in a Plastic Facility. Hortic. Sci. Technol. 2023, 41, 617–633. [Google Scholar] [CrossRef]
- Esan, V.I.; Ogunbode, T.O.; Ogunlaran, O.M.; Ayegboyin, M.H.; Omilani, O.O.; Sangoyomi, T.E.; Akande, J.A. Genetic Variability and Morpho-Agronomic Characterization of Some Mango (Mangifera indica L.) Cultivars and Varieties in Nigeria. Int. J. Fruit Sci. 2024, 24, 256–272. [Google Scholar] [CrossRef]
- Yadav, D.; Pal, A.K.; Singh, S.P. Vegetative Methods of Plant Propagation: II- Grafting Cutting Layering and Budding in Mango. Int. J. Pure Appl. Biosci. 2018, 6, 575–586. [Google Scholar] [CrossRef]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms Underlying Graft Union Formation and Rootstock Scion Interaction in Horticultural Plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Adhikari, P.B.; Xu, Q.; Notaguchi, M. Compatible Graft Establishment in Fruit Trees and Its Potential Markers. Agronomy 2022, 12, 1981. [Google Scholar] [CrossRef]
- Lopes, R.d.C.; Pereira, R.N.; Silva, L.d.S.; Lobo, J.T.; Araújo e Amariz, R.; Cavalcante, Í.H.L. Impact of First Mechanical Fructification Pruning on Mango Orchards. Int. J. Fruit Sci. 2021, 21, 1059–1072. [Google Scholar] [CrossRef]
- Osuna-Enciso, T.; Pérez-Barraza, M.H.; Martínez-Alvarado, C.O.; Hernández-Verdugo, S.; Osuna-Rodríguez, J.M.; Martín-Matheis, H.S. Retraso de Floración y Calidad del Fruto de Mango Kent en el Sur de Sinaloa, México. Rev. Fitotec. Mex. 2023, 46, 389. [Google Scholar] [CrossRef]
- Alshallash, K.S.; Sharaf, M.; Hmdy, A.E.; Khalifa, S.M.; Abdel-Aziz, H.F.; Sharaf, A.; Ibrahim, M.T.S.; Alharbi, K.; Elkelish, A. Hydrogel Improved Growth and Productive Performance of Mango Trees under Semi-Arid Condition. Gels 2022, 8, 602. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.5.2; R Core Team: Vienna, Austria, 2024.
- Schielzeth, H.; Dingemanse, N.J.; Nakagawa, S.; Westneat, D.F.; Allegue, H.; Teplitsky, C.; Réale, D.; Dochtermann, N.A.; Garamszegi, L.Z.; Araya-Ajoy, Y.G. Robustness of Linear Mixed-Effects Models to Violations of Distributional Assumptions. Methods Ecol. Evol. 2020, 11, 1141–1152. [Google Scholar] [CrossRef]
- Lenth, R.V.; Bolker, B.; Buerkner, P.; Giné-Vázquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Piaskowski, J.; Riebl, H.; et al. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2024. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 12 July 2025).
- Husson, F.; Josse, J.; Le, S.; Mazet, J. FactoMineR: Multivariate Exploratory Data Analysis and Data Mining. 2024. Available online: https://cran.r-project.org/web/packages/FactoMineR/index.html (accessed on 12 July 2025).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J.; Freidank, M.; Cai, J.; Protivinsky, T. Corrplot: Visualization of A Correlation Matrix. 2024. Available online: https://cran.r-project.org/web/packages/corrplot/index.html (accessed on 12 July 2025).
- Dubey, A.K.; Sharma, R.M.; Deepak; Kumar, A. Long Term Performance of Mango Varieties on Five Polyembryonic Rootstocks under Subtropical Conditions: Effect on Vigour, Yield, Fruit Quality and Nutrient Acquisition. Sci. Hortic. 2021, 280, 109944. [Google Scholar] [CrossRef]
- El Shahawy, S.A.; Nomier, S.A.A.; Mohsen, F.M.S.; Gad, M.M. Identification of the Promising Mango Rootstocks and Their Response to Grafting of Some Foreign Mango Cultivarsin the Nursery. Zagazig J. Agric. Res. 2023, 50, 43–53. [Google Scholar] [CrossRef]
- Hamza, A.; Razzaq, K.; Umair, M.; Hussain, A.; Zafar, M.S.; Rehman, A.U. Standardization of Grafting Height for Growth and Development of Mango (Mangifera indica L.) Nursery Plants. Data Plus 2023, 1, 65–70. [Google Scholar] [CrossRef]
- Minja, R.R.; Kimaro, A.A.; Mpanda, M.; Moshy, S.; Mwaijande, V.; Ngereza, A.; Ambrose, J.; Ndee, A.; Kihula, B.; Nyalusi, G. Effects of Rootstock Type and Scion Cultivar on Grafting Success and Growth of Mango (Mangifera indica L.) Seedlings. J. Exp. Agric. Int. 2017, 32, 179–189. [Google Scholar] [CrossRef]
- Instituto Nacional de Innovación Agraria (INIA). Manejo Integrado del Cultivo de Mango Kent; Instituto Nacional de Innovación Agraria: Lima, Peru, 2019. [Google Scholar]
- Cui, Q.; Xie, L.; Dong, C.; Gao, L.; Shang, Q. Stage-Specific Events in Tomato Graft Formation and the Regulatory Effects of Auxin and Cytokinin. Plant Sci. 2021, 304, 110803. [Google Scholar] [CrossRef]
- Sharma, A.; Zheng, B. Molecular Responses during Plant Grafting and Its Regulation by Auxins, Cytokinins, and Gibberellins. Biomolecules 2019, 9, 397. [Google Scholar] [CrossRef]
- Nanda, A.K.; Melnyk, C.W. The Role of Plant Hormones during Grafting. J. Plant Res. 2018, 131, 49–58. [Google Scholar] [CrossRef]
- Emire, A.; Demise, S.; Giri, T.; Tadele, W. Growth and Yield Performance Evaluation of Mango (Mangifera indica L.) Varieties in Adola Rede District, Guji Zone, Southern Ethiopia. Am. J. Plant Biol. 2022, 7, 136–142. [Google Scholar] [CrossRef]
- Scuderi, D.; Gianguzzi, G.; Tinebra, I.; Gugliuzza, G.; Farina, V. Phenology and Fruit Growth Dynamics of Mango (Mangifera indica L.) in Greenhouse and Open Air in Mediterranean Climate. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2023, 93, 731–741. [Google Scholar] [CrossRef]
- Rebolledo-Martínez, A.; Peralta-Antonio, N.; Rebolledo-Martínez, L.; Becerril-Román, E.A.; Rebolledo-García, R.L. Effect of Rootstock in Tree Growth, Dry Matter, Flowering, Yield and Quality of ‘Manila’ Mango. Sci. Hortic. 2019, 251, 155–161. [Google Scholar] [CrossRef]
- Shivran, M.; Sharma, N.; Dubey, A.K.; Singh, S.K.; Sharma, N.; Muthusamy, V.; Jain, M.; Singh, B.P.; Singh, N.; Kumar, N.; et al. Scion/Rootstock Interaction Studies for Quality Traits in Mango (Mangifera indica L.) Varieties. Agronomy 2023, 13, 204. [Google Scholar] [CrossRef]
- Reddy, Y.T.N.; Kurian, R.M.; Ramachander, P.R.; Singh, G.; Kohli, R.R. Long-Term Effects of Rootstocks on Growth and Fruit Yielding Patterns of ‘Alphonso’ Mango (Mangifera indica L.). Sci. Hortic. 2003, 97, 95–108. [Google Scholar] [CrossRef]
- Vittal, H.; Sharma, N.; Dubey, A.K.; Shivran, M.; Singh, S.K.; Meena, M.C.; Kumar, N.; Sharma, N.; Singh, N.; Pandey, R.; et al. Rootstock-Mediated Carbohydrate Metabolism, Nutrient Contents, and Physiological Modifications in Regular and Alternate Mango (Mangifera indica L.) Scion Varieties. PLoS ONE 2023, 18, e0284910. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Liu, T.; Folta, K.; Sarkhosh, A. Physiological, Biochemical, and Molecular Aspects of Grafting in Fruit Trees. Hortic. Res. 2022, 9, uhac032. [Google Scholar] [CrossRef] [PubMed]
- Mizani, A.; Bally, I.S.E.; Ibell, P.T.; Wright, C.; Maddox, C. The Effect of Rootstocks on Mango Tree Vigour, Scion Architecture, Yield, Percentage of Flowering Terminals in Young Unpruned Trees. Acta Hortic. 2022, 1346, 183–190. [Google Scholar] [CrossRef]
- Halder, R.; Varma, S.; Singh, M.; Dahiya, A. From Orchard to Table: Understanding Climate Change Impacts on Mango Production in India: A Review. Int. J. Adv. Biochem. Res. 2024, 8, 472–478. [Google Scholar] [CrossRef]
- Normand, F.; Lauri, P.-E.; Legave, J.-M. Climate Change and its Probable Effects on Mango Production and Cultivation. Acta Hortic. 2015, 1075, 21–31. [Google Scholar] [CrossRef]
- Ogunbode, T.O.; Esan, V.I.; Ayegboyin, M.H.; Ogunlaran, O.M.; Sangoyomi, E.T.; Akande, J.A. Understanding the Perception of Mango (Mangifera indica) Farmers on the Impact of Climate Change on Mango Farming in Nigeria. Int. J. Agron. 2024, 2024, 6486998. [Google Scholar] [CrossRef]
- Aldana, C.; Saavedra, Y.; Gonzales, J.; Gálvez, D.; Palacios, C.; Aldana, W.; Moncada, W. Producción agrícola espacial-temporal del Citrus x limon y Mangifera indica, mediante firmas espectrales e imágenes de satélite. Sci. Agropecu. 2021, 12, 557–570. [Google Scholar] [CrossRef]
- Gouveia, C.M.; Justino, F.; Gurjao, C.; Zita, L.; Alonso, C. Revisiting Climate-Related Agricultural Losses across South America and Their Future Perspectives. Atmosphere 2023, 14, 1303. [Google Scholar] [CrossRef]


| Treatment | Rootstock | Interstock | Scion |
|---|---|---|---|
| T1 | Chulucanas | Chulucanas | Kent |
| T2 | Chulucanas | Chato | Kent |
| T3 | Chulucanas | Julie | Kent |
| T4 | Chulucanas | Irwin | Kent |
| T5 | Chato | Chulucanas | Kent |
| T6 | Chato | Chato | Kent |
| T7 | Chato | Julie | Kent |
| T8 | Chato | Irwin | Kent |
| Variable | Rootstock | Interstock | Mean | sd | Min | Max |
|---|---|---|---|---|---|---|
| Fruit Weight (g) | Chato | Chato | 452.53 | 79.92 | 305 | 639 |
| Chato | Chulucanas | 462.34 | 69.82 | 340 | 620 | |
| Chato | Irwin | 482.03 | 87.3 | 350 | 700 | |
| Chato | Julie | 465.97 | 92.07 | 300 | 645 | |
| Chulucanas | Chato | 484.97 | 101.98 | 316 | 765 | |
| Chulucanas | Chulucanas | 468.17 | 118.73 | 230 | 665 | |
| Chulucanas | Irwin | 447.23 | 70.09 | 310 | 605 | |
| Chulucanas | Julie | 484.7 | 93.46 | 367 | 717 | |
| Fruit length (mm) | Chato | Chato | 105.97 | 6.78 | 93 | 120 |
| Chato | Chulucanas | 106.28 | 6.8 | 94 | 119 | |
| Chato | Irwin | 106.07 | 6.47 | 95 | 124 | |
| Chato | Julie | 107.67 | 7.54 | 91 | 120 | |
| Chulucanas | Chato | 106.53 | 7.21 | 95 | 123 | |
| Chulucanas | Chulucanas | 106.2 | 10.87 | 86 | 126 | |
| Chulucanas | Irwin | 106.43 | 7.28 | 92 | 122 | |
| Chulucanas | Julie | 109.73 | 8.03 | 98 | 129 | |
| Fruit diameter (mm) | Chato | Chato | 85.23 | 4.72 | 76 | 97.5 |
| Chato | Chulucanas | 86.09 | 3.61 | 78.5 | 93.5 | |
| Chato | Irwin | 87.63 | 5.07 | 79.5 | 100 | |
| Chato | Julie | 86.23 | 5.56 | 75.5 | 97.5 | |
| Chulucanas | Chato | 87.7 | 5.4 | 78 | 100.5 | |
| Chulucanas | Chulucanas | 86.08 | 6.61 | 69.5 | 99 | |
| Chulucanas | Irwin | 84.53 | 4.03 | 77.5 | 92.5 | |
| Chulucanas | Julie | 86.3 | 4.68 | 79.5 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas-Niño, S.; Vilchez-Navarro, S.; Morocho-Romero, H.; Cárdenas-Huamán, G.; Nuñez-Ticliahuanca, E.-O.; Montañez-Artica, A.-G.; Velarde-Apaza, L.; Ramirez Rojas, M.; Rojas, J.C.; Lozano-Isla, F. Impact of Interstock and Rootstock on the Growth and Productivity of Mango (Mangifera indica L.) Cultivar Kent in the San Lorenzo Valley, Peru. Int. J. Plant Biol. 2025, 16, 134. https://doi.org/10.3390/ijpb16040134
Casas-Niño S, Vilchez-Navarro S, Morocho-Romero H, Cárdenas-Huamán G, Nuñez-Ticliahuanca E-O, Montañez-Artica A-G, Velarde-Apaza L, Ramirez Rojas M, Rojas JC, Lozano-Isla F. Impact of Interstock and Rootstock on the Growth and Productivity of Mango (Mangifera indica L.) Cultivar Kent in the San Lorenzo Valley, Peru. International Journal of Plant Biology. 2025; 16(4):134. https://doi.org/10.3390/ijpb16040134
Chicago/Turabian StyleCasas-Niño, Sebastian, Sandy Vilchez-Navarro, Henry Morocho-Romero, Gabriela Cárdenas-Huamán, Esdwin-Oberti Nuñez-Ticliahuanca, Ana-Gabriela Montañez-Artica, Leslie Velarde-Apaza, Max Ramirez Rojas, Juan Carlos Rojas, and Flavio Lozano-Isla. 2025. "Impact of Interstock and Rootstock on the Growth and Productivity of Mango (Mangifera indica L.) Cultivar Kent in the San Lorenzo Valley, Peru" International Journal of Plant Biology 16, no. 4: 134. https://doi.org/10.3390/ijpb16040134
APA StyleCasas-Niño, S., Vilchez-Navarro, S., Morocho-Romero, H., Cárdenas-Huamán, G., Nuñez-Ticliahuanca, E.-O., Montañez-Artica, A.-G., Velarde-Apaza, L., Ramirez Rojas, M., Rojas, J. C., & Lozano-Isla, F. (2025). Impact of Interstock and Rootstock on the Growth and Productivity of Mango (Mangifera indica L.) Cultivar Kent in the San Lorenzo Valley, Peru. International Journal of Plant Biology, 16(4), 134. https://doi.org/10.3390/ijpb16040134





