High-Resolution Melting (HRM) Analysis for Screening Edited Lines: A Case Study in Vitis spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material (Gene Transfer Experiments, In Vitro and Greenhouse Cultivation)
2.2. DNA Extraction
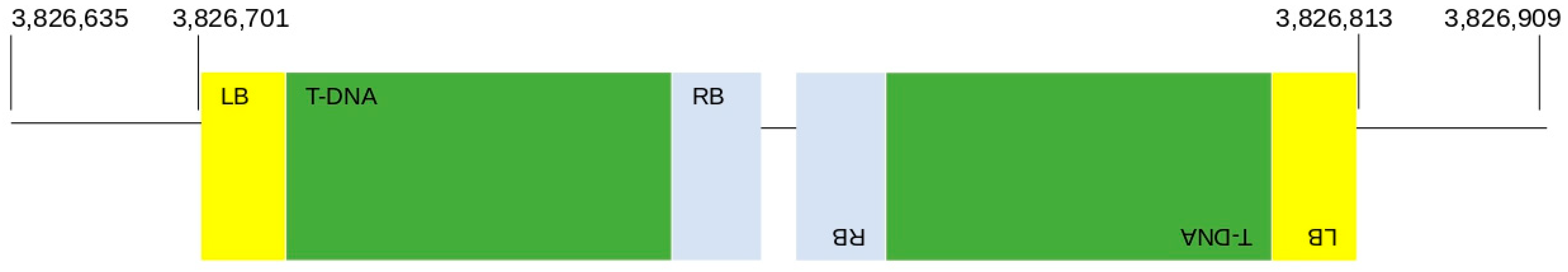
2.3. Detection of T DNA Integration Point
2.4. qPCR-HRM Assay
2.5. HRM Data Analysis
2.6. Statistical Analysis
2.7. Droplet Digital PCR Quantification
3. Results
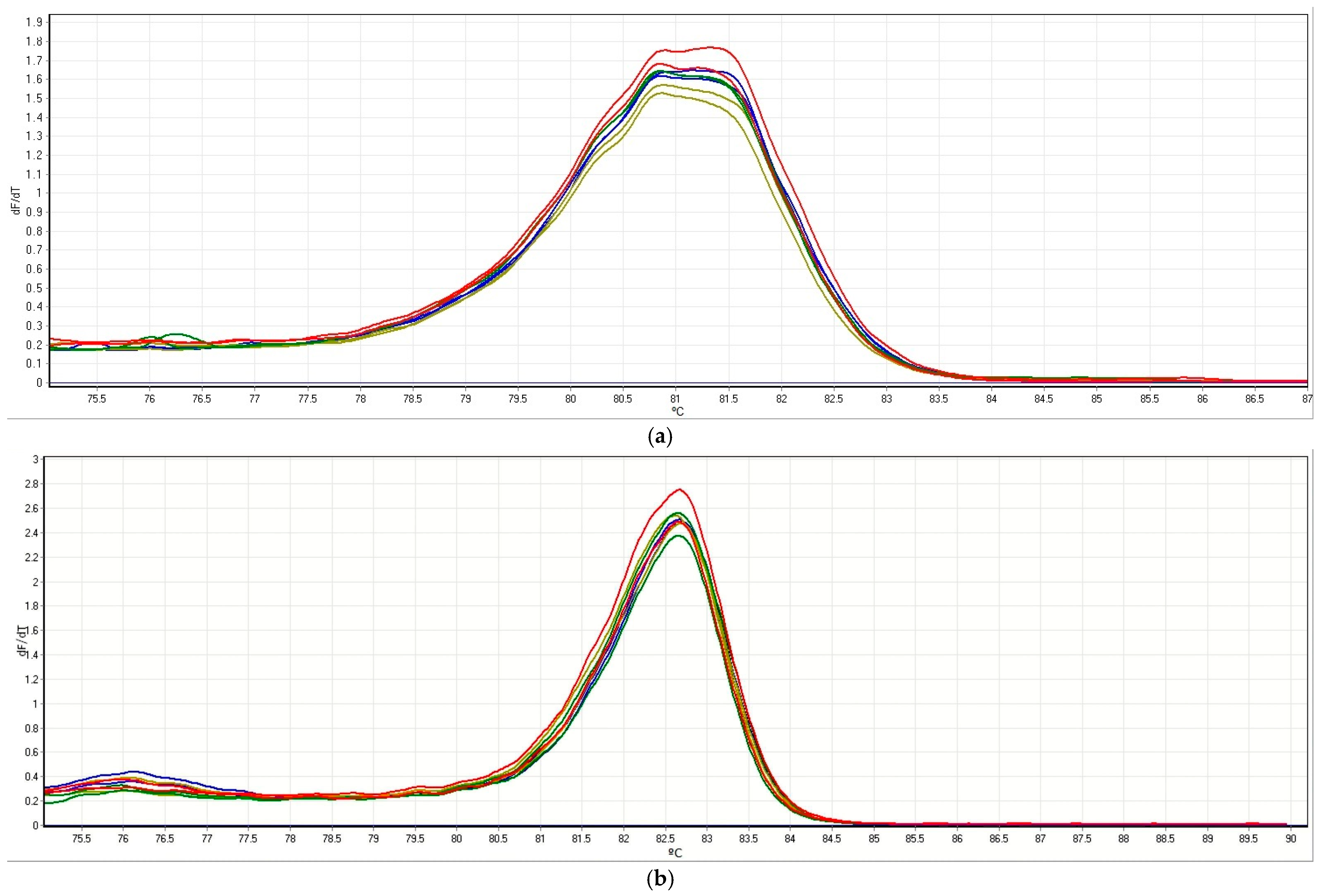
3.1. Set Up of a HRM Method to Evaluate Specific Target Gene Sequences in Grapevine
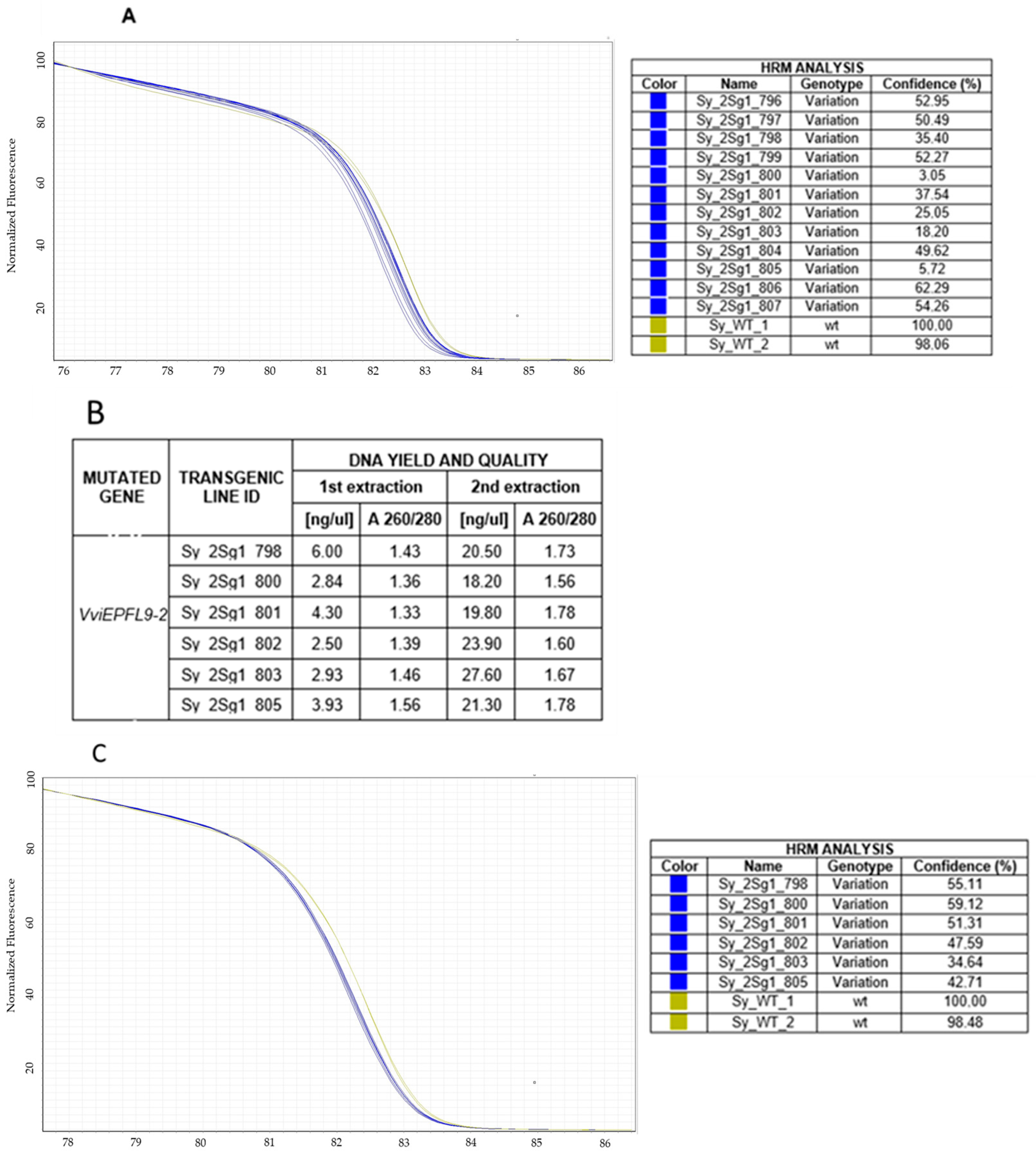
3.2. Screening of the Mutations In Vitro and Greenhouse-Cultivated Edited Lines
3.3. Case Study: Clones Discrimination
3.4. Off-Target Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.R.; Chadha-Mohanty, P. Novel Alleles of Rice eIF4G Generated by CRISPR/Cas9-Targeted Mutagenesis Confer Resistance to Rice Tungro Spherical Virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef]
- Ortigosa, A.; Gimenez-Ibanez, S.; Leonhardt, N.; Solano, R. Design of a Bacterial Speck Resistant Tomato by CRISPR/Cas9-Mediated Editing of SlJAZ2. Plant Biotechnol. J. 2019, 17, 665–673. [Google Scholar] [CrossRef]
- Zhang, Z.; Ge, X.; Luo, X.; Wang, P.; Fan, Q.; Hu, G.; Xiao, J.; Li, F.; Wu, J. Simultaneous Editing of Two Copies of GH14-3-3D Confers Enhanced Transgene-Clean Plant Defense against Verticillium Dahliae in Allotetraploid Upland Cotton. Front. Plant Sci. 2018, 9, 842. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis in Grape in the First Generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.D.; Joung, J.K. CRISPR-Cas Systems for Editing, Regulating and Targeting Genomes. Nat. Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Kumawat, S.; Kumar, V.; Patil, G.B.; Joshi, T.; Sonah, H.; Sharma, T.R.; Deshmukh, R. Genome Editing in Plants: Exploration of Technological Advancements and Challenges. Cells 2019, 8, 1386. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex Genome Engineering Using CRISPR/Cas Systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The New Frontier of Genome Engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S. A Guide to Genome Engineering with Programmable Nucleases. Nat. Rev. Genet. 2014, 15, 321–334. [Google Scholar] [CrossRef]
- Clemens, M.; Faralli, M.; Lagreze, J.; Bontempo, L.; Piazza, S.; Varotto, C.; Malnoy, M.; Oechel, W.; Rizzoli, A.; Dalla Costa, L. VvEPFL9-1 Knock-Out via CRISPR/Cas9 Reduces Stomatal Density in Grapevine. Front. Plant Sci. 2022, 13, 878001. [Google Scholar] [CrossRef] [PubMed]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Jeff Xi, J.; Qiu, J.-L.; et al. Targeted Genome Modification of Crop Plants Using a CRISPR-Cas System. Nat. Biotechnol. 2013, 31, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-Free Genome Editing of Bread Wheat Using CRISPR/Cas9 Ribonucleoprotein Complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Olsson, N.; Fält, A.S.; Ohlsson, P.; Gonzalez, M.N.; Samuelsson, M.; Hofvander, P. Genome Editing in Potato via CRISPR-Cas9 Ribonucleoprotein Delivery. Physiol. Plant. 2018, 164, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-Free Genome Editing in Plants with Preassembled CRISPR-Cas9 Ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, S.W.; Lee, S.; Koo, Y. Optimized Protocols for Protoplast Isolation, Transfection, and Regeneration in the Solanum Genus for the CRISPR/Cas-Mediated Transgene-Free Genome Editing. Appl. Biol. Chem. 2024, 67, 21. [Google Scholar] [CrossRef]
- Murovec, J.; Guček, K.; Bohanec, B.; Avbelj, M.; Jerala, R. DNA-Free Genome Editing of Brassica Oleracea and B. Rapa Protoplasts Using CRISPR-Cas9 Ribonucleoprotein Complexes. Front. Plant Sci. 2018, 871, 1594. [Google Scholar] [CrossRef]
- Gambino, G.; Nuzzo, F.; Moine, A.; Chitarra, W.; Pagliarani, C.; Petrelli, A.; Boccacci, P.; Delliri, A.; Velasco, R.; Nerva, L.; et al. Genome Editing of a Recalcitrant Wine Grape Genotype by Lipofectamine-Mediated Delivery of CRISPR/Cas9 Ribonucleoproteins to Protoplasts. Plant J. 2024, 119, 404–412. [Google Scholar] [CrossRef]
- Najafi, S.; Bertini, E.; D’Incà, E.; Fasoli, M.; Zenoni, S. DNA-Free Genome Editing in Grapevine Using CRISPR/Cas9 Ribonucleoprotein Complexes Followed by Protoplast Regeneration. Hortic. Res. 2023, 10, uhac240. [Google Scholar] [CrossRef]
- Scintilla, S.; Salvagnin, U.; Giacomelli, L.; Zeilmaker, T.; Malnoy, M.A.; Rouppe van der Voort, J.; Moser, C. Regeneration of Non-Chimeric Plants from DNA-Free Edited Grapevine Protoplasts. Front. Plant Sci. 2022, 13, 1078931. [Google Scholar] [CrossRef]
- Tricoli, D.M.; Debernardi, J.M. An Efficient Protoplast-Based Genome Editing Protocol for Vitis Species. Hortic. Res. 2024, 11, uhad266. [Google Scholar] [CrossRef] [PubMed]
- DIRECTIVE 2001/18/EC of the European Parliament and of the council of 12 March 2001 on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC. Off. J. L 2001, 106, 1–39.
- European Network of GMO Laboratories (ENGL). Detection of Food and Feed Plant Products Obtained by New Mutagenesis Techniques; European Union Reference Laboratory for GM Food and Feed: Ispra, Italy, 2019. [Google Scholar]
- Zischewski, J.; Fischer, R.; Bortesi, L. Detection of On-Target and off-Target Mutations Generated by CRISPR/Cas9 and Other Sequence-Specific Nucleases. Biotechnol. Adv. 2017, 35, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Druml, B.; Cichna-Markl, M. High Resolution Melting (HRM) Analysis of DNA-Its Role and Potential in Food Analysis. Food Chem. 2014, 158, 245–254. [Google Scholar] [CrossRef]
- Wittwer, C.T.; Hemmert, A.C.; Kent, J.O.; Rejali, N.A. DNA Melting Analysis. Mol. Asp. Med. 2024, 97, 101268. [Google Scholar] [CrossRef]
- Dufresne, S.D.; Belloni, D.R.; Wells, W.A.; Tsongalis, G.J. BRCA1 and BRCA2 Mutation Screening Using SmartCycler II High-Resolution Melt Curve Analysis. Arch. Pathol. Lab. Med. 2006, 130, 185–187. [Google Scholar] [CrossRef]
- Reed, G.H.; Wittwer, C.T. Sensitivity and Specificity of Single-Nucleotide Polymorphism Scanning by High-Resolution Melting Analysis. Clin. Chem. 2004, 50, 1748–1754. [Google Scholar] [CrossRef]
- Thomas, H.R.; Percival, S.M.; Yoder, B.K.; Parant, J.M. High-Throughput Genome Editing and Phenotyping Facilitated by High Resolution Melting Curve Analysis. PLoS ONE 2014, 9, e114632. [Google Scholar] [CrossRef]
- Distefano, G.; La Malfa, S.; Gentile, A.; Wu, S.-B. EST-SNP Genotyping of Citrus Species Using High-Resolution Melting Curve Analysis. Tree Genet. Genomes 2013, 9, 1271–1281. [Google Scholar] [CrossRef]
- Pereira, L.; Martins-Lopes, P. Vitis vinifera L. Single-Nucleotide Polymorphism Detection with High-Resolution Melting Analysis Based on the UDP-Glucose:Flavonoid 3-O-Glucosyltransferase Gene. J. Agric. Food Chem. 2015, 63, 9165–9174. [Google Scholar] [CrossRef]
- Denbow, C.J.; Lapins, S.; Dietz, N.; Scherer, R.; Nimchuk, Z.L.; Okumoto, S. Gateway-Compatible CRISPR-Cas9 Vectors and a Rapid Detection by High-Resolution Melting Curve Analysis. Front. Plant Sci. 2017, 8, 1171. [Google Scholar] [CrossRef]
- Li, R.; Ba, Y.; Song, Y.; Cui, J.; Zhang, X.; Zhang, D.; Yuan, Z.; Yang, L. Rapid and Sensitive Screening and Identification of CRISPR/Cas9 Edited Rice Plants Using Quantitative Real-Time PCR Coupled with High Resolution Melting Analysis. Food Control 2020, 112, 107088. [Google Scholar] [CrossRef]
- Denbow, C.; Ehivet, S.; Okumoto, S. High Resolution Melting Temperature Analysis to Identify CRISPR/Cas9 Mutants from Arabidopsis. Bio-Protocol 2018, 8, e2944. [Google Scholar] [CrossRef]
- Sanzani, S.; Miazzi, M.; Di Rienzo, V.; Fanelli, V.; Gambacorta, G.; Taurino, M.; Montemurro, C. A Rapid Assay to Detect Toxigenic Penicillium Spp. Contamination in Wine and Musts. Toxins 2016, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Samaras, A.; Madesis, P.; Karaoglanidis, G.S. Detection of sdhB Gene Mutations in SDHI-Resistant Isolates of Botrytis Cinerea Using High Resolution Melting (HRM) Analysis. Front. Microbiol. 2016, 7, 1815. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Gomes, S.; Castro, C.; Eiras-Dias, J.E.; Brazão, J.; Graça, A.; Fernandes, J.R.; Martins-Lopes, P. High Resolution Melting (HRM) Applied to Wine Authenticity. Food Chem. 2017, 216, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, U.; Videau, P.; Coulonnier, E.; Papon, C.; Navarro-Payá, D.; Valenzuela, A.V.; Matus, J.T.; Malnoy, M.; Zekri, O.; Fiorani, F.; et al. Reduced Stomatal Density Improves Water-Use Efficiency in Grapevine under Climate Scenarios of Decreased Water Availability. Plant Cell Rep. 2025, 44, 195. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Zhou, Y.; Jin, W.; Xie, K.; Chen, L.L. CRISPR-P 2.0: An Improved CRISPR-Cas9 Tool for Genome Editing in Plants. Mol Plant. 2017, 10, 530–532. [Google Scholar] [CrossRef]
- Dalla Costa, L.; Piazza, S.; Pompili, V.; Salvagnin, U.; Cestaro, A.; Moffa, L.; Vittani, L.; Moser, C.; Malnoy, M. Strategies to Produce T-DNA Free CRISPRed Fruit Trees via Agrobacterium Tumefaciens Stable Gene Transfer. Sci. Rep. 2020, 10, 20155. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Liew, M.; Pryor, R.; Palais, R.; Meadows, C.; Erali, M.; Lyon, E.; Wittwer, C. Genotyping of Single-Nucleotide Polymorphisms by High-Resolution Melting of Small Amplicons. Clin. Chem. 2004, 50, 1156–1164. [Google Scholar] [CrossRef]
- Reed, G.H.; Kent, J.O.; Wittwer, C.T. High-Resolution DNA Melting Analysis for Simple and Efficient Molecular Diagnostics. Pharmacogenomics 2007, 8, 597–608. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal Component Analysis. WIREs Comp. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Groth, D.; Hartmann, S.; Klie, S.; Selbig, J. Principal Components Analysis. Methods Mol. Biol. 2013, 930, 527–547. [Google Scholar] [CrossRef] [PubMed]
- Gatto, F.; Savini, C.; Sacco, M.G.; Vinciguerra, D.; Buttinger, G.; Corbisier, P.; Mazzara, M.; Emons, H. Single and Multi-Laboratory Validation of a Droplet Digital PCR Method. Food Control 2022, 140, 109117. [Google Scholar] [CrossRef]
- Pecoraro, S.; Berben, G.; Burns, M.; Corbisier, P.; De Giacomo, M.; De Loose, M.; Dagand, E.; Dobnik, D.; Eriksson, R.; Holst-Jensen, A.; et al. Overview and Recommendations for the Application of Digital PCR; European Network of GMO Laboratories (ENGL): Ispra, Italy, 2019. [Google Scholar] [CrossRef]
- Dalla Costa, L.; Vinciguerra, D.; Giacomelli, L.; Salvagnin, U.; Piazza, S.; Spinella, K.; Malnoy, M.; Moser, C.; Marchesi, U. Integrated Approach for the Molecular Characterization of Edited Plants Obtained via Agrobacterium Tumefaciens-Mediated Gene Transfer. Eur. Food Res. Technol. 2022, 248, 289–299. [Google Scholar] [CrossRef]
- Guertler, P.; Pallarz, S.; Belter, A.; Eckermann, K.N.; Grohmann, L. Detection of Commercialized Plant Products Derived from New Genomic Techniques (NGT)-Practical Examples and Current Perspectives. Food Control 2023, 152, 109869. [Google Scholar] [CrossRef]
- Shillito, R.D.; Whitt, S.; Ross, M.; Ghavami, F.; De Vleesschauwer, D.; D’halluin, K.; Van Hoecke, A.; Meulewaeter, F. Detection of Genome Edits in Plants-from Editing to Seed. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 595–608. [Google Scholar] [CrossRef]
- Razzaq, A.; Saleem, F.; Kanwal, M.; Mustafa, G.; Yousaf, S.; Arshad, H.M.I.; Hameed, M.K.; Khan, M.S.; KhanJoyia, F.A. Modern Trends in Plant Genome Editing: An Inclusive Review of the CRISPR/Cas9 Toolbox. Int. J. Mol. Sci. 2019, 20, 4045. [Google Scholar] [CrossRef]
- A Guide to High Resolution Melting (HRM) Analysis. Available online: https://documents.thermofisher.com/TFS-Assets/LSG/manuals/cms_070283.pdf (accessed on 1 November 2025).
- Carrubba, A.; Abbate, L.; Sarno, M.; Sunseri, F.; Mauceri, A.; Lupini, A.; Mercati, F. Characterization of Sicilian Rosemary (Rosmarinus officinalis L.) Germplasm through a Multidisciplinary Approach. Planta 2020, 251, 37. [Google Scholar] [CrossRef]
- Lee, B.B.; Schott, E.J.; Behringer, D.C.; Bojko, J.; Kough, A.; Plough, L.V. Rapid Genetic Identification of the Blue Crab Callinectes Sapidus and Other Callinectes spp. Using Restriction Enzyme Digestion and High Resolution Melt (HRM) Assays. Front. Mar. Sci. 2020, 7, 633. [Google Scholar] [CrossRef]
- Graham, N.; Patil, G.B.; Bubeck, D.M.; Dobert, R.C.; Glenn, K.C.; Gutsche, A.T.; Kumar, S.; Lindbo, J.A.; Maas, L.; May, G.D.; et al. Plant Genome Editing and the Relevance of Off-Target Changes. Plant Physiol. 2020, 183, 1453–1471. [Google Scholar] [CrossRef]
- Garritano, S.; Gemignani, F.; Voegele, C.; Nguyen-Dumont, T.; Le Calvez-Kelm, F.; De Silva, D.; Lesueur, F.; Landi, S.; Tavtigian, S.V. Determining the Effectiveness of High Resolution Melting Analysis for SNP Genotyping and Mutation Scanning at the TP53 Locus. BMC Genet. 2009, 10, 5. [Google Scholar] [CrossRef]
- Fernandes, T.J.R.; Costa, J.; Oliveira, M.B.P.P.; Mafra, I. COI Barcode-HRM as a Novel Approach for the Discrimination of Hake Species. Fish. Res. 2018, 197, 50–59. [Google Scholar] [CrossRef]
- Fitzcharles, E.M. Rapid Discrimination between Four Antarctic Fish Species, Genus Macrourus, Using HRM Analysis. Fish. Res. 2012, 127–128, 166–170. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinella, K.; Dalla Costa, L.; La Rocca, D.; Ciuffa, S.; Verginelli, D.; Shahbaz, U.; Videau, P.; Zekri, O.; Marchesi, U. High-Resolution Melting (HRM) Analysis for Screening Edited Lines: A Case Study in Vitis spp. Int. J. Plant Biol. 2025, 16, 126. https://doi.org/10.3390/ijpb16040126
Spinella K, Dalla Costa L, La Rocca D, Ciuffa S, Verginelli D, Shahbaz U, Videau P, Zekri O, Marchesi U. High-Resolution Melting (HRM) Analysis for Screening Edited Lines: A Case Study in Vitis spp. International Journal of Plant Biology. 2025; 16(4):126. https://doi.org/10.3390/ijpb16040126
Chicago/Turabian StyleSpinella, Katia, Lorenza Dalla Costa, Davide La Rocca, Sara Ciuffa, Daniela Verginelli, Umar Shahbaz, Pierre Videau, Olivier Zekri, and Ugo Marchesi. 2025. "High-Resolution Melting (HRM) Analysis for Screening Edited Lines: A Case Study in Vitis spp." International Journal of Plant Biology 16, no. 4: 126. https://doi.org/10.3390/ijpb16040126
APA StyleSpinella, K., Dalla Costa, L., La Rocca, D., Ciuffa, S., Verginelli, D., Shahbaz, U., Videau, P., Zekri, O., & Marchesi, U. (2025). High-Resolution Melting (HRM) Analysis for Screening Edited Lines: A Case Study in Vitis spp. International Journal of Plant Biology, 16(4), 126. https://doi.org/10.3390/ijpb16040126







