Physiological and Transcriptome Analysis of Drought-Tolerant Mutant ds-1 of Blue Fescue (Festuca glauca) Under Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Drought Treatment
2.2. Measurement of Physiological Indicators
2.3. Transcriptome Sequencing
2.4. qRT-PCR Verification of DEGs
2.5. Data Analysis
3. Results
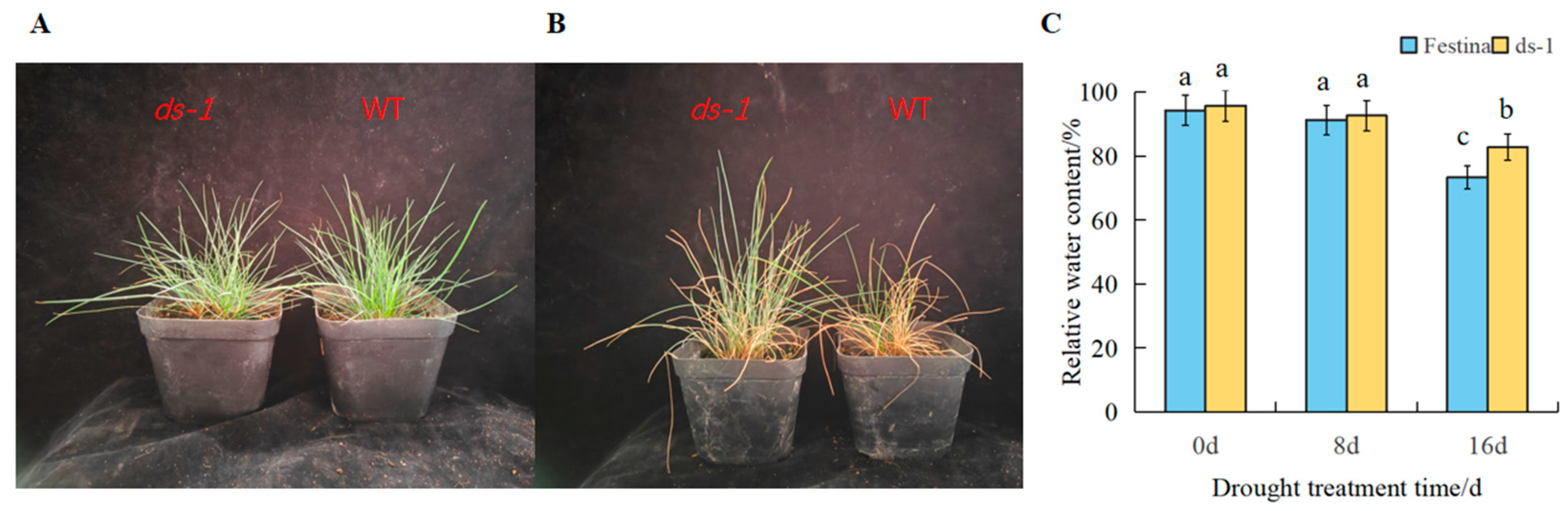
3.1. Phenotype Characteristics and Relative Water Content
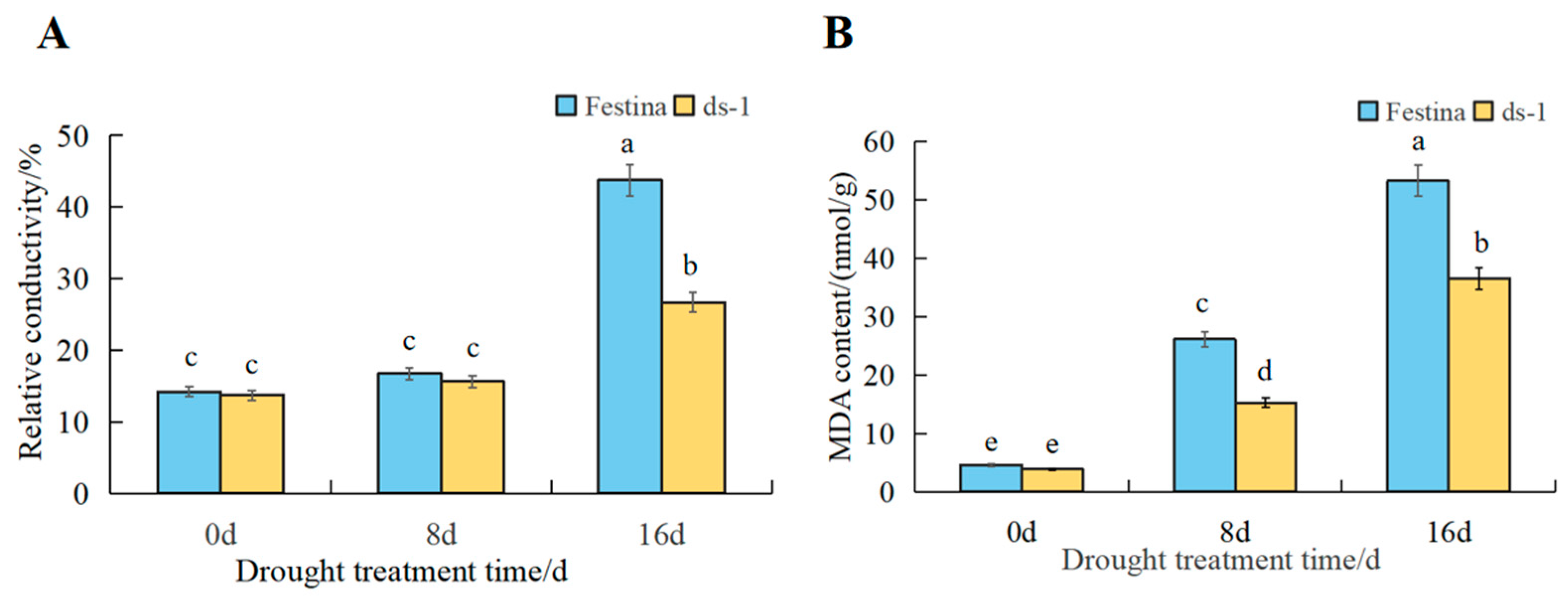
3.2. Integrity of Plasma Membrane
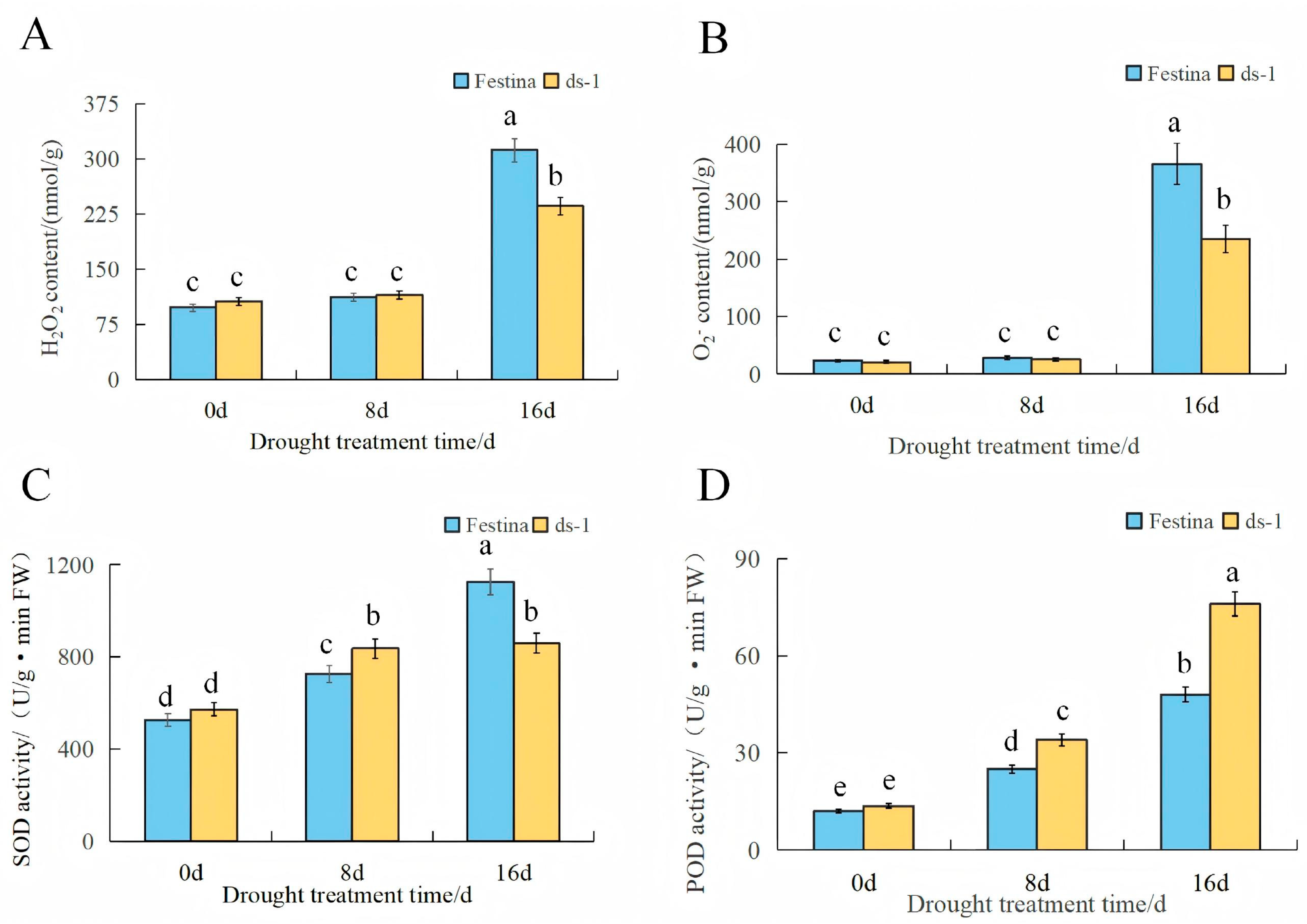
3.3. Changes in ROS Content and Antioxidant Enzyme Activity
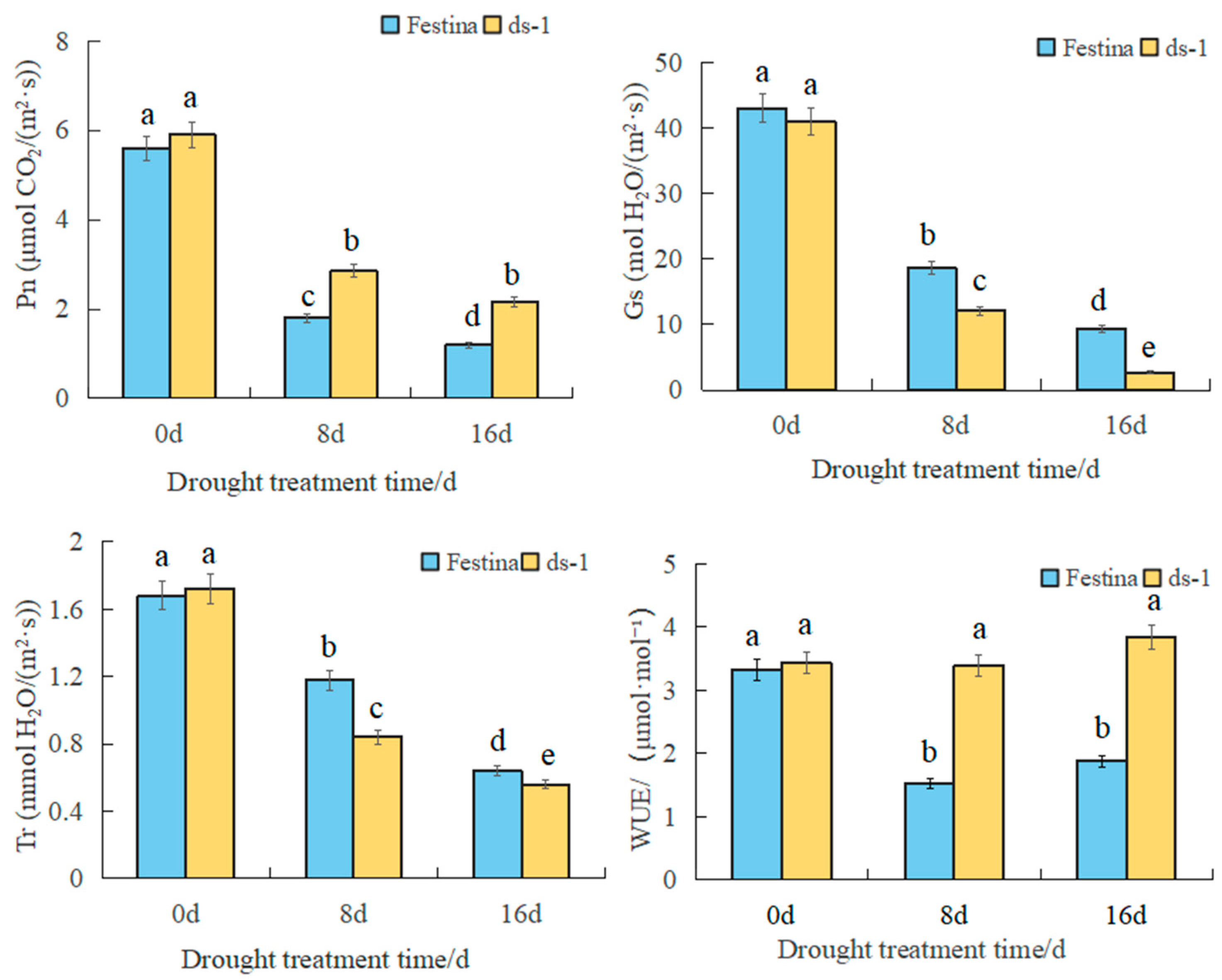
3.4. Changes in Photosynthetic Physiological Characteristics
3.5. Quality Assessment of Sequencing Data
3.6. Expression Analysis of DEGs
3.7. GO Enrichment Analysis of Common DEGs
3.8. KEGG Enrichment Analysis of Common DEGs
3.9. qRT-PCR Verification of Transcriptome
4. Discussion
4.1. Identification of Drought Tolerance of ds-1
4.2. Transcriptional Mechanism of ds-1 Drought Tolerance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gibson, D.; Taylor, I. Festuca longifolia Thuill.(F. glauca auct. non Vill., F. glauca var. caesia (Sm.) Howarth, F. caesia Sm.). J. Ecol. 2005, 93, 214–226. [Google Scholar] [CrossRef]
- Jarecka, K.; Sosnowski, J. The effect of a growth stimulant based on lodine nanoparticles on Festuca glauca. J. Ecol. Eng. 2021, 22, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, P.; Zhao, R.; Yu, S.; Liu, H.; Wu, H.; Weng, J.; Zhang, H. RNA-Seq Transcriptomics and iTRAQ Proteomics Analysis Reveal the Dwarfing Mechanism of Blue Fescue (Festuca glauca). Plants 2024, 13, 3357. [Google Scholar] [CrossRef]
- Feng, D.; Liu, W.; Chen, K.; Ning, S.; Gao, Q.; Chen, J.; Liu, J.; Sun, X.; Xu, W. Exogenous substances used to relieve plants from drought stress and their associated underlying mechanisms. Int. J. Mol. Sci. 2024, 25, 9249. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, S.; Sun, H. Paclobutrazol modulates physiological and hormonal changes in Amorpha fruticosa under drought stress. Russ. J. Plant Physl. 2020, 67, 122–130. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Cheng, Y.; Lu, G.; Fu, G.; Ma, H.; Liu, Q.; Zhang, X.; Zou, X.; Li, C. Exogenous melatonin alleviates damage from drought stress in Brassica napus L. (rapeseed) seedlings. Acta Physiol. Plant. 2018, 40, 43. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Adhikari, A.; Lee, I.-J.; Loake, G.J.; Yun, B.-W. A novel DUF569 gene is a positive regulator of the drought stress response in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 5316. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, H.; Liu, X.; Gan, X.; Nie, F.; Yang, W.; Zhang, L.; Chen, Y.; Song, Y.; Zhang, H. Ectopic expression of HaNAC1, an ATAF transcription factor from Haloxylon ammodendron, improves growth and drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 151, 535–544. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Z.; Zhou, Q.; Wang, X.; Song, S.; Dong, S. Physiological response of soybean plants to water deficit. Front. Plant Sci. 2022, 12, 809692. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Lata, C.; Singh Chauhan, P.; Prasad, V.; Prasad, M. A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr. Genom. 2017, 18, 469–482. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd_Allah, E.F.; Khan, A.L.; Al-Harrasi, A.S. Early events in plant abiotic stress signaling: Interplay between calcium, reactive oxygen species and phytohormones. J. Plant Growth Regul. 2018, 37, 1033–1049. [Google Scholar] [CrossRef]
- López-Cordova, A.; Ramírez-Medina, H.; Silva-Martinez, G.-A.; González-Cruz, L.; Bernardino-Nicanor, A.; Huanca-Mamani, W.; Montero-Tavera, V.; Tovar-Aguilar, A.; Ramírez-Pimentel, J.-G.; Durán-Figueroa, N.-V. LEA13 and LEA30 are Involved in Tolerance to Water Stress and Stomata Density in Arabidopsis thaliana. Plants 2021, 10, 1694. [Google Scholar] [CrossRef]
- Xuan, H.; Huang, Y.; Zhou, L.; Deng, S.; Wang, C.; Xu, J.; Wang, H.; Zhao, J.; Guo, N.; Xing, H. Key soybean seedlings drought-responsive genes and pathways revealed by comparative transcriptome analyses of two cultivars. Int. J. Mol. Sci. 2022, 23, 2893. [Google Scholar] [CrossRef]
- Gahlaut, V.; Jaiswal, V.; Kumar, A.; Gupta, P.K. Transcription factors involved in drought tolerance and their possible role in developing drought tolerant cultivars with emphasis on wheat (Triticum aestivum L.). Theor. Appl. Genet. 2016, 129, 2019–2042. [Google Scholar] [CrossRef] [PubMed]
- Hrmova, M.; Hussain, S.S. Plant transcription factors involved in drought and associated stresses. Int. J. Mol. Sci. 2021, 22, 5662. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525. [Google Scholar] [CrossRef]
- Jung, S.E.; Kim, T.H.; Shim, J.S.; Bang, S.W.; Yoon, H.B.; Oh, S.H.; Kim, Y.S.; Oh, S.-J.; Seo, J.S.; Kim, J.-K. Rice NAC17 transcription factor enhances drought tolerance by modulating lignin accumulation. Plant Sci. 2022, 323, 111404. [Google Scholar] [CrossRef]
- Ngara, R.; Goche, T.; Swanevelder, D.Z.; Chivasa, S. Sorghum’s whole-plant transcriptome and proteome responses to drought stress: A review. Life 2021, 11, 704. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, H.; Gan, X.; Zhang, L.; Chen, Y.; Nie, F.; Shi, L.; Li, M.; Guo, Z.; Zhang, G. Transcriptome profiling of the potato (Solanum tuberosum L.) plant under drought stress and water-stimulus conditions. PLoS ONE 2015, 10, e0128041. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Ham, L.H.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.-S.P. Differential gene expression in soybean leaf tissues at late developmental stages under drought stress revealed by genome-wide transcriptome analysis. PLoS ONE 2012, 7, e49522. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Ghany, S.E.; Ullah, F.; Ben-Hur, A.; Reddy, A.S. Transcriptome analysis of drought-resistant and drought-sensitive sorghum (Sorghum bicolor) genotypes in response to PEG-induced drought stress. Int. J. Mol. Sci. 2020, 21, 772. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, S.; Chen, H.; Li, S.; Shan, X.; Li, H.; Liu, H.; Dong, H.; Yuan, Y. Transcriptome analysis of drought-responsive and drought-tolerant mechanisms in maize leaves under drought stress. Physiol. Plant 2023, 175, e13875. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Abbas, K.; Wang, L.; Gong, B.; Hou, S.; Wang, W.; Dai, B.; Xia, H.; Wu, X.; Lü, G. Drought resistance index screening and evaluation of lettuce under water deficit conditions on the basis of morphological and physiological differences. Front. Plant Sci. 2023, 14, 1228084. [Google Scholar] [CrossRef]
- Dou, L.; Sun, Y.; Li, S.; Ge, C.; Shen, Q.; Li, H.; Wang, W.; Mao, J.; Xiao, G.; Pang, C. Transcriptomic analyses show that 24-epibrassinolide (EBR) promotes cold tolerance in cotton seedlings. PLoS ONE 2021, 16, e0245070. [Google Scholar] [CrossRef]
- Zaiyou, J.; Xiaomin, T.; Hongsheng, W.; Guifang, X. Evaluate the photosynthesis and chlorophyll fluorescence of Epimedium brevicornu Maxim. Sci. Rep. 2022, 12, 19470. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, H.; Liu, M.; Zhang, W.; Song, H.; Lan, H.; Wei, Y.; Niu, B.; Schmidt, B.; Liu, W. RabbitQC: High-speed scalable quality control for sequencing data. Bioinformatics 2021, 37, 573–574. [Google Scholar] [CrossRef]
- Pertea, G.; Huang, X.; Liang, F.; Antonescu, V.; Sultana, R.; Karamycheva, S.; Lee, Y.; White, J.; Cheung, F.; Parvizi, B. TIGR Gene Indices clustering tools (TGICL): A software system for fast clustering of large EST datasets. Bioinformatics 2003, 19, 651–652. [Google Scholar] [CrossRef]
- Poullet, M.; Orlando, L. Assessing DNA sequence alignment methods for characterizing ancient genomes and methylomes. Front. Ecol. Evol. 2020, 8, 105. [Google Scholar] [CrossRef]
- Zang, H.; Guo, S.; Dong, S.; Song, Y.; Li, K.; Fan, X.; Qiu, J.; Zheng, Y.; Jiang, H.; Wu, Y. Construction of a Full-Length Transcriptome of Western Honeybee Midgut Tissue and Improved Genome Annotation. Genes 2024, 15, 728. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.-Y.; Dillies, M.-A. SARTools: A DESeq2-and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. S2), W316–W322. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yu, J.; Zhang, H.; Yang, Z. Relationship between the Phenylpropanoid Pathway and Dwarfism of Paspalum seashore Based on RNA-Seq and iTRAQ. Int. J. Mol. Sci. 2021, 22, 9568. [Google Scholar] [CrossRef] [PubMed]
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luo, X.; Nawaz, G.; Yin, J.; Yang, J. Physiological and biochemical responses of four cassava cultivars to drought stress. Sci. Rep. 2020, 10, 6968. [Google Scholar] [CrossRef]
- Ebrahimiyan, M.; Majidi, M.M.; Mirlohi, A.; Noroozi, A. Physiological traits related to drought tolerance in tall fescue. Euphytica 2013, 190, 401–414. [Google Scholar] [CrossRef]
- Schmid-Siegert, E.; Stepushenko, O.; Glauser, G.; Farmer, E.E. Membranes as structural antioxidants: Recycling of malondialdehyde to its source in oxidation-sensitive chloroplast fatty acids. J. Biol. Chem. 2016, 291, 13005–13013. [Google Scholar] [CrossRef]
- Mohideen, K.; Chandrasekar, K.; Ramsridhar, S.; Rajkumar, C.; Ghosh, S.; Dhungel, S. Assessment of oxidative stress by the estimation of lipid peroxidation marker malondialdehyde (MDA) in patients with chronic periodontitis: A systematic review and meta-analysis. Int. J. Dent. 2023, 2023, 6014706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-h.; ZHANG, J.-l. Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agr. 2018, 17, 336–347. [Google Scholar] [CrossRef]
- Irani, S.; Majidi, M.M.; Mirlohi, A.; Zargar, M.; Karami, M. Assessment of drought tolerance in sainfoin: Physiological and drought tolerance indices. Agron. J. 2015, 107, 1771–1781. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Abogadallah, G.M. Insights into the significance of antioxidative defense under salt stress. Plant Signal. Behav. 2010, 5, 369–374. [Google Scholar] [CrossRef]
- Tian, X.; Lei, Y.B. Physiological responses of wheat seedlings to drought and UV-B radiation. Effect of exogenous sodium nitroprusside application. Russ. J. Plant Physl. 2007, 54, 676–682. [Google Scholar] [CrossRef]
- Fu, L.; Ding, Z.; Han, B.; Hu, W.; Li, Y.; Zhang, J. Physiological investigation and transcriptome analysis of polyethylene glycol (PEG)-induced dehydration stress in cassava. Int. J. Mol. Sci. 2016, 17, 283. [Google Scholar] [CrossRef] [PubMed]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Kausar, A.; Al Zeidi, M.; Asekova, S.; Siddique, K.H.; Farooq, M. Plant photosynthetic responses under drought stress: Effects and management. J. Agron. Crop. Sci. 2023, 209, 651–672. [Google Scholar] [CrossRef]
- Huang, B.; Gao, H. Physiological responses of diverse tall fescue cultivars to drought stress. Hortscience 1999, 34, 897–901. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Wu, X.; Wang, W. Identification of drought tolerant mechanisms in a drought-tolerant maize mutant based on physiological, biochemical and transcriptomic analyses. BMC Plant Biol. 2020, 20, 315. [Google Scholar] [CrossRef]
- Tong, R.; Zhou, B.; Cao, Y.; Ge, X.; Jiang, L. Metabolic profiles of moso bamboo in response to drought stress in a field investigation. Sci. Total Environ. 2020, 720, 137722. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Dossa, K.; Zhou, K.; Zhu, M.; Xie, H.; Tang, S.; Yu, Y.; Guo, X.; Zhou, B. Identification of putative drought-responsive genes in rice using gene co-expression analysis. Bioinformation 2019, 15, 480. [Google Scholar] [CrossRef]
- Klein, M.; Geisler, M.; Suh, S.J.; Kolukisaoglu, H.Ü.; Azevedo, L.; Plaza, S.; Curtis, M.D.; Richter, A.; Weder, B.; Schulz, B. Disruption of AtMRP4, a guard cell plasma membrane ABCC-type ABC transporter, leads to deregulation of stomatal opening and increased drought susceptibility. Plant J. 2004, 39, 219–236. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in stomatal defense against biotic and drought stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, T.; Liu, S.-W.; Uddin, S.; Amjid, M.W.; Niu, S.-H.; Wu, H.X. The transcriptional landscape and hub genes associated with physiological responses to drought stress in Pinus tabuliformis. Int. J. Mol. Sci. 2021, 22, 9604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Jiang, L.; Xin, Q.; Liu, Y.; Tan, J.X.; Chen, Z.Z. Structural basis and functions of abscisic acid receptors PYLs. Front. Plant Sci. 2015, 6, 88. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, Z.; Zhou, X.E.; Shi, H.; Hong, Y.; Cao, M.; Chan, Z.; Liu, X.; Xu, H.E.; Zhu, J.-K. Structure determination and activity manipulation of the turfgrass ABA receptor FePYR1. Sci. Rep. 2017, 7, 14022. [Google Scholar] [CrossRef]
- Mathura, S.R.; Sutton, F.; Bowrin, V. Characterization and expression analysis of SnRK2, PYL, and ABF/AREB/ABI5 gene families in sweet potato. PLoS ONE 2023, 18, e0288481. [Google Scholar] [CrossRef]
- Navarro, C.; Moore, J.; Ott, A.; Baumert, E.; Mohan, A.; Gill, K.S.; Sandhu, D. Evolutionary, comparative and functional analyses of the brassinosteroid receptor gene, BRI1, in wheat and its relation to other plant genomes. PLoS ONE 2015, 10, e0127544. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Park, C.-W.; Lee, K.M.; Hong, C.-O.; Son, H.-J.; Kim, K.K.; Park, H.C.; Kim, Y.-J. The roles of MYC2 transcription factor in JA-signaling pathway in Plants. J. Plant Biol. 2025, 68, 113–131. [Google Scholar] [CrossRef]
- Pu, Z.; Qin, T.; Wang, Y.; Wang, X.; Shi, N.; Yao, P.; Liu, Y.; Bai, J.; Bi, Z.; Sun, C. Genome-wide analysis of the JAZ gene family in potato and functional verification of StJAZ23 under drought stress. Int. J. Mol. Sci. 2025, 26, 2360. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H.; Ma, S.; Xiang, D.; Liu, R.; Xiong, L. OsJAZ1 attenuates drought resistance by regulating JA and ABA signaling in rice. Front. Plant Sci. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, X.; Bao, J.; Zhang, C.; Yan, H.; Li, K.; Gong, M.; Li, S.; Ma, S. Identification and expression analysis of chlorophyll a/b binding protein gene family in grape (Vitis vinifera). Physiol. Mol. Biol. Plants 2022, 28, 1147–1158. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Liu, R.; Yan, L.; Liu, Z.-Q.; Jiang, S.-C.; Shen, Y.-Y.; Wang, X.-F.; Zhang, D.-P. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 2012, 63, 1095–1106. [Google Scholar] [CrossRef]
- Wang, L.; Wei, J.; Shi, X.; Qian, W.; Mehmood, J.; Yin, Y.; Jia, H. Identification of the light-harvesting chlorophyll a/b binding protein gene family in peach (Prunus persica L.) and their expression under drought stress. Genes 2023, 14, 1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, K.; Wang, J.; Ding, Z.-t.; Wang, H.; Bi, C.-h.; Zhang, Y.-w.; Sun, H.-w. Proteomic analysis of Camellia sinensis (L.) reveals a synergistic network in the response to drought stress and recovery. J. Plant Physiol. 2017, 219, 91–99. [Google Scholar] [CrossRef]
- Guo, P.; Baum, M.; Grando, S.; Ceccarelli, S.; Bai, G.; Li, R.; Von Korff, M.; Varshney, R.K.; Graner, A.; Valkoun, J. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J. Exp. Bot. 2009, 60, 3531–3544. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 2015, 20, 56–64. [Google Scholar] [CrossRef]
- Dudhate, A.; Shinde, H.; Tsugama, D.; Liu, S.; Takano, T. Transcriptomic analysis reveals the differentially expressed genes and pathways involved in drought tolerance in pearl millet [Pennisetum glaucum (L.) R. Br]. PLoS ONE 2018, 13, e0195908. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, M.; Gao, Z.; Ren, W.; Yang, F.; He, H.; Zhao, J. RNA-seq analysis reveals MAPKKK family members related to drought tolerance in maize. PLoS ONE 2015, 10, e0143128. [Google Scholar] [CrossRef]
- Danquah, A.; De Zélicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef]
- Klein, T.; Hoch, G.; Yakir, D.; Körner, C. Drought stress, growth and nonstructural carbohydrate dynamics of pine trees in a semi-arid forest. Tree Physiol. 2014, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, R.; Song, Z.; Fu, W.; Yun, L.; Gao, J.; Hu, G.; Wang, Z.; Wu, H.; Zhang, G. Iris lactea var. chinensis plant drought tolerance depends on the response of proline metabolism, transcription factors, transporters and the ROS-scavenging system. BMC Plant Biol. 2023, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, B.; Peng, Q.; Liu, W.; He, X.; Liang, Z.; Lin, Y.E. Transcriptome analyses in different cucumber cultivars provide novel insights into drought stress responses. Int. J. Mol. Sci. 2018, 19, 2067. [Google Scholar] [CrossRef]
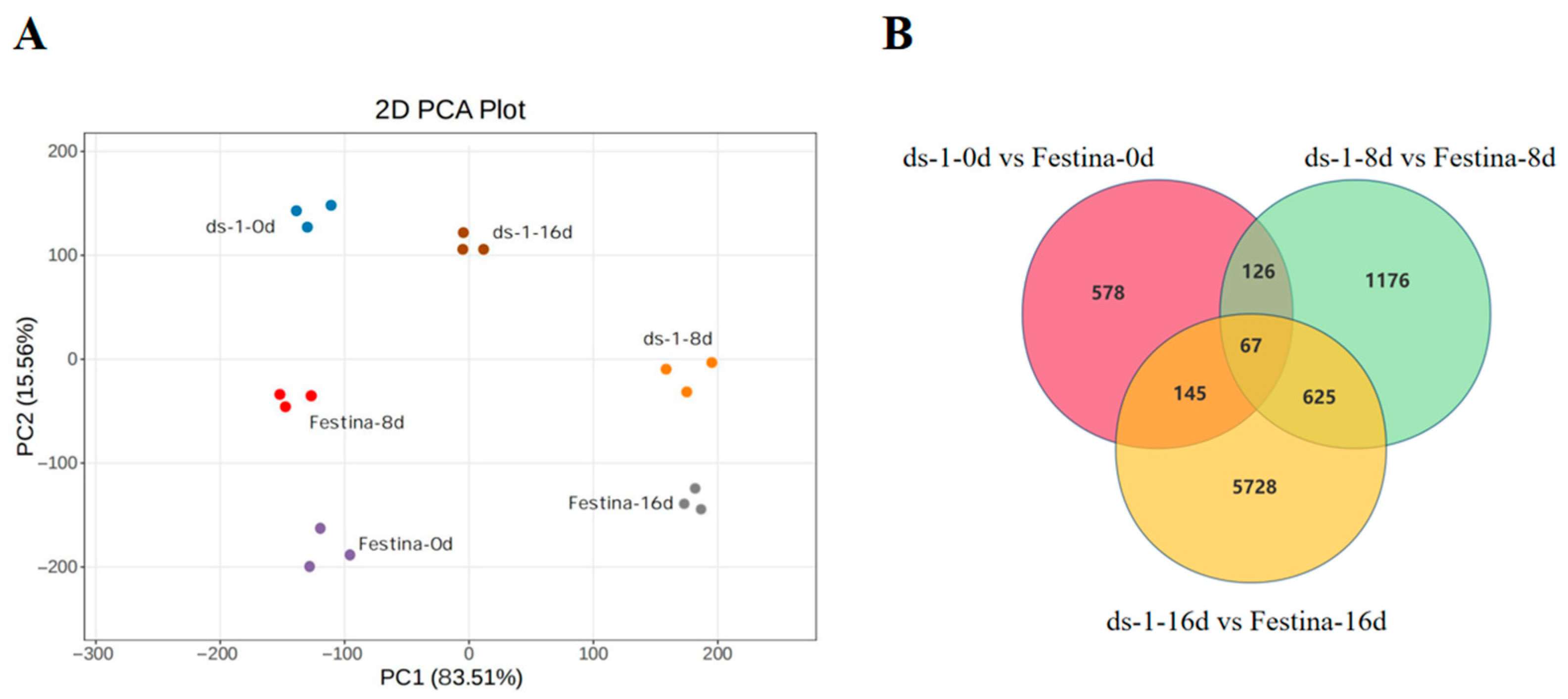
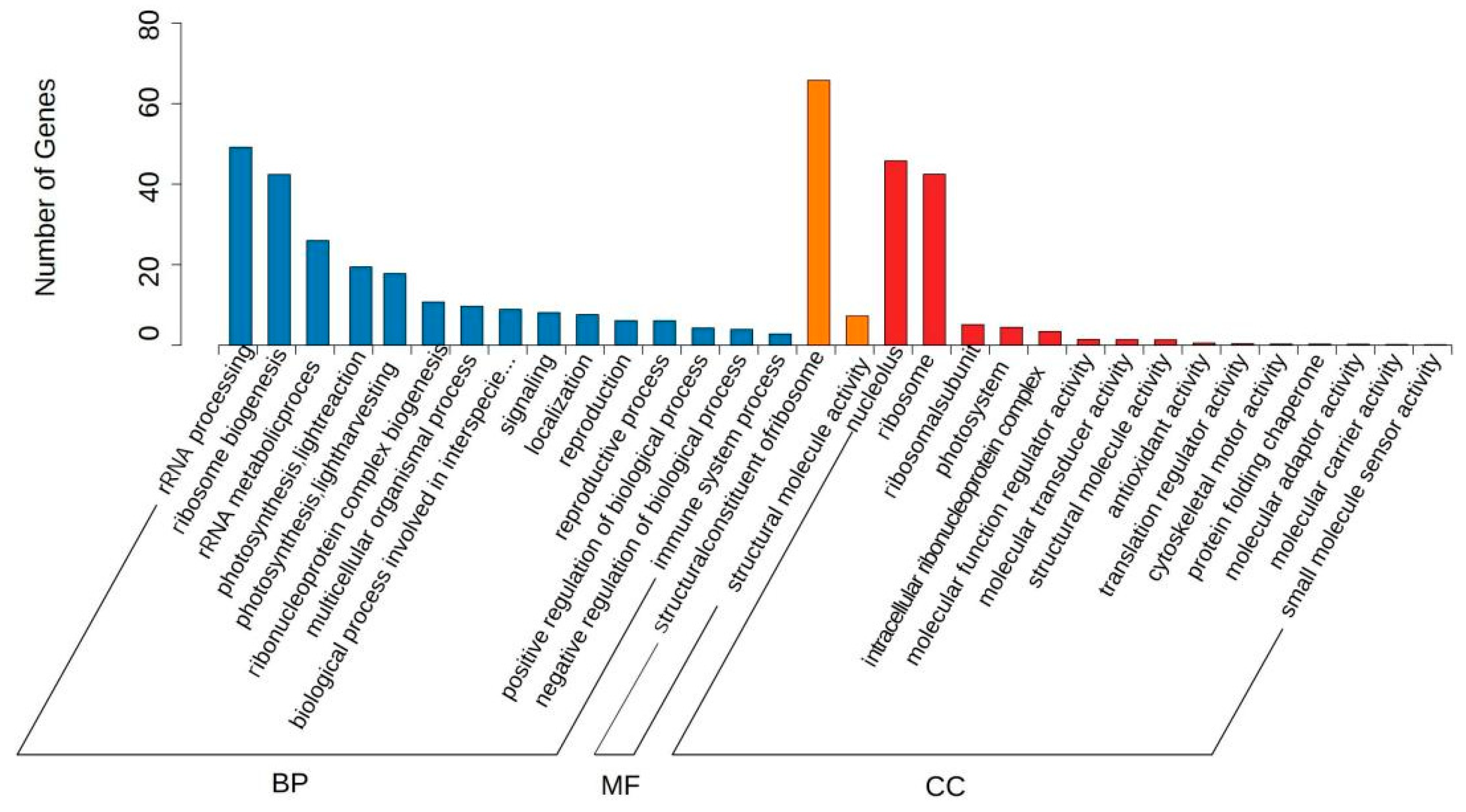
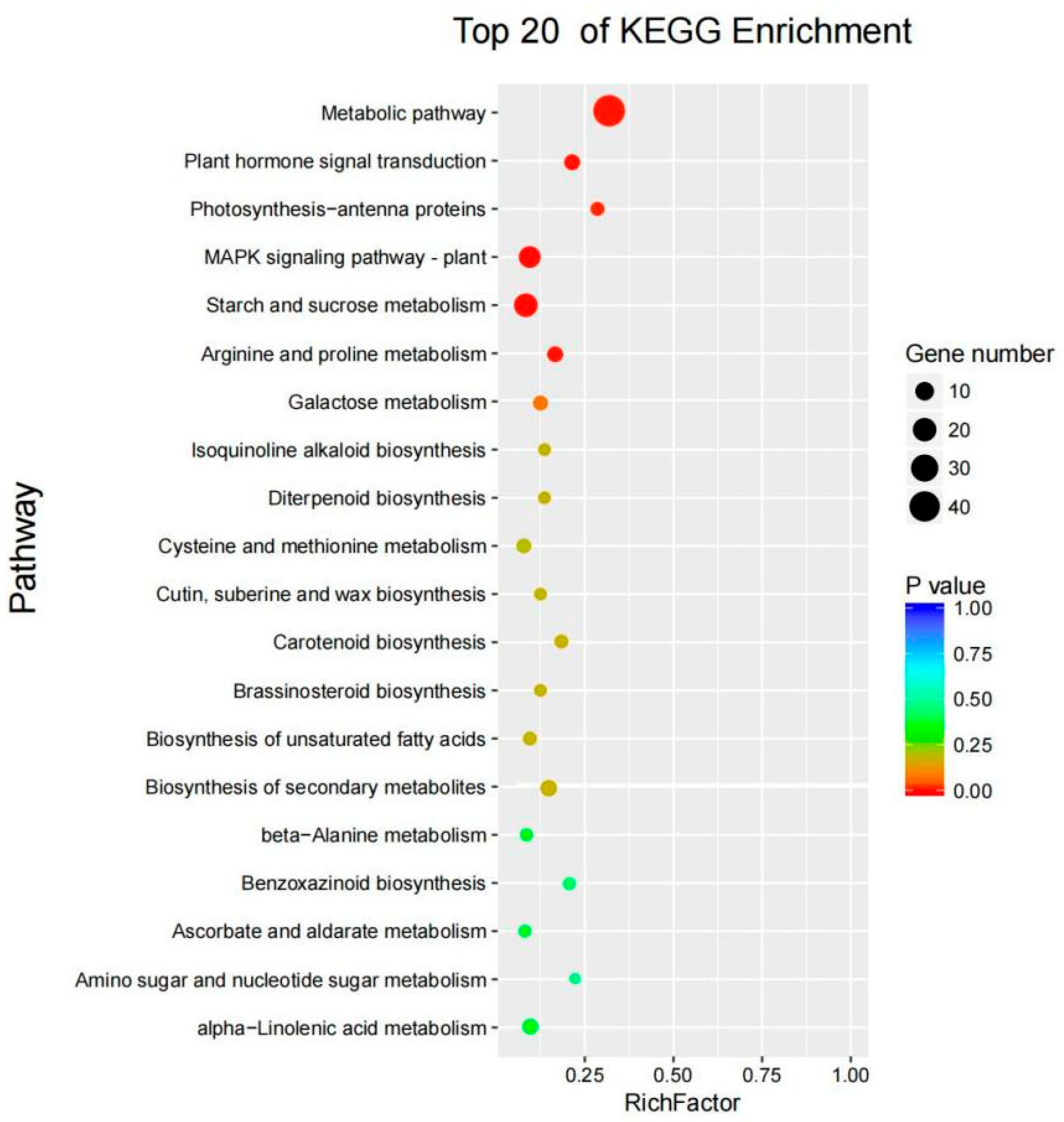
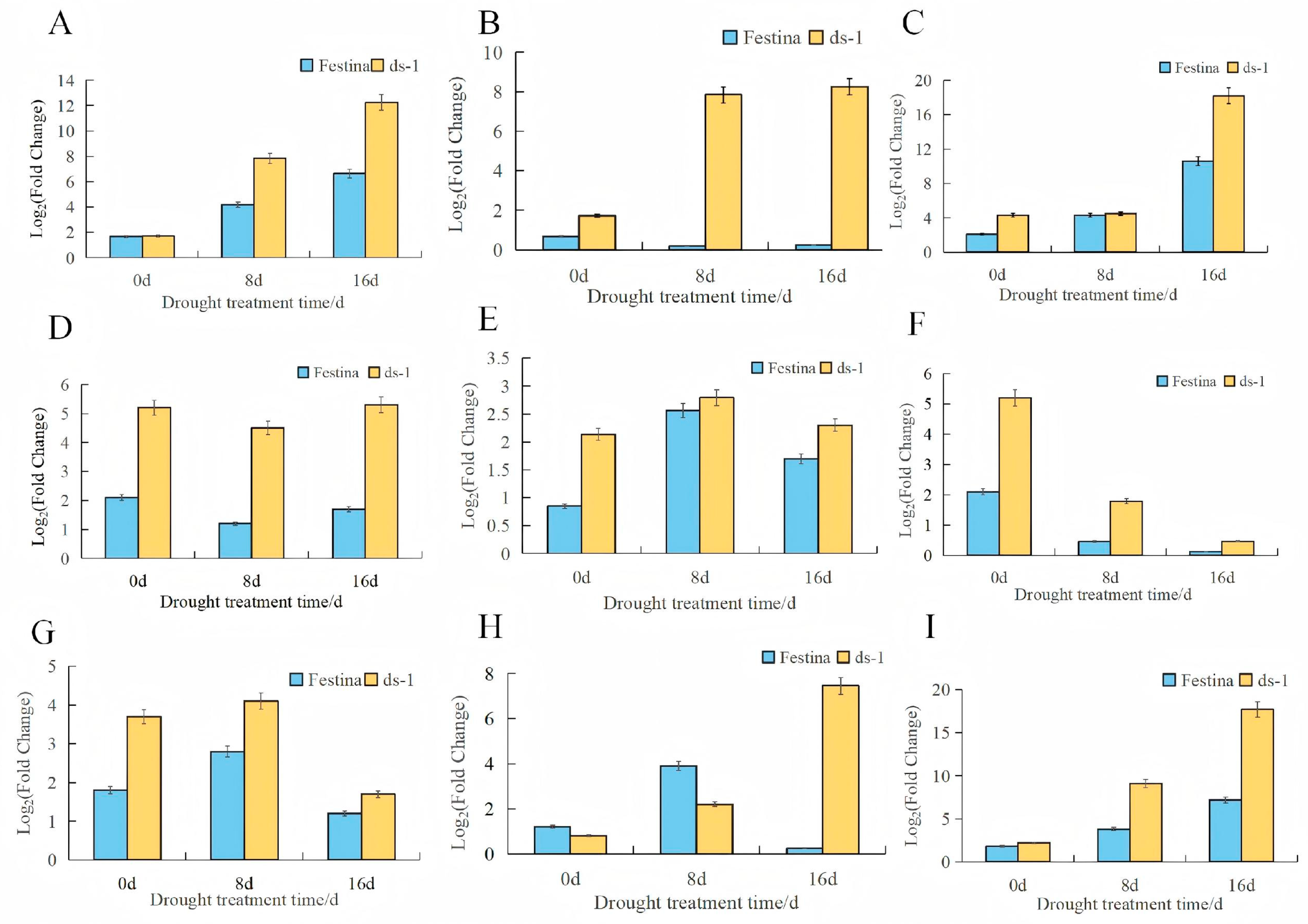
| Comparison Group | Total DEGs | Upregulated DEGs | Downregulated DEGs |
|---|---|---|---|
| ds-1—0d vs. Festina—0d | 916 | 262 | 654 |
| ds-1—8d vs. Festina—8d | 1994 | 1213 | 781 |
| ds-1—16d vs. Festina—16d | 6565 | 3589 | 2976 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Han, P.; Xiao, X.; Chen, W.; Liu, H.; Zhang, H.; Xu, L. Physiological and Transcriptome Analysis of Drought-Tolerant Mutant ds-1 of Blue Fescue (Festuca glauca) Under Drought Stress. Int. J. Plant Biol. 2025, 16, 116. https://doi.org/10.3390/ijpb16040116
Zhang Y, Han P, Xiao X, Chen W, Liu H, Zhang H, Xu L. Physiological and Transcriptome Analysis of Drought-Tolerant Mutant ds-1 of Blue Fescue (Festuca glauca) Under Drought Stress. International Journal of Plant Biology. 2025; 16(4):116. https://doi.org/10.3390/ijpb16040116
Chicago/Turabian StyleZhang, Yong, Peng Han, Xuefeng Xiao, Wei Chen, Hang Liu, Hengfeng Zhang, and Lu Xu. 2025. "Physiological and Transcriptome Analysis of Drought-Tolerant Mutant ds-1 of Blue Fescue (Festuca glauca) Under Drought Stress" International Journal of Plant Biology 16, no. 4: 116. https://doi.org/10.3390/ijpb16040116
APA StyleZhang, Y., Han, P., Xiao, X., Chen, W., Liu, H., Zhang, H., & Xu, L. (2025). Physiological and Transcriptome Analysis of Drought-Tolerant Mutant ds-1 of Blue Fescue (Festuca glauca) Under Drought Stress. International Journal of Plant Biology, 16(4), 116. https://doi.org/10.3390/ijpb16040116






