Abstract
Stomatal blockers are hydrophobic polymers applied to leaves to physically block stomatal pores and restrict gas exchange, and which have potential as plant growth regulators to retard growth. Three experiments in a heated glasshouse, one sown in autumn and two sown in winter, were conducted with pot-grown rapeseed plants at the four-leaf stage to evaluate retardant potential of two bio-based polymers: di-1-p-menthene (DPM) and extracted cauliflower leaf wax. Both stomatal blockers reduced stomatal conductance and plant dry weight in the autumn-sown experiment, when solar radiation was high during leaf development and stomatal conductance of water-treated plants was relatively high. Wax was more effective than DPM at reducing plant dry weight, despite no difference in stomatal conductance. In the two winter-sown experiments, when solar radiation was lower during leaf development, stomatal conductance in water-treated plants was less than in the autumn-sown experiment. Stomatal conductance was reduced by the blockers in the winter-sown experiments, but plant dry weight was unaffected. It was concluded that stomatal blockers may have potential to act as plant growth regulators to retard growth in rapeseed, but further research is necessary to define the circumstances when a response will occur.
Keywords:
oilseed rape; canola; porometer; terpene; epicuticular wax; PGR; delay; hold; slow; inhibit 1. Introduction
Rapeseed is the sixth largest crop by area, grown on 43 Mha in 2023, 11 Mha of which was in Europe [1]. Much of the rapeseed is winter-sown in Europe, and with climate change, most winters are now warm, leading to excessive vegetative growth in almost all years. This can lead to lodging, sub-optimal light interception and reduced yield [2]. It is common to reduce these problems by applying PGRs in the autumn, such as metconazole. Metconazole retards rapeseed vegetative growth, reduces subsequent lodging risk and improves yield [3]. Metconazole is, however, derived from petroleum, and there is increasing interest in more environmentally friendly bio-based agrochemicals [4]. In this paper, we propose possible bio-based alternatives to metconazole as PGRs for rapeseed.
Stomatal blockers are hydrophobic polymers sprayed on leaves to form a film and block stomata, and there is a large body of well-established evidence that stomatal blockers reduce gas exchange [5]. For many years, the primary commercial use of blocking stomata with these polymers has been as film antitranspirants to reduce plant water loss, mainly on ornamental species where any effects of reduced photosynthesis are less important than in crop species [6]. More recently, it has been demonstrated that the restricted gas exchange from spraying the bio-based blocker di-1-p-menthene (DPM) retards grapevine vegetative growth [7], although this is not a commercial use as far as the authors are aware. Thus, stomatal blockers can retard growth by a different mechanism to conventional PGRs. Stomatal blockers reduce photosynthesis and the supply of assimilate for growth by physically blocking stomata [5], whereas conventional PGRs retard growth by inhibition of the plant hormones which stimulate growth: gibberellins in the case of metconazole [3]. The advantage of stomatal blockers over conventional PGRs is that some are bio-based, and thus likely to be less persistent in the environment and possibly more acceptable to consumers [4].
The purpose of the study described in this paper was to conduct three proof-of-concept experiments on young glasshouse-grown rapeseed plants to discover if two bio-based stomatal blockers (DPM and extracted leaf wax) may have potential as growth retardants for rapeseed. DPM, the terpene which has been shown to retard grapevine vegetative growth [7], is formulated from pinene [8] which is derived from pine resin [9]. The other polymer tested was a novel bio-based leaf wax formulation extracted from waste cauliflower leaves [10]. The novel leaf wax formulation was chosen for two reasons: first, because leaf wax is a natural hydrophobic polymer on the leaf cuticle which reduces water loss [11] and therefore the wax should have potential as a stomatal blocker; second, extracting the wax from waste leaves might enable a cheaper stomatal blocker to be produced than existing commercial products such as DPM. As far as the authors are aware, there is no commercial use of extracted leaf wax as a stomatal blocker, and this is the first report of research on extracted leaf wax as a stomatal blocker for retarding growth. The two objectives of the experiments were to compare the average effect of the two stomatal blockers with water, and if significant, to compare between the two blockers. Stomatal conductance was measured to assess effects on stomatal blockage, and plant dry weight was measured to assess growth effects. The two null hypotheses tested were that bio-based stomatal blockers cannot retard rapeseed growth, and that there is no difference in growth inhibition between DPM and extracted leaf wax.
2. Materials and Methods
The schedules and specific timings to perform the main tasks of this experiment are described in Table 1. One plant per 1 L pot of rapeseed (Brassica napus cv. Excalibur) was grown in John Innes Number 2 compost [12] in a heated glasshouse at Harper Adams University (52°46′ N, 2°25′ W) and watered approximately to saturation every other day. The glasshouse was heated to maintain a minimum temperature of 15 °C/5 °C day/night. Additional lighting was provided by sodium vapour lamps (Osram Ltd., Reading, UK, model: Vialox NAV-T 400) for 16 h per day. Internal glasshouse environment data was not recorded, but solar radiation was recorded at a weather station approximately 0.5 km from the glasshouse. Treatments were applied at the 4th-leaf stage and a photograph of similar plants at this stage, from a separate study on drought conducted at the same time, is shown in a previously published paper [10]. Treatments were replicated in six (Experiment [Exp] 1) or eight (Exp 2 and 3) randomized blocks and were water (for control), 1% v/v Vapor Gard (DPM 96%, Miller Chemical and Fertilizer LLC, Hanover, PA, USA) in water, and 1% v/v leaf wax in water + 0.5% v/v Wetcit (alcohol ethoxylate 9%, ORO AGRI Inc, Trophy Club, TX, USA). Details of the extraction method, chemical composition and formulation of the leaf wax have been described previously [10]. For Exps 1 and 2, each plant was moved one at a time away from the glasshouse bench for spraying. The adaxial surface of the leaves of each plant was uniformly sprayed using a 0.5 L hand-held trigger sprayer [13] at a distance of a few cm until the surface of the leaves was fully covered. This method was chosen for Exps 1 and 2 to ensure complete coverage of the adaxial leaf surface. The assessment of coverage of each plant during spraying is, however, subjective and, therefore, to reduce variation between plants in the quantity of spray applied in Exp 3, the plants were sprayed using an automatic pot sprayer in an enclosed chamber [10] that simulates a field crop sprayer more closely than the hand-held sprayer. Plants were arranged in a line under the track of the sprayer without any overlapping leaves and sprayed using nozzles at 50 cm height from the plants, 3 bar pressure at 1 m s−1 speed using Flat Fan 015 nozzles (Teejet, Glendale Heights, IL, USA) delivering the equivalent of 200 L ha−1. Both methods of spraying delivered approximately 1 mL of spray liquid to each plant.

Table 1.
Dates of main tasks.
Because it is not possible to see and count blocked stomata due to reflection from the polymer film, stomatal conductance was used as an indirect measure of stomatal blockage. Three readings of adaxial stomatal conductance were taken from the 3rd leaf of each plant with a transient state diffusion porometer (AP4, Delta-T Devices, Cambridge, UK), and the mean calculated. Stomatal conductance measurements were taken during the period 11.00 am to 2.00 pm to coincide with stomatal opening in the stomatal circadian rhythm. Measurements were conducted on the three plants in one block before moving to the three plants in the next block. Thus, most of any short-term changes in stomatal opening between plants measured during the 11 am to 2 pm period of measurement would be removed by the block effect in the statistical analysis. Solar radiation was measured in a weather station about 0.5 km from the glasshouse. Two treatment orthogonal contrasts corresponding to the two objectives and null hypotheses (water vs. mean of DPM and wax, and DPM vs. wax) were calculated in ANOVA using GenStat (23rd Edition, VSNi, Hemel Hempstead, UK) with validity checked by examining residual plots. One extreme outlier was removed from the data for the DPM treatment in Exp 3.
3. Results and Discussion
Leaf development was faster in the autumn-sown experiment (Exp 1), which took 24 days from sowing to reach the four-leaf stage, compared with the two winter-sown experiments (Exps 2 and 3), which both took 39 days from sowing to reach the four-leaf stage. Rapeseed leaf development is mainly influenced by temperature [14], and although the glasshouse heating was set to minimum temperatures, the daytime minimum may have been exceeded if solar radiation was high. Glasshouse internal environment data was not available for these experiments, but solar radiation was available and varied between the experiments because of the different sowing dates (Figure 1). The mean daily radiation from sowing to spraying was greater in Exp 1, sown in October (3.75 MJ m−2 per day), compared with Exp 2, sown in December (1.90 MJ m−2 per day), and Exp 3, sown in January (2.81 MJ m−2 per day). Thus, the faster leaf development in Exp 1 may have resulted from the higher solar radiation than in the two winter-sown experiments (Exp 2 and 3), and the higher solar radiation would probably have been associated with the glasshouse temperature being higher than the heating minimum setting of 15 °C during daylight.
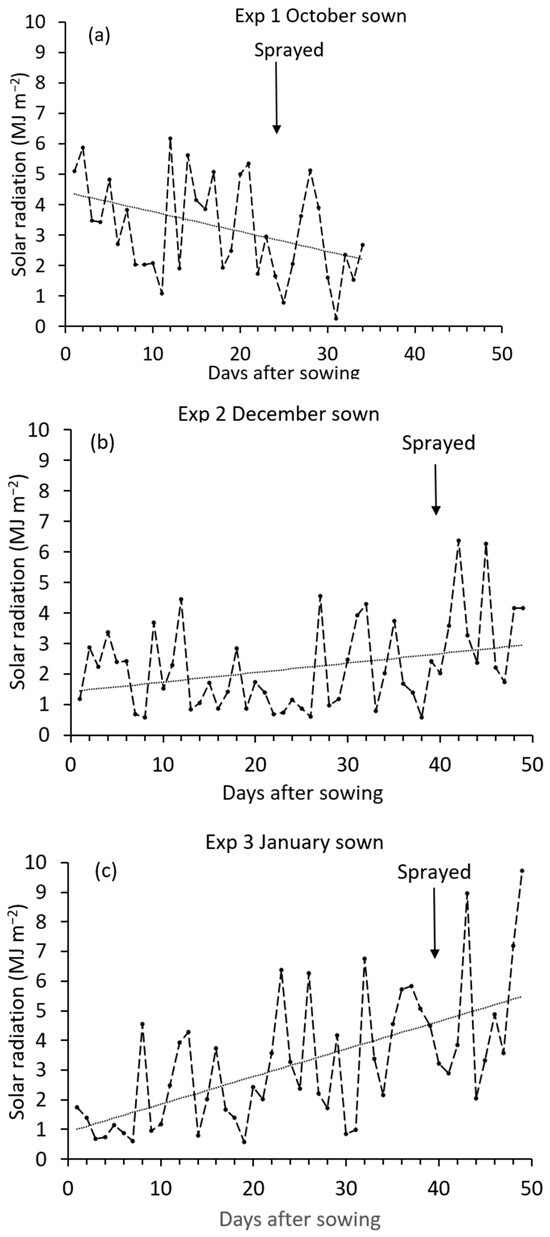
Figure 1.
Daily solar radiation for (a) Exp 1, (b) Exp 2, and (c) Exp 3. Arrow is date of spraying and dotted line is fitted linear regression: Exp 1, solar radiation = 4.42 − 0.065 days after sowing, R2 = 16.5%; p < 0.015; Exp 2, solar radiation = 1.43 + 0.031 days after sowing, R2 = 9.3%; p = 0.033; Exp 3, solar radiation = 0.92 + 0.093 days after sowing R2 = 35.9%; p < 0.001.
Stomatal conductance in water-treated plants was substantially greater in Exp 1 (Figure 2a) than in the other two experiments (Figure 2b,c), with Exps 2 and 3 only having about 55% of the conductance of Exp 1. This is possibly because of the higher mean daily solar radiation from sowing to spraying in Exp 1 than in the other two winter-sown experiments. Higher solar radiation during leaf development is known to increase stomatal density [15], which would be expected to give greater stomatal conductance. Stomatal conductance was reduced by the blockers in all three experiments (Table 2, Figure 2), and the mean reduction in conductance varied from 75% in Exp 2 to 30% in Exp 3. This reduction was expected from the large body of research in the 20th century on these materials demonstrating reduced stomatal conductance [5]. There was, however, no difference between the two blockers in any experiment.
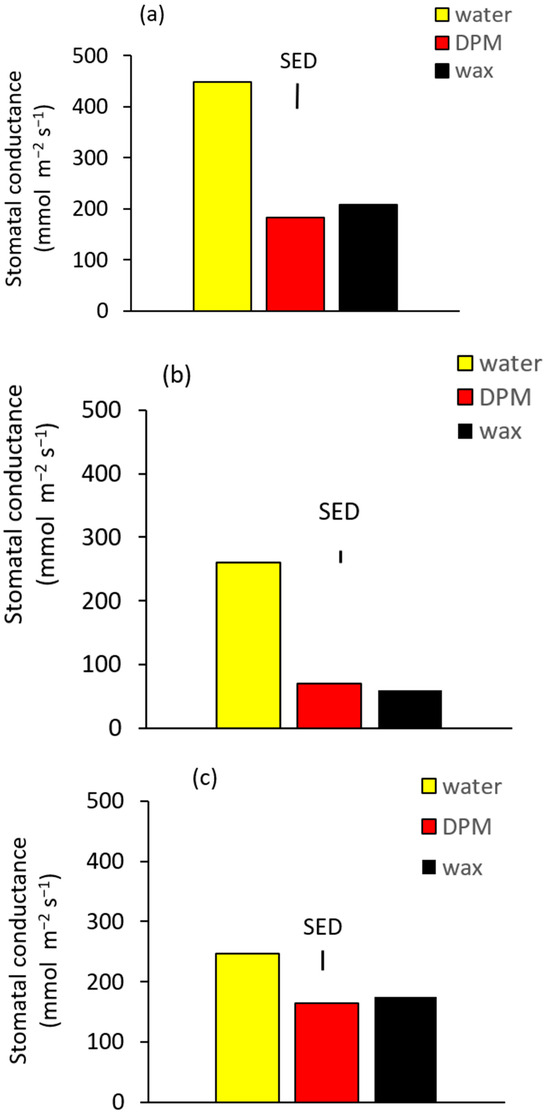
Figure 2.
Adaxial stomatal conductance at 1 day after application in (a) Exp 1 and (b) Exp 2, and 2 days after application in (c) Exp 3. Vertical bars are SEDs (10 DF Exp 1, 14 DF Exp 2, 13 DF Exp 3).

Table 2.
Orthogonal contrast p values.
Dry weight of water-treated plants was greatest in Exp 3 (Figure 3c) and lowest in Exp 2 (Figure 3b). It is well-established that cumulative solar radiation is the main determinant of dry matter production when there are no other limiting factors [16], and the dry weight of water-treated plants reflects the differences in cumulative solar radiation received during the entire period of the experiment from sowing to harvest. Cumulative solar radiation was greatest in Exp 3 (159.4 MJ m−2), because of the increasing trend for higher solar radiation as days lengthened towards the end of this experiment in March, lower in Exp 1 (113.9 MJ m−2) with solar radiation high at the start in October but declining as daylength reduced in the autumn, and the lowest in Exp 2, sown in December and harvested in February (108.4 MJ m−2).
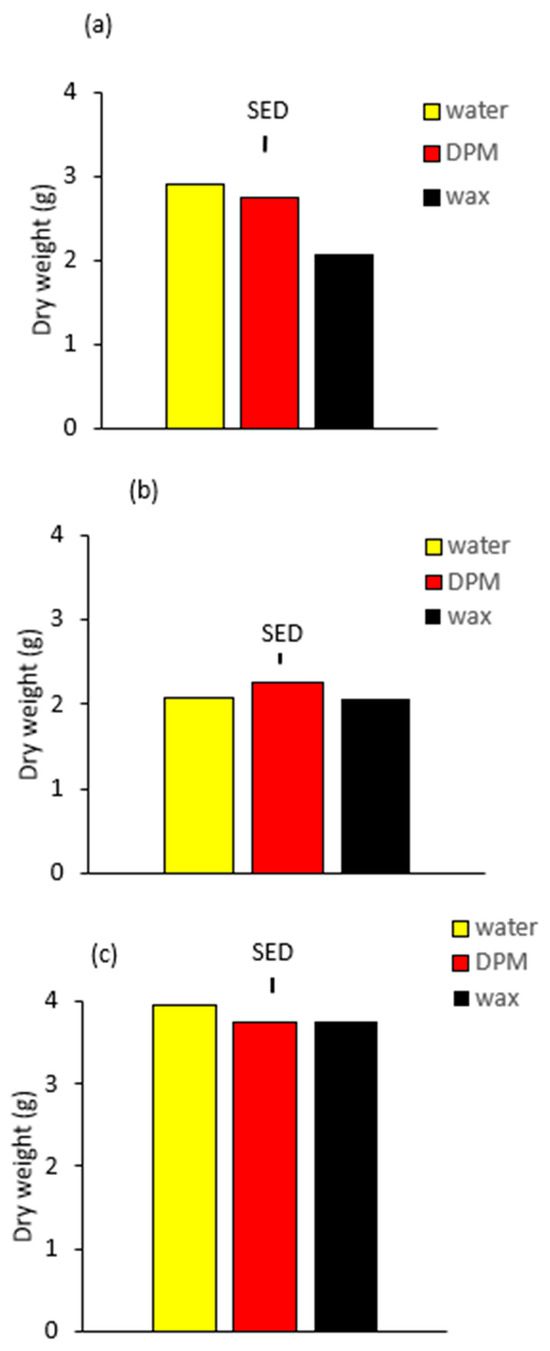
Figure 3.
Plant dry weight 10 days after application for (a) Exp 1, (b) Exp 2 and (c) Exp 3. Vertical bars are SEDs (10 DF Exp 1, 14 DF Exp 2 and Exp 3).
Plant dry weight was reduced in Exp 1 by the stomatal blockers (Table 2, Figure 3a), refuting the first null hypothesis that stomatal blockers cannot retard rapeseed growth. Wax was more effective than DPM, refuting the second null hypothesis that there is no difference. Wax gave a 29% reduction in plant dry weight compared with 6% for DPM, in contrast to the lack of difference in stomatal conductance. There was no significant effect of blockers on dry weight in either Exp 2 (Figure 3b) or Exp 3 (Figure 3c), although there was an indication of a slight reduction in Exp 3. The effectiveness of blockers in only one out of three experiments could indicate a chance occurrence; however, there are clear differences in the environment of Exp 1 compared with Exps 2 and 3, which would be expected to give an effect of the blocker in reducing dry weight in Exp 1 but not in Exps 2 and 3. The greater mean daily solar radiation between sowing and spraying for Exp 1 (3.75 MJ m−2 per day) would have been expected to give a greater growth rate, and therefore a reduction in gas exchange by the blockers would have a greater effect in reducing growth than in Exps 2 and 3, which had lower mean daily solar radiation from sowing to spraying (1.90 MJ m−2 per day and 2.81 MJ m−2 per day, respectively). Since there was no difference in the effect of wax and DPM on stomatal conductance, the greater inhibition of dry weight by wax may have resulted from the effects of the wax which are not related to stomatal conductance. One possible explanation for the greater effect of wax in reducing dry weight compared with DPM could be that the applied wax changed the optical properties of the leaves to reflect more of the photosynthetically active radiation. It has been shown in wheat that epicuticular wax reflects light [17].
These results provide, as far as the authors are aware, the first published indication that stomatal blockers can act as growth retardants in rapeseed. These results are consistent with research on grapevines showing the ability of the stomatal blocker DPM to reduce vine growth [7]. Much further research will be needed to discover if this retardation can persist to reduce lodging after stem extension and increase yield, as occurs with metconazole. The retardation from DPM was small, and it may be possible to increase the response by increasing the concentration from the 1% used. Previous research on DPM sprayed on rapeseed as an antitranspirant has shown greater reductions in stomatal conductance from higher concentrations up to the highest concentration tested of 3% [18]. In grapevine research, which found consistent growth reduction over two years in the field from DPM, other aspects of the treatment in addition to greater concentration were different to those in our experiments and could also possibly contribute to a greater response. These included a repeat spray, an additional surfactant and spraying a larger volume to run-off rather than spraying to complete coverage [7].
4. Conclusions
These experiments give a preliminary indication that bio-based stomatal blockers can retard rapeseed growth and indicate that sufficient solar radiation intensity during leaf emergence may be necessary for a response. Further research is required to confirm the retardation by blockers and to further explore the effect of solar radiation intensity.
Author Contributions
Conceptualization, J.M.M., P.K., and R.M.; formal analysis, P.K., M.F., and M.W.; funding acquisition, P.K., R.M. and M.M.; investigation, M.F. and M.W.; methodology, M.F., M.W., G.-S.L., M.M.; project administration, P.K. and R.M.; resources, R.M., G.-S.L. and M.M.; supervision, P.K. and R.M.; visualization, P.K.; writing—original draft, P.K. and M.F.; writing—review and editing: P.K., M.F. and J.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by grant number POC15_02 of the Food Processing Waste and By-Products Utilization Network (FoodWasteNet) of the UK Biological Sciences and Biotechnology Research Council (BBSRC).
Data Availability Statement
Solar radiation and individual plant data are available on figshare at: https://doi.org/10.6084/m9.figshare.29625968 (accessed on 23 July 2025).
Acknowledgments
Miller Chemical and Fertilizer (USA) provided Vapor Gard.
Conflicts of Interest
Authors Gee-Sian Leung and Ray Marriott were employees of Suprex Limited at the time of the work. Since the work was conducted, Suprex Limited has been dissolved and the companies where they are presently employees had no connection with the work and are given as contact addresses. Melville Miles was an employee of Freshtime Limited at the time of the work but is no longer employed there. For all these authors, the paper reflects their views as scientists not the views of the companies.
Abbreviations
The following abbreviations are used in this manuscript:
| DPM | di-1-p-menthene |
| PGR | Plant growth regulator |
| Exp | Experiment |
References
- FAOSTAT. Food and Agriculture Data. Available online: https://www.fao.org/faostat/en/#home (accessed on 7 August 2025).
- Berry, P.M.; Spink, J.H. A physiological analysis of oilseed rape yields: Past and future. J. Agric. Sci. 2006, 144, 381–392. [Google Scholar] [CrossRef]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Priya, A.K.; Alagumalai, A.; Balaji, D.; Song, H. Bio-based agricultural products: A sustainable alternative to agrochemicals for promoting a circular economy. RSC Sustain. 2023, 1, 746–762. [Google Scholar] [CrossRef]
- Solarova, J.; Pospisilova, J.; Slavik, B. Gas exchange regulation by changing of epidermal conductance with antitranspirants. Photosynthetica 1981, 15, 365–400. Available online: https://kramerius.lib.cas.cz/view/uuid:3298e183-4ce4-11e1-1431-001143e3f55c?page=uuid:3298e321-4ce4-11e1-1431-001143e3f55c (accessed on 13 March 2025).
- Mphande, W.; Farrell, A.D.; Kettlewell, P.S. Commercial uses of antitranspirants in crop production: A review. Outlook Agric. 2023, 52, 3–10. [Google Scholar] [CrossRef]
- Palliotti, A.; Poni, S.; Berrios, J.G.; Bernizzoni, F. Vine performance and grape composition as affected by early-season source limitation induced with anti-transpirants in two red Vitis vinifera L. cultivars. Aust. J. Grape Wine Res. 2010, 16, 426–433. [Google Scholar] [CrossRef]
- Miller Chemical. Vapor Gard Life Cycle Assessment. Available online: https://www.millerchemical.com/wp-content/uploads/2021/03/Miller-Life-Cycle-Assessment-Vapor-Gard-Final-Rev.pdf (accessed on 23 May 2025).
- Park, B.B.; An, J.Y.; Park, S.U. Recent studies on pinene and its biological and pharmacological activities. EXCLI J. 2021, 20, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Faralli, M.; Weerasinghe, M.; Leung, G.-S.; Marriott, R.; Miles, M.; Kettlewell, P. Wax extracted from waste cauliflower leaves shows potential antitranspirant efficacy when applied to rapeseed plants. Agronomy 2022, 12, 455. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- RHS John Innes Potting Compost. Available online: https://www.rhs.org.uk/soil-composts-mulches/john-innes-compost (accessed on 5 January 2022).
- Hozelock. Spraymist Trigger Sprayer. Available online: https://www.hozelock.com/product/spraymist-trigger-sprayer/ (accessed on 11 August 2025).
- Robertson, M.J.; Lilley, J.M. Simulation of growth, development and yield of canola (Brassica napus) in APSIM. Crop Pasture Sci. 2016, 67, 332–344. [Google Scholar] [CrossRef]
- Ticha, I. Photosynthetic characteristics during ontogenesis of leaves. VII: Stomata density and sizes. Photosynthetica 1982, 16, 375–471. Available online: https://kramerius.lib.cas.cz/periodical/uuid:28acad14-4ce4-11e1-88e8-005056a60003 (accessed on 13 June 2025).
- Monteith, J.L. Physical limitations to crop growth. Agric. Prog. 1966, 41, 9–23. Available online: https://repository.rothamsted.ac.uk/item/8w2z3/physical-limitations-to-crop-growth (accessed on 11 July 2025).
- Camarillo-Castillo, F.; Huggins, T.D.; Mondal, S.; Reynolds, M.P.; Tilley, M.; Hays, D.B. High-resolution spectral information enables phenotyping of leaf epicuticular wax in wheat. Plant Meth. 2021, 17, 58. [Google Scholar] [CrossRef]
- Xiang, J.; Vickers, L.H.; Hare, M.C.; Kettlewell, P.S. Increasing the concentration of film antitranspirant increases yields of rapeseed under terminal drought by improving plant water status. Agric. Water Man. 2023, 284, 108350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).