Plant Tissue Culture In Vitro: A Long Journey with Lingering Challenges
Abstract
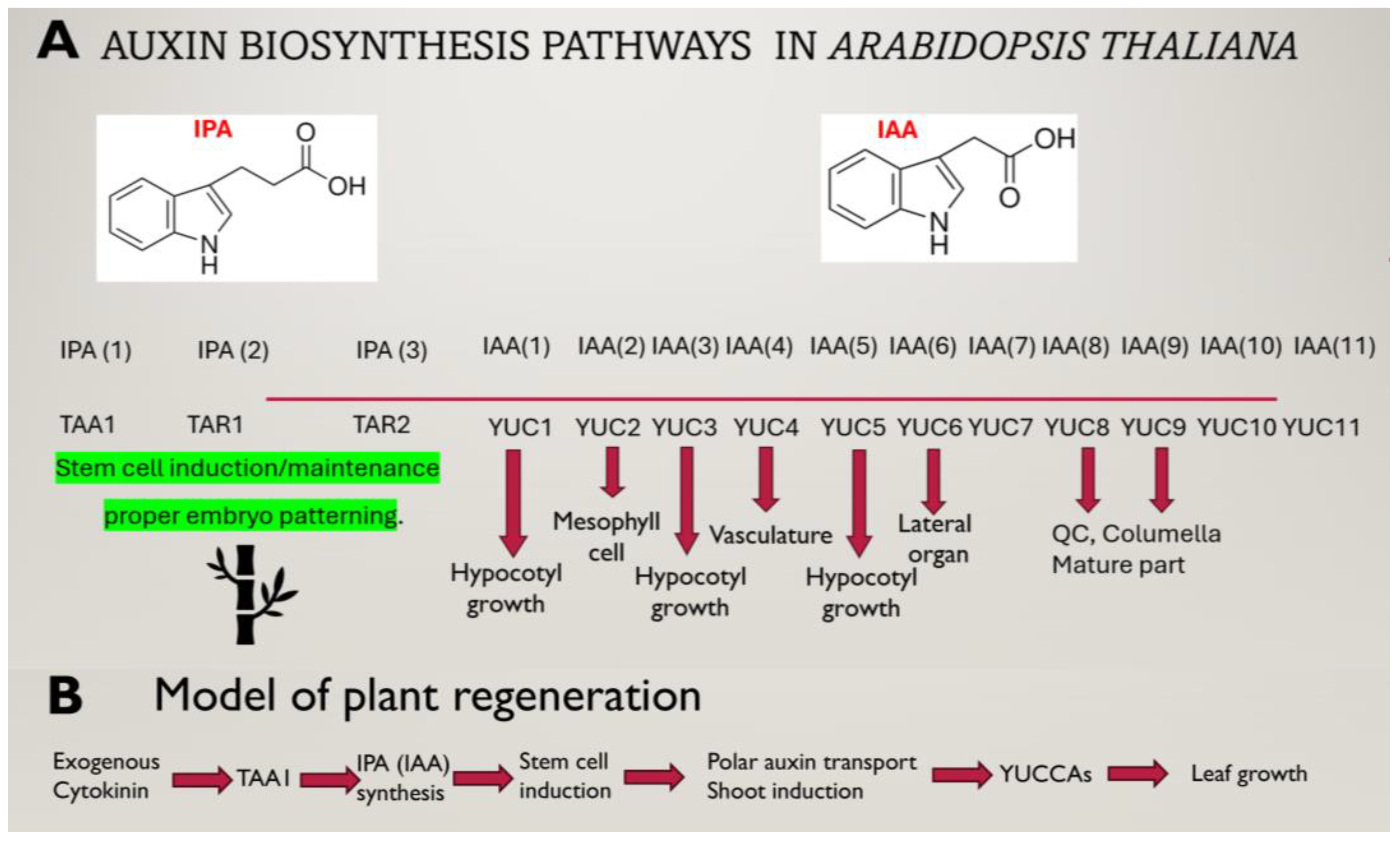
1. Auxin: The Main Force in Micropropagation (Shoot Formation and Rooting)
2. Nutrients Balance as a Key Factor in Plant Growth Regulation and the Limitations of MS Medium
3. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TAA | TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS |
| YUCCA | indole-3-pyruvate monooxygenase gene–auxin biosynthesis gene |
| SAM | shoot apical meristem |
References
- Haberlandt, G. Culturversuche mit isolierten pflanzenzellen. Sitzungsber. Akad. Wiss. Math.-Naturwiss. Kl. 1902, 111, 69–91. [Google Scholar]
- Haberlandt, G. Experiments on the culture of isolated plant cells. Bot. Rev. 1969, 35, 68–88. [Google Scholar] [CrossRef]
- Skoog, F. Miller CO Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar]
- Melnyk, C.W. Quantitative regeneration: Skoog and Miller revisited. Quant. Plant Biol. 2023, 4, e10. [Google Scholar] [CrossRef]
- Welsch, R.; Touraev, A.; Palme, K. Small molecules mediate cellular reprogramming across two kingdoms. J. Exp. Bot. 2021, 72, 7645–7647. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Shahzad, A. Regeneration of plant organs in vitro and its mechanistic basis. Front. Plant Sci. 2025, 16, 1568614. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Van Der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol.–Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An academic and technical overview on plant micropropagation challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Maruyama, K.; Ikeuchi, M. Multifaceted controls on auxin metabolism during cellular reprogramming and organ regeneration in plants. J. Exp. Bot. 2025, eraf251. [Google Scholar] [CrossRef] [PubMed]
- Sassi, M.; Vernoux, T. Auxin and self-organization at the shoot apical meristem. J. Exp. Bot. 2013, 64, 2579–2592. [Google Scholar] [CrossRef]
- Vanneste, S.; Pei, Y.; Friml, J. Mechanisms of auxin action in plant growth and development. Nat. Rev. Mol. Cell Biol. 2025, 26, 648–666. [Google Scholar] [CrossRef]
- Cao, X.; Yang, H.; Shang, C.; Ma, S.; Liu, L.; Cheng, J. The roles of auxin biosynthesis YUCCA gene family in plants. Int. J. Mol. Sci. 2019, 20, 6343. [Google Scholar] [CrossRef]
- Wang, J.L.; Di, D.W.; Luo, P.; Zhang, L.; Li, X.F.; Guo, G.Q.; Wu, L. The roles of epigenetic modifications in the regulation of auxin biosynthesis. Front. Plant Sci. 2022, 13, 959053. [Google Scholar] [CrossRef]
- Expósito-Rodríguez, M.; Borges, A.A.; Borges-Pérez, A.; Hernández, M.; Pérez, J.A. Cloning and biochemical characterization of ToFZY, a tomato gene encoding a flavin monooxygenase involved in a tryptophan-dependent auxin biosynthesis pathway. J. Plant Growth Regul. 2007, 26, 329–340. [Google Scholar] [CrossRef]
- Tivendale, N.D.; Ross, J.J.; Cohen, J.D. The shifting paradigms of auxin biosynthesis. Trends Plant Sci. 2014, 19, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kneuper, I.; Teale, W.; Dawson, J.E.; Tsugeki, R.; Katifori, E.; Palme, K.; Ditengou, F.A. Auxin biosynthesis and cellular efflux act together to regulate leaf vein patterning. J. Exp. Bot. 2021, 72, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Chen, S.; Xu, T. The Central Role of Auxin in Orchestrating Apical Stem Cells in Plants. Plant Cell Environ. 2025, 48, 5053–5072. [Google Scholar] [CrossRef]
- Yadav, S.; Kumar, H.; Mahajan, M.; Sahu, S.K.; Singh, S.K.; Yadav, R.K. Local auxin biosynthesis promotes shoot patterning and stem cell differentiation in Arabidopsis shoot apex. Development 2023, 150, dev202014. [Google Scholar] [CrossRef]
- Nguyen, V.; Gutzat, R. Epigenetic regulation in the shoot apical meristem. Curr. Opin. Plant Biol. 2022, 69, 102267. [Google Scholar] [CrossRef]
- Pasternak, T.; Paponov, I.A.; Kondratenko, S. Optimizing protocols for Arabidopsis shoot and root protoplast cultivation. Plants 2021, 10, 375. [Google Scholar] [CrossRef]
- SSakamoto, Y.; Kawamura, A.; Suzuki, T.; Segami, S.; Maeshima, M.; Polyn, S.; De Veylder, L.; Sugimoto, K. Transcriptional activation of auxin biosynthesis drives developmental reprogramming of differentiated cells. Plant Cell 2022, 34, 4348–4365. [Google Scholar] [CrossRef] [PubMed]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar] [CrossRef]
- Yu, T.Y.; Wang, P.; Lv, Y.; Wang, B.; Zhao, M.R.; Dong, X.W. Auxin Orchestrates Germ Cell Specification in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 3257. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.L.; Guo, H.R.; Xie, L.; Zeng, R.Z.; Zhang, X.Q.; Zhang, Z.S. Transcriptomic and hormonal analyses reveal that YUC-mediated auxin biogenesis is involved in shoot regeneration from rhizome in Cymbidium. Front. Plant Sci. 2017, 8, 1866. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wrobel-Marek, J.; Heidmann, I.; Horstman, A.; Chen, B.; Reis, R.; Angenent, G.C.; Boutilier, K. Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis. Plant Physiol. 2022, 188, 1095–1110. [Google Scholar] [CrossRef]
- Lee, K.; Yoon, H.; Park, O.-S.; Lim, J.; Kim, S.G.; Seo, P.J. ESR2–HDA6 complex negatively regulates auxin biosynthesis to delay callus initiation in Arabidopsis leaf explants during tissue culture. Plant Comm. 2024, 5, 100892. [Google Scholar] [CrossRef] [PubMed]
- Soeno, K.; Sato, A.; Shimada, Y. Chemical Biology in the Auxin Biosynthesis Pathway via Indole-3-Pyruvic Acid. Jpn. Agric. Res. Q. JARQ 2024, 58, 1–11. [Google Scholar] [CrossRef]
- Xiao, T.T.; Müller, S.; Shen, D.; Liu, J.; Adema, K.; van Seters, A.; Franssen, H.; Bisseling, T.; Kulikova, O.; Kohlen, W. Nodule organogenesis in Medicago truncatula requires local stage-specific auxin biosynthesis and transport. Plant Physiol. 2025, 197, kiaf133. [Google Scholar] [CrossRef]
- Chen, L.; Tong, J.; Xiao, L.; Ruan, Y.; Liu, J.; Zeng, M.; Huang, H.; Wang, J.W.; Xu, L. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016, 67, 4273–4284. [Google Scholar] [CrossRef]
- Sang, Y.L.; Cheng, Z.J.; Zhang, X.S. Endogenous auxin biosynthesis and de novo root organogenesis. J. Exp. Bot. 2016, 67, 4011–4013. [Google Scholar] [CrossRef]
- Kondratenko, S.I.; Pasternak, T.P.; Samovol, O.P.; Mogilna, O.M.; Sergienko, O.V. Modeling of asymmetric division of somatic cell in protoplasts culture of higher plants. Regul. Mech. Biosyst. 2020, 11, 2. [Google Scholar] [CrossRef]
- O’Neill, D.P.; Ross, J.J. Auxin regulation of the gibberellin pathway in pea. Plant Physiol. 2002, 130, 1974–1982. [Google Scholar] [CrossRef]
- Willige, B.C.; Isono, E.; Richter, R.; Zourelidou, M.; Schwechheimer, C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport–dependent growth and development in Arabidopsis thaliana. Plant Cell 2011, 23, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Khanday, I.; Santos-Medellín, C.; Sundaresan, V. Somatic embryo initiation by rice BABY BOOM1 involves activation of zygote-expressed auxin biosynthesis genes. New Phytol. 2023, 238, 673–687. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol.-Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Geilfus, C.M. Chloride: From nutrient to toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.; Miller, R.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Bedarev, V.A. Hyperhydricity in Plant Tissue Culture. Plants 2022, 11, 3313. [Google Scholar] [CrossRef]
- Lu, Z.; Ren, T.; Li, J.; Hu, W.; Zhang, J.; Yan, J.; Li, X.; Cong, R.; Guo, S.; Lu, J.; et al. Nutrition-mediated cell and tissue-level anatomy triggers the covariation of leaf photosynthesis and leaf mass per area. J. Exp. Bot. 2020, 71, 6524–6537. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhu, C.; Li, Q.; Su, L.; Li, Y.; Wu, H.; Jiang, W.; Lu, T.; Yu, H. Chloride modulates carbohydrate metabolism and ethylene synthesis in tomato fruits. Plant J. 2025, 122, e70132. [Google Scholar] [CrossRef]
- Dracup, M.; Greenway, H. Regulation of turgor pressure by suspension-cultured tobacco cells. J. Exp. Bot. 1988, 39, 1591–1603. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; Klerk, G.J.D. The anatomy and morphology of tissue cultured plants. In Plant Propagation by Tissue Culture: Volume 1. The Background; Springer: Dordrecht, The Netherlands, 2008; pp. 465–477. [Google Scholar]
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasternak, T.; Steinmacher, D. Plant Tissue Culture In Vitro: A Long Journey with Lingering Challenges. Int. J. Plant Biol. 2025, 16, 97. https://doi.org/10.3390/ijpb16030097
Pasternak T, Steinmacher D. Plant Tissue Culture In Vitro: A Long Journey with Lingering Challenges. International Journal of Plant Biology. 2025; 16(3):97. https://doi.org/10.3390/ijpb16030097
Chicago/Turabian StylePasternak, Taras, and Douglas Steinmacher. 2025. "Plant Tissue Culture In Vitro: A Long Journey with Lingering Challenges" International Journal of Plant Biology 16, no. 3: 97. https://doi.org/10.3390/ijpb16030097
APA StylePasternak, T., & Steinmacher, D. (2025). Plant Tissue Culture In Vitro: A Long Journey with Lingering Challenges. International Journal of Plant Biology, 16(3), 97. https://doi.org/10.3390/ijpb16030097





