A Comparative Analysis of Fruit Quality and Flavor in Capsicum chinense and Capsicum annuum from Myanmar, Peru, and Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
| Accession | Supplier | Origin | Species |
|---|---|---|---|
| C34 | NARO Genebank | Peru | C. chinense |
| C37 | Yokohama City University | Peru | C. chinense |
| C39 | Yokohama City University | Peru | C. chinense |
| SP4 | Kyoto University | Peru | C. chinense |
| C44 | Collected material [14] | Sagaing Region, Myanmar | C. chinense |
| C47 | Collected material | Sagaing Region, Myanmar | C. chinense |
| A7 | Collected material | Minamiashigara, Japan | C. annuum |
2.2. Morphological Characterization
2.3. Capsaicinoid Quantification
2.4. Ascorbic Acid Analysis
2.5. Carotenoid Content
2.6. Total Polyphenols
2.7. Aromatic Composition
2.8. Statistical Analysis
3. Results and Discussion
3.1. Morphology
3.2. Spiciness in chili pepper
3.3. Antioxidant Activity
3.4. Aroma Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meckelmann, S.W.; Riegel, D.W.; Van Zonneveld, M.J.; Ríos, L.; Peña, K.; Ugas, R.; Quinonez, L.; Mueller-Seitz, E.; Petz, M. Compositional Characterization of Native Peruvian Chili Peppers (Capsicum spp.). J. Agric. Food Chem. 2013, 61, 2530–2537. [Google Scholar] [CrossRef]
- Eshbaugh, W.H. The Taxonomy of the Genus Capsicum. In Peppers: Botany, Production and Uses; Russo, V.M., Ed.; CABI International: Boston, MA, USA, 2012; pp. 14–28. [Google Scholar]
- Bosland, P.; Votava, E. Peppers: Vegetable and Spice Capsicums; Crop Production Science in Horticulture Series: London, UK, 2012; pp. 1–38. [Google Scholar]
- Manikharda; Takahashi, M.; Arakaki, M.; Yonamine, K.; Hashimoto, F.; Takara, K.; Wada, K. Influence of Fruit Ripening of Color, Organic Acid Contents, Capsaicinoids, Aroma Compounds, and Antioxidant Capacity of Shimatogarashi (Capsicum frutescens). J. Oleo Sci. 2018, 67, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Morales-Soriano, E.; Kebede, B.; Ugas, R.; Grauwet, T.; Van Loey, A.; Hendrickx, M. Flavor Characterization of Native Peruvian Chili Peppers Through Integrated Aroma Fingerprinting and Pungency Profiling. Food Res. Int. 2018, 109, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, R. Capsaicinoids Production and Accumulation in Epidermal Cells on the Internal Side of the Fruit Pericarp in ‘Bhut Jolokia’ (Capsicum chinense). Cytologia 2017, 82, 303–306. [Google Scholar] [CrossRef]
- Luo, X.J.; Peng, J.; Li, Y.J. Recent Advances in the Study on Capsaicinoids and Capsinoids. Eur. J. Pharmacol. 2010, 650, 1–7. [Google Scholar] [CrossRef]
- Eggink, P.M.; Maliepaard, C.; Tikunov, Y.; Haanstra, J.P.W.; Bovy, A.G.; Visser, R.G.F. A taste of sweet pepper: Volatile and Non-Volatile Chemical Composition of Fresh Sweet Pepper (Capsicum annuum) in Relation to Sensory Evaluation of Taste. Food Chem. 2012, 132, 301–310. [Google Scholar] [CrossRef]
- Tripodi, P.; Kumar, S. The Capsicum Crop: An Introduction. In The Capsicum Genome; Ramchiary, N., Kole, C., Eds.; Spinger Nature: Cham, Switzerland, 2019; pp. 1–8. [Google Scholar]
- Vitamin C, Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional (accessed on 14 April 2025).
- Oboh, G.; Rocha, J.B.T. Distribution and Antioxidant Activity of Polyphenols in Ripe and Unripe Tree Pepper (Capsicum pubescens). J. Food Biochem. 2006, 31, 456–473. [Google Scholar] [CrossRef]
- Topuz, A.; Ozdemir, F. Assessment of Carotenoids, Capsaicinoids and Ascorbic Acid Composition of Some Selected Pepper Cultivars (Capsicum annuum L.) Grown in Turkey. J. Food Compos. Anal. 2007, 20, 596–602. [Google Scholar] [CrossRef]
- Lima Sampiao, A.P.; Gonzáles Aguilera, J.; da Silva Mendes, A.M.; Argentel-Martínez, L.; Zuffo, A.M.; Teodoro, P.E. The Role of the Genetic Diversity of Capsicum spp. in the Conservation of the Species: Qualitative and Quantitative Characterization. Cienc. Agrotec. 2023, 47, e009122. [Google Scholar]
- Naito, K.; Aye, S.S.; Thein, M.S.; Hein, A.P.; Takei, E.; Osada, T.; Domon, E.; Watanabe, K.; Kawase, M. A Field Study to Explore Plant Genetic Resources in the Sagaing Region and Shan State of Myanmar in 2016. Annu. Rep. Explor. Introd. Plant Genet. Resour. 2017, 33, 265–293. [Google Scholar]
- International Plant Genetic Resources Institute (IPGRI). Descriptors for Capsicum (Capsicum Spp.); International Plant Genetic Resources Institute: Rome, Italy, 1995; pp. 23–37. [Google Scholar]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophan Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Grother, P. NIST Special Database 20. NIST Scientific and Technical Document Database; World Wide Web-Internet and Web Information Systems; 2008. Available online: https://www.nist.gov/publications/nist-special-database-20-nist-scientific-and-technical-document-database (accessed on 23 January 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 20 February 2025).
- Alvares, P.; Almeida, L.; Da Silva, A.; Araujo, P.; Pimienta, S.; Pombo, C.; Erpen-Dalla, L.; Azeredo, L.; Rodrigues, R. Biomorphological Characterization of Brazilian Capsicum chinense Jacq. Germplasm. Agronomy 2020, 10, 447. [Google Scholar] [CrossRef]
- Thul, S.T.; Lal, R.K.; Shasany, A.K.; Darokar, M.P.; Gupta, A.K.; Gupta, M.M.; Verma, R.K.; Khanuja, S.P.S. Estimation of Phenotypic Divergence in a Collection of Capsicum Species for Yield-Related Traits. Euphytica 2009, 168, 189–196. [Google Scholar] [CrossRef]
- Weiss, E.A. Spice Crops; CABI Publishing International: New York, NY, USA, 2002; p. 411. [Google Scholar]
- González-Zamora, A.; Sierra-Campos, E.; Luna-Ortega, J.G.; Pérez-Morales, R.; Ortiz, J.C.R.; García-Hernández, J.L. Characterization of Different Capsicum Varieties by Evaluation of their Capsaicinoids Content by High Performance Liquid Chromatography Determination of Pungency and Effect of High Temperature. Molecules 2013, 18, 13471–13486. [Google Scholar] [CrossRef]
- Usman, M.G.; Rafii, M.Y.; Ismail, M.R.; Malek, M.A.; Latif, M.A. Capsaicin and Dihydrocapsacin Determination in Chili Pepper Genotypes Using Ultra-Fast Liquid Chromatography. Molecules 2014, 19, 6474–6488. [Google Scholar] [CrossRef]
- Koeda, S.; Sato, K.; Tomi, K.; Tanaka, Y.; Takisawa, R.; Hosokawa, M.; Doi, M.; Nakazaki, T.; Kitajima, A. Analysis of Non-Pungency, Aroma, and Origin of a Capsicum chinense Cultivar from a Caribbean Island. Hort. J. 2014, 83, 244–251. [Google Scholar] [CrossRef]
- Zewdie, Y.; Bosland, P.W. Capsaicinoid Profiles are Not Good Chemataxonomic Indicators for Capsicum species. Biochem. Syst. Ecol. 2001, 29, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Jayaprakasha, G.K.; Crosby, K.; Yoo, K.S.; Leskovar, D.I.; Jifon, J.; Patil, B.S. Ascorbic Acid, Capsaicinoid, and Flavonoid Aglycone Concentrations as a Function of Fruit Maturity Stage in Greenhouse-Grown Peppers. J. Food Compos. Anal. 2014, 33, 195–202. [Google Scholar] [CrossRef]
- Veras, A.O.M.; Béttega, R.; Freire, F.B.; Barrozo, M.A.S.; Freire, J.T. Drying Kinetics, Structural Characteristics, and Vitamin C Retention of Dedo-de-Moça Pepper (Capsicum baccatum) During Convective and Freeze Drying. Braz. J. Chem. Eng. 2012, 29, 741–750. [Google Scholar] [CrossRef]
- Ribes-Moya, A.M.; Raigón, M.D.; Moreno-Peris, E.; Fita, A.; Rodriguez-Burruezo, A. Response to Organic Cultivation of Heirloom Capsicum Peppers: Variation in the Level of Bioactive Compounds and Effect of Ripening. PLoS ONE 2018, 13, e0207888. [Google Scholar] [CrossRef]
- Institute of Medicine (IOM). Dietary Reference Intakes for Vitamin A, Vitamin K, Arsecnic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academy Press: Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 20 April 2025).
- Biacs, P.A.; Czinkotai, B.; Hoschke, A. Factors Affecting Stability of Colored Substances in Paprika Powders. J. Agric. Food Chem. 1992, 40, 363–367. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F.; Bonesi, M.; Conforti, F.; De Luca, D.; Menichini, F. Air-Dried Capsicum annuum var. acuminatum Medium and Big: Determination of Bioactive Constituents, Antioxidant Activity and Carbohydrate-Hydrolyzing Enzymes Inhibition. Food Res. Int. 2012, 45, 170–176. [Google Scholar]
- Kusumiyati, K.; Putri, I.E.; Hamdani, J.S.; Suhandy, D. Real-Time Detection of the Nutritional Compounds in Green ‘Ratuni UNPAD’ Cayenne Pepper. Horticulturae 2022, 8, 554. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.; Gonzales-Mas, M.d.C.; Nuez, F. Carotenoid Composition and Vitamin A Value in Aji (Capsicum baccatum L.) and Rocoto (C. pubescens R&P), 2 Pepper Species from the Andean Region. J. Food Sci. 2010, 75, S446–S453. [Google Scholar]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, Additive, and Antagonistic Effects of Food Mixtures on Total Antioxidant Capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.R.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P.J.; Menichi, F. The Influence of Fruit Ripening on the Phytochemical Content and Biological Activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Kasampalis, D.S.; Tsouvaltzis, P.; Ntouros, K.; Gertsis, A.; Gitas, I.; Moshou, D.; Siomos, A.S. Nutritional Composition Changes in Bell Pepper as Affected by the Ripening Stage of Fruits at Harvest or Postharvest Storage and Assessed Non-Destructively. J. Sci. Food Agric. 2021, 102, 445–454. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 Richest Dietary Sources of Polyphenols: An Application of the Phenol-Explorer Database. Eur. J. Clin. Nutr. 2010, 64, S112–S120. [Google Scholar] [CrossRef]
- Rodríguez-Burruezo, A.; Kollmannsberger, H.; González-Mas, M.C.; Nitz, S.; Nuez, F. HS-SPME Comparative Analysis of Genotypic Diversity in the Volatile Fraction and Aroma-Contributing Compounds of Capsicum Fruits from the Annuum-Chinense-Frutescens Complex. J. Agric. Food Chem. 2010, 58, 4388–4400. [Google Scholar] [CrossRef]
- Trovato, E.; Vento, F.; Creti, D.; Dugo, P.; Mondello, L. Elucidation of Analytical-Compositional Fingerprinting of Three Different Species of Chili Pepper by Using Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry Analysis, and Sensory Profile Evaluation. Molecules 2022, 27, 2355. [Google Scholar] [CrossRef]
- Pino, J.; González, M.; Ceballos, L.; Centurión-Yah, A.R.; Trujillo-Aguirre, J.; Latournerie-Moreno, L.; Sauri-Duch, E. Characterization of Total Capsaicinoids, Color and Volatile Compounds of Habanero Chilli Pepper (Capsicum chinense Jack.) Cultivars Grown in Yucatan. Food Chem. 2007, 104, 1682–1686. [Google Scholar] [CrossRef]
- Kollmannsberger, H.; Rodríguez-Barruezo, A.; Nitz, S.; Nuez, F. Volatile and Capsaicinoid Composition of Ají (Capsicum baccatum) and Rocoto (Capsicum pubescens), Two Andean Species of Chile Peppers. J. Sci. Food Agric. 2011, 91, 1598–1611. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Ota, M.; Hirano, H.; Nakagawa, K. Characterization of Newly Developed Pepper Cultivars (Capsicum chinense) ‘Dieta0011-0301’, ‘Dieta0011-0602’, ‘Dieta0041-0401’, and ‘Dieta0041-0601’ Containing High Capsinoid Concentrations and a Strong Fruity Aroma. Biosci. Biotechnol. Biochem. 2020, 84, 1870–1885. [Google Scholar] [CrossRef]
- Murakami, Y.; Iwabuchi, H.; Ohba, Y.; Fukami, H. Analysis of Volatile Compounds from Chili Peppers and Characterization of Habanero (Capsicum chinense) Volatiles. J. Oleo Sci. 2019, 68, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Munda, S.; Sarma, N.; Begum, T.; Pandey, S.K.; Chanda, S.K.; Sastry, G.N.; Lal, M. Estimation of Genetic Variation in Yield, its Contributing Characters and Capsaicin Content of Capsicum chinense Jacq. (ghost pepper) Germplasm from Northeast India. PeerJ 2023, 11, e15521. [Google Scholar] [CrossRef]
- Garcés-Claver, A.; Gil-Ortega, R.; Álvarez-Fernández, A.; Arnedo-Andrés, M.S. Inheritance of Capsaicin and Dihydrocapsaicin, Determined by HPLC-ESI/MS, in an Intraspecific Cross of Capsicum annuum L. J. Agric. Food Chem. 2007, 55, 6951–6957. [Google Scholar] [CrossRef]
- Shirasawa, K.; Ban, T.; Nagata, N.; Murakana, T. Impact of Genomics on Capsicum Breeding. In The Capsicum Genome; Ramchiary, N., Kole, C., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 209–220. [Google Scholar]
- Moreno-Peris, E.; Cortés-Olmos, C.; Díez-Díaz, M.; González-Mas, M.; de Luis-Margarit, A.; Fita, A.; Rodríguez-Burruezo, A. Hybridization in Peppers (Capsicum spp.) to Improve the Volatile Composition in Fully Ripe Fruits: The Effects of Parent Combinations and Fruit Tissues. Agronomy 2020, 10, 751. [Google Scholar] [CrossRef]
- Yamazaki, A.; Hosokawa, M. Increased Percentage of Fruit Set of F1 Hybrid of Capsicum chinense During High-Temperature Period. Sci. Hortic. 2019, 243, 421–427. [Google Scholar] [CrossRef]
- Hazarika, G.; Phukan, R.; Sarma, D.; Sarma, R.N.; Deka, S.D.; Neog, B.; Sarma, A.; Gogoi, S.; Das, R.T.; Ojha, H. Genetic Relatedness Among Interspecific Hybrids in the Genus Capsicum and their Implications in Breeding King Chilli. S. Afr. J. Bot. 2023, 163, 744–755. [Google Scholar] [CrossRef]
- Kreissl, J.; Mall, V.; Steinhaus, P.; Steinhaus, M. Leibniz-LSB@TUM Odorant Database, Version 1.2. Leibniz Institute for Food Systems Biology at the Technical University of Munich: Freising, Germany. 2022. Available online: https://www.leibniz-lsb.de/en/databases/leibniz-lsbtum-odorant-database (accessed on 5 April 2025).
- The Good Scents Company Information System. Available online: https://www.thegoodscentscompany.com (accessed on 3 April 2025).
- Flavornet and Human Odor Space. Available online: http://www.flavornet.org (accessed on 4 April 2025).

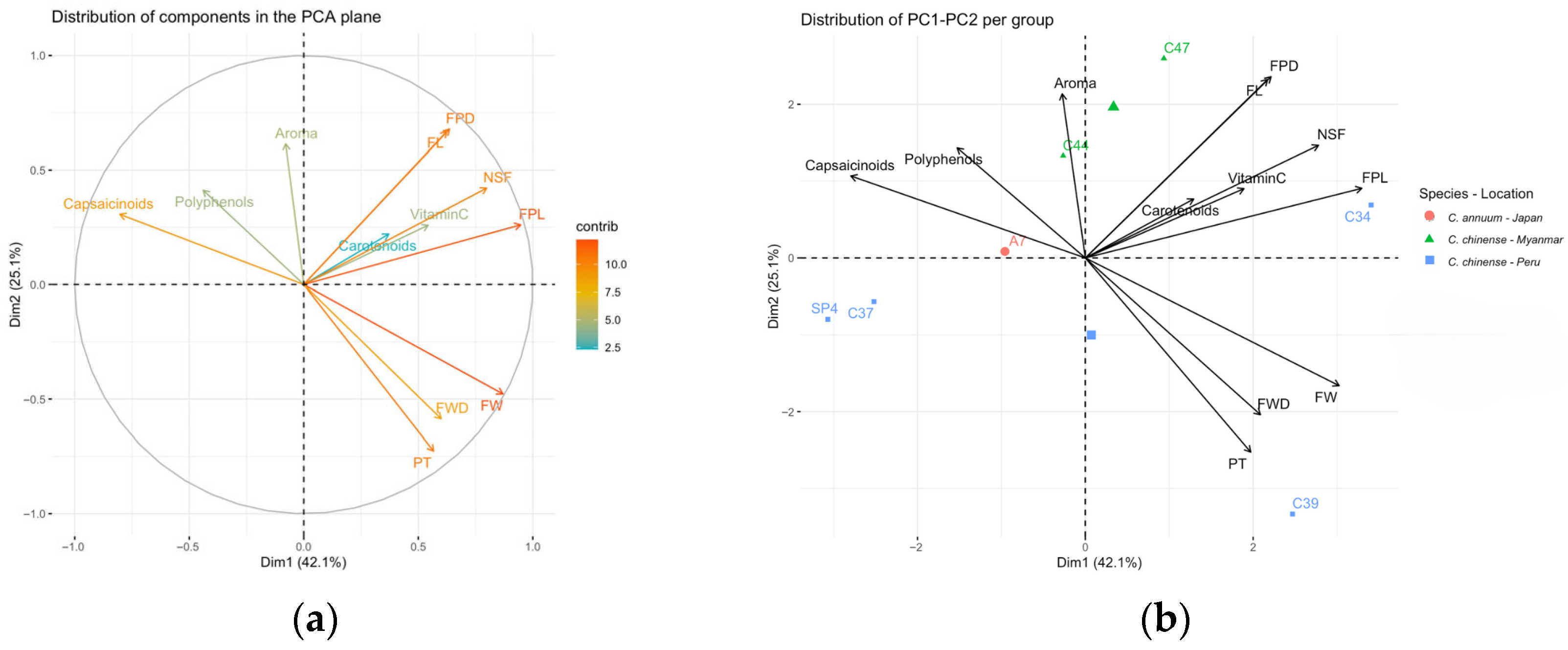
| Accession | FPL | FPD | NSF | PT | FW | FL | FWD |
|---|---|---|---|---|---|---|---|
| C34 | 4.04 ± 0.16 b | 2.12 ± 0.19 a,b | 79.4 ± 14.16 a | 1.9 ± 0.24 b | 13.98 ± 4.10 b | 62.82 ± 17.03 b | 21.06 ± 5.53 b |
| C37 | 1.78 ± 0.10 b,c | 0.9 ± 0.06 b,c | 6.2 ± 1.02 b | 1.06 ± 0.10 b | 0.69 ± 0.06 c | 15.02 ± 0.61 b,c | 10.64 ± 0.57 b,c |
| C39 | 3.28 ± 0.24 b,c | 1.54 ± 0.09 c,d | 28.2 ± 5.85 b | 2.64 ± 0.15 c | 19.28 ± 1.09 c | 38.54 ± 4.69 b,c | 39.52 ± 2.41 cd |
| SP4 | 1.5 ± 0.03 d | 1.02 ± 0.14 e | 15.2 ± 1.99 b | 1.62 ± 0.09 c | 0.6 ± 0.05 c | 10.46 ± 0.29 d | 9.22 ± 0.37 e |
| C44 | 2.92 ± 0.26 c | 2.22 ± 0.20 d | 22.8 ± 9.85 b | 1.16 ± 0.09 c | 5.46 ± 0.58 c | 54.5 ± 3.32 c | 25.72 ± 0.74 d,e |
| A7 | 3.42 ± 0.06 a | 2.54 ± 0.09 a | 58.6 ± 5.11 a | 1.04 ± 0.16 a | 3.71 ± 0.55 a | 91 ± 4.25 a | 8 ± 0.57 a |
| C47 | 2.86 ± 0.19 d | 1.78 ± 0.08 e | 11.8 ± 2.94 b | 0.94 ± 0.05 c | 3.64 ± 0.36 c | 56.26 ± 1.50 d | 17.34 ± 0.92 e |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Accession | CAP | DIH | NDH | Total | SHUs | Classification 1 |
|---|---|---|---|---|---|---|
| C34 | 53.75 ± 0.09 a | 50.99 ± 0.06 a | 41.97 ± 0 b | 146.7 ± 0.14 b | 20,765.56 ± 22.78 a | Moderately pungent |
| C37 | 728.91 ± 4.00 b | 158.8 ± 0.94 b | 60.18 ± 0.16 c | 947.89 ± 5.10 c | 148,518.3 ± 810.71 b | Very highly pungent |
| C39 | 23.9 ± 0 c | 40.97 ± 0 c | 41.97 ± 0 d | 106.83 ± 0 d | 14,346.38 ± 0 c | Moderately pungent |
| SP4 | 404.05 ± 3.97 f | 119.52 ± 0.59 f | 111.72 ± 0.36 f | 635.3 ± 4.88 g | 94,685.2 ± 761.45 f | Very highly pungent |
| C44 | 659.97 ± 3.28 d | 169.01 ± 0.72 d | 63.56 ± 0.20 e | 892.54 ± 4.19 e | 139,376.75 ± 661.24 d | Very highly pungent |
| A7 | 306.71 ± 1.43 a | 138.37 ± 0.61 a | 52.08 ± 0.13 a | 497.16 ± 2.16 a | 76,501.27 ± 339.39 a | Highly pungent |
| C47 | 726.87 ± 4.18 e | 168.42 ± 1.10 e | 66.42 ± 0.18 f | 961.71 ± 5.33 f | 150,319.36 ± 845.97 e | Very highly pungent |
| p-value | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Accession | Carotenoid | Polyphenols | Vitamin C |
|---|---|---|---|
| C34 | 89.97 ± 0.44 b | 1254.4 ± 424.16 a | 768 ± 23.30 a |
| C37 | 32.05 ± 0.25 c | 1071.75 ± 6.52 a | 777 ± 15.40 b |
| C39 | 35.6 ± 0.96 d | 967.38 ± 19.57 a | 645 ± 17.059 b,c |
| SP4 | 44.88 ± 0.32 g | 2258.97 ± 733.88 a | 269 ± 5 e |
| C44 | 64.61 ± 0.85 e | 2435.09 ± 733.88 a | 680 ± 13.45 c |
| A7 | 66.56 ± 0.19 a | 1534.9 ± 403.12 a | 506 ± 6.08 a |
| C47 | 28.97 ± 0.71 f | 1554.47 ± 0 a | 715 ± 10.58 d |
| p-value | p < 0.05 | n.s. | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega Morales, C.F.; Irie, K.; Kawase, M. A Comparative Analysis of Fruit Quality and Flavor in Capsicum chinense and Capsicum annuum from Myanmar, Peru, and Japan. Int. J. Plant Biol. 2025, 16, 90. https://doi.org/10.3390/ijpb16030090
Ortega Morales CF, Irie K, Kawase M. A Comparative Analysis of Fruit Quality and Flavor in Capsicum chinense and Capsicum annuum from Myanmar, Peru, and Japan. International Journal of Plant Biology. 2025; 16(3):90. https://doi.org/10.3390/ijpb16030090
Chicago/Turabian StyleOrtega Morales, Claudia F., Kenji Irie, and Makoto Kawase. 2025. "A Comparative Analysis of Fruit Quality and Flavor in Capsicum chinense and Capsicum annuum from Myanmar, Peru, and Japan" International Journal of Plant Biology 16, no. 3: 90. https://doi.org/10.3390/ijpb16030090
APA StyleOrtega Morales, C. F., Irie, K., & Kawase, M. (2025). A Comparative Analysis of Fruit Quality and Flavor in Capsicum chinense and Capsicum annuum from Myanmar, Peru, and Japan. International Journal of Plant Biology, 16(3), 90. https://doi.org/10.3390/ijpb16030090





