Isolation and Molecular Characterization of Potential Plant Growth-Promoting Bacteria from Groundnut and Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Samples from Agricultural Farmlands
2.2. Sample Preparation and Microbial Analysis
2.3. Screening of Rhizo- and Endophytic Bacteria for Plant Growth Promotion
2.3.1. Indole Acetic Acid (IAA) Production
2.3.2. Exopolysaccharides Production
2.3.3. Hydrogen Cyanide Production
2.3.4. Ammonia Production
2.3.5. Nitrogen Fixation Production
2.3.6. Phosphate Solubilization
2.4. Other Tests
2.5. Identification of Isolated Bacteria by Molecular Techniques
2.6. Statistical Analysis
3. Results
3.1. Bacterial Plate Cellular Morphology and Biochemical Tests
3.2. Qualitative and Quantitative Screening of Plant Growth-Promoting Rhizo- and Endophytic Bacteria
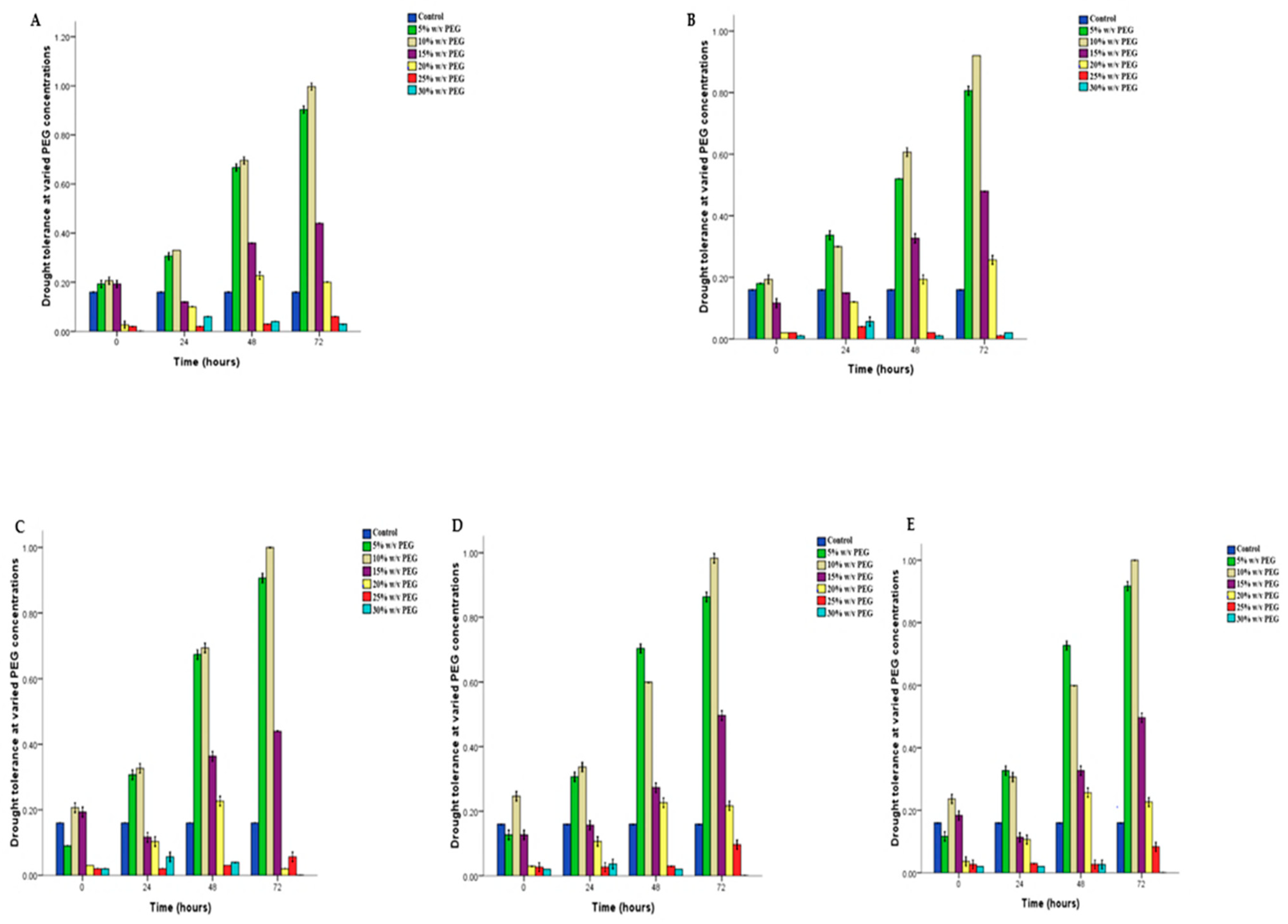
3.3. Drought Tolerance Screening
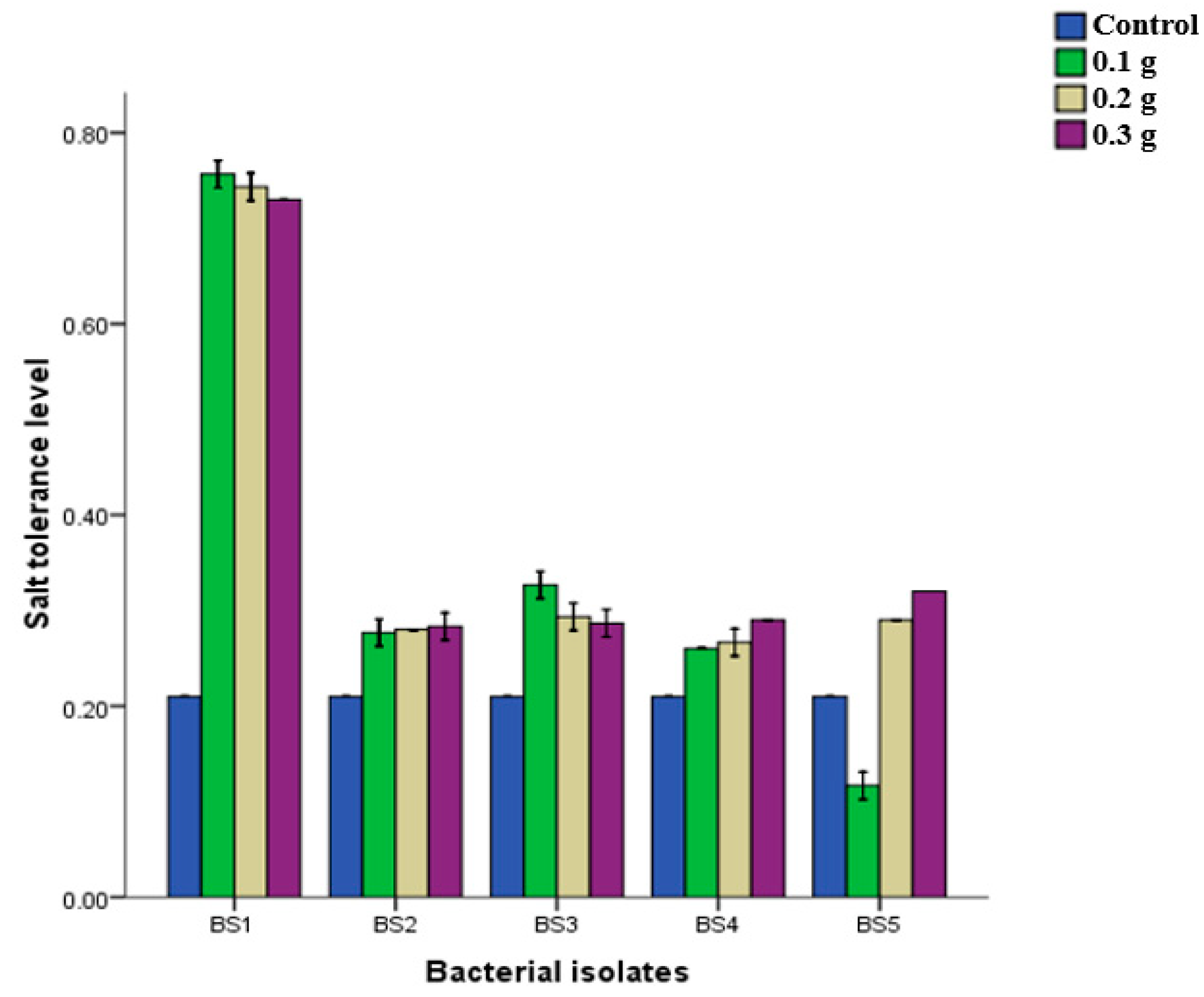
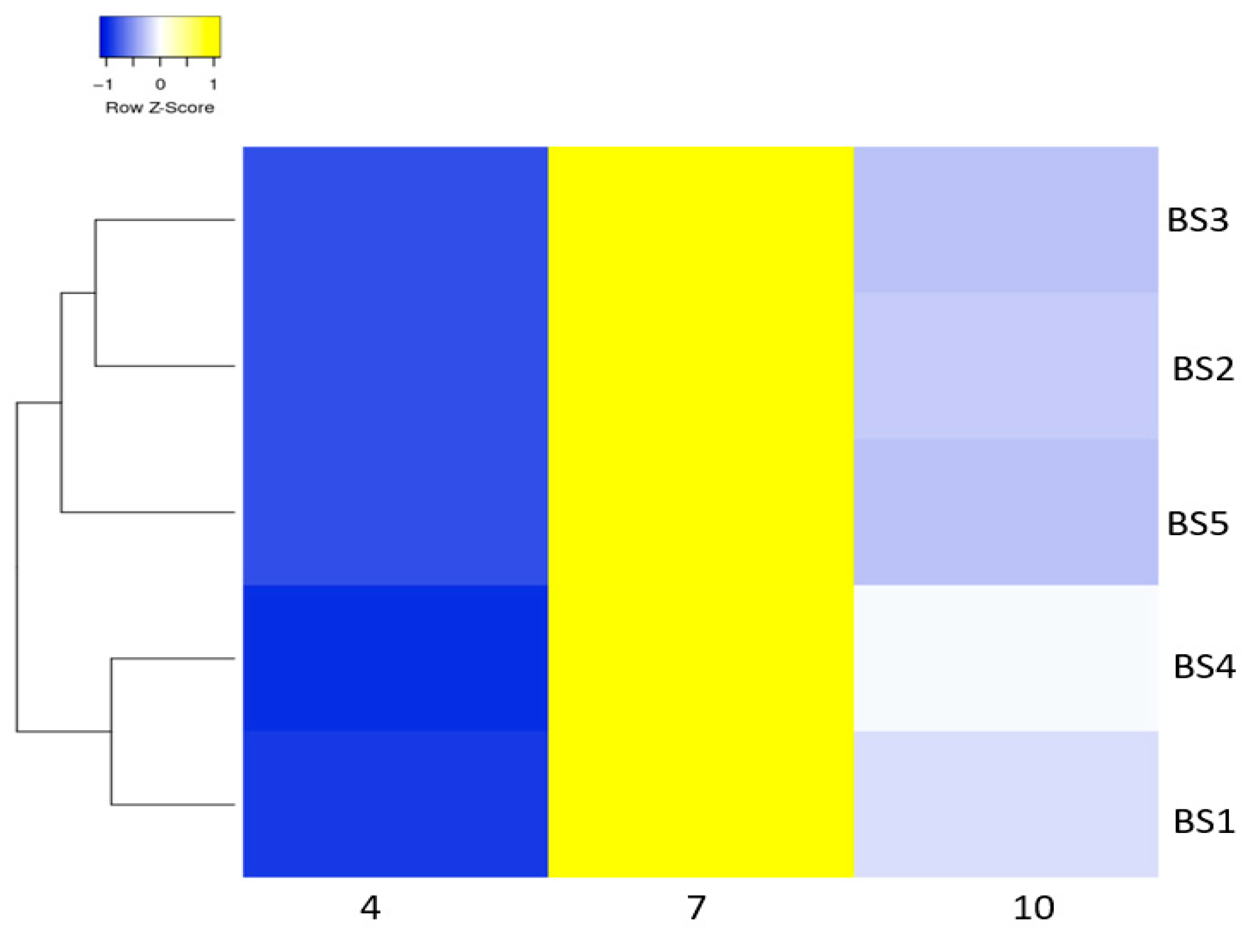
3.4. Bacteria Subjected to Different Salt Concentrations and Varied pH
3.5. Optimization of Process Parameters of Nutrient Source Requirements
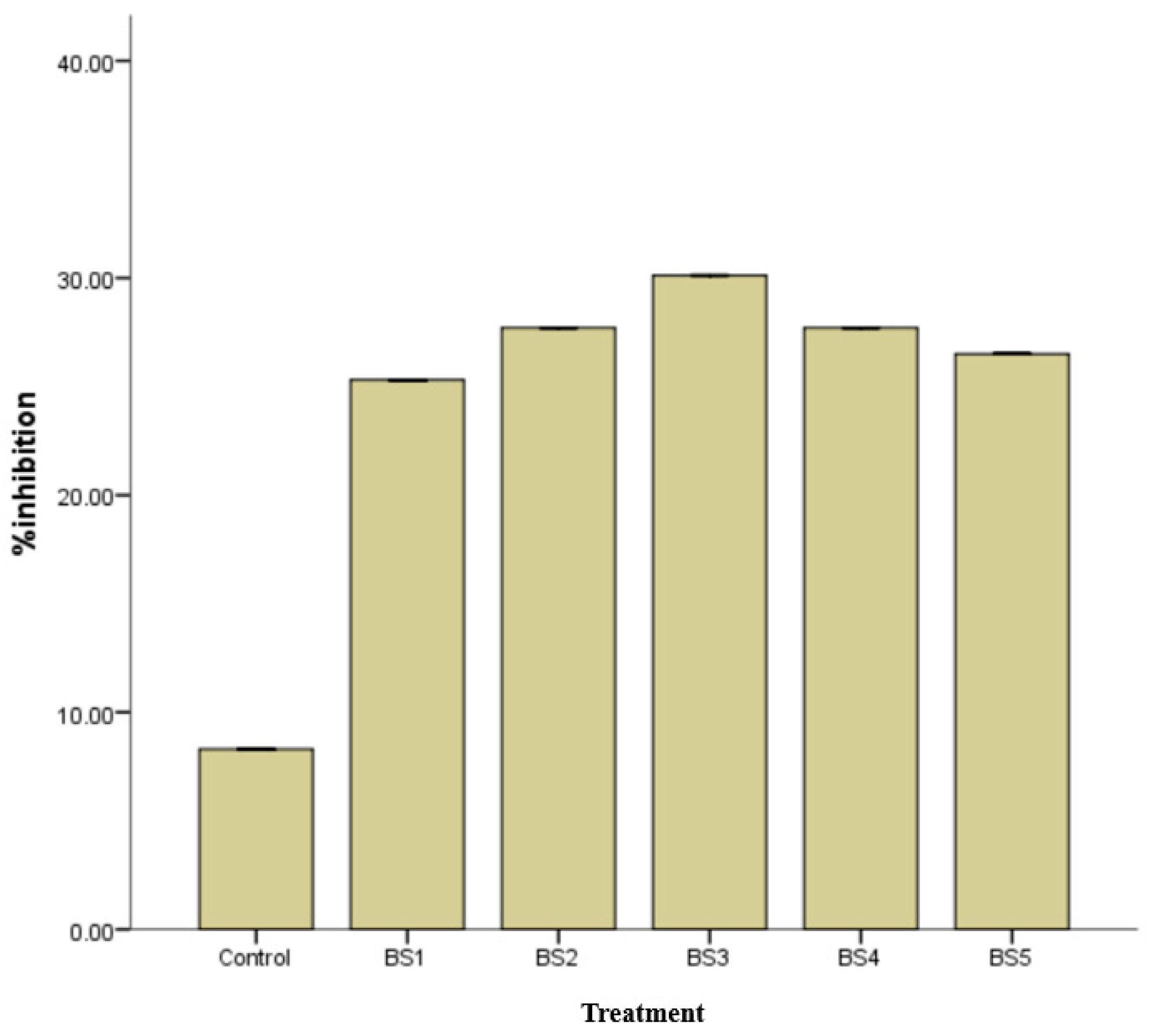
3.6. Antifungal Activities
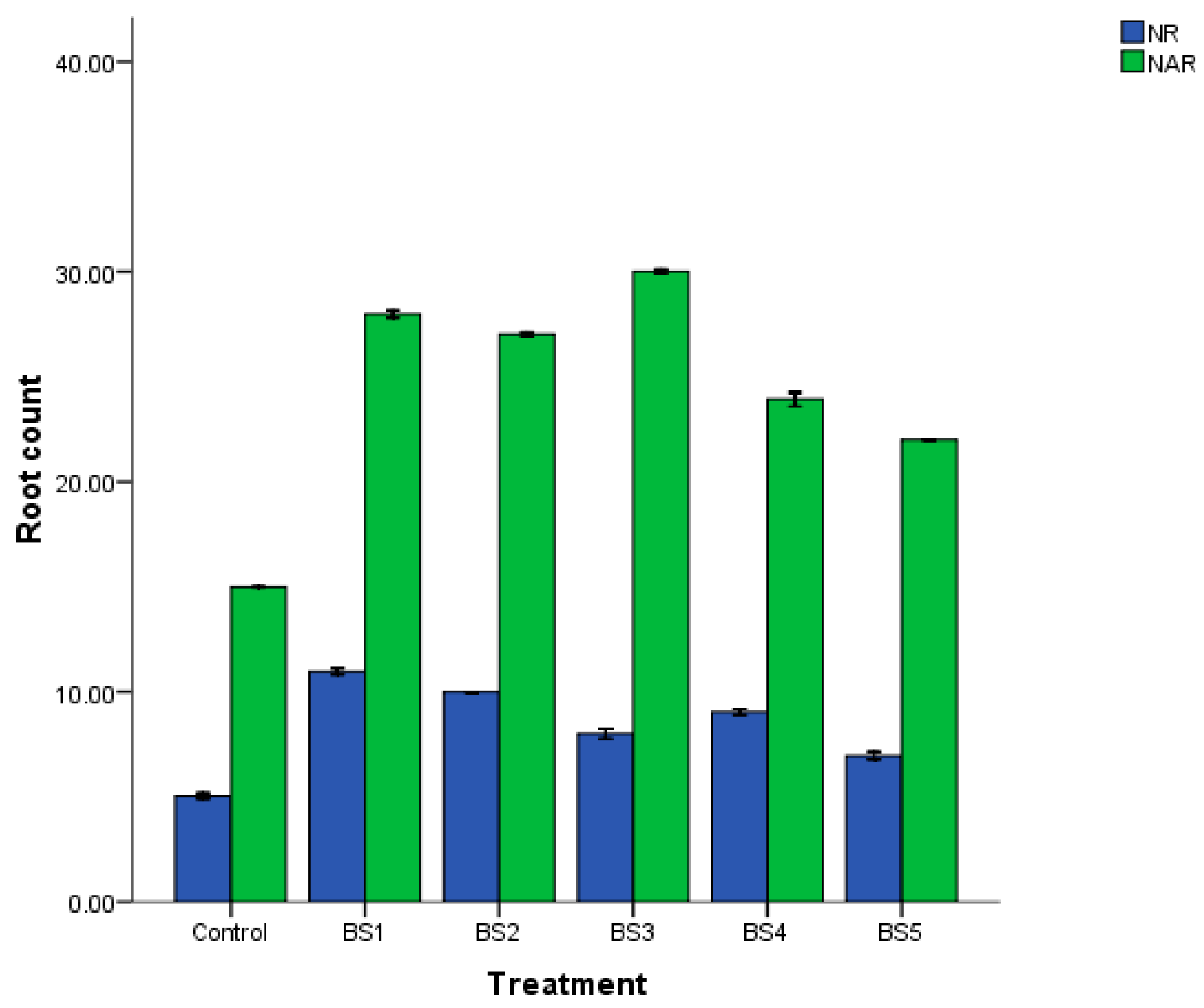
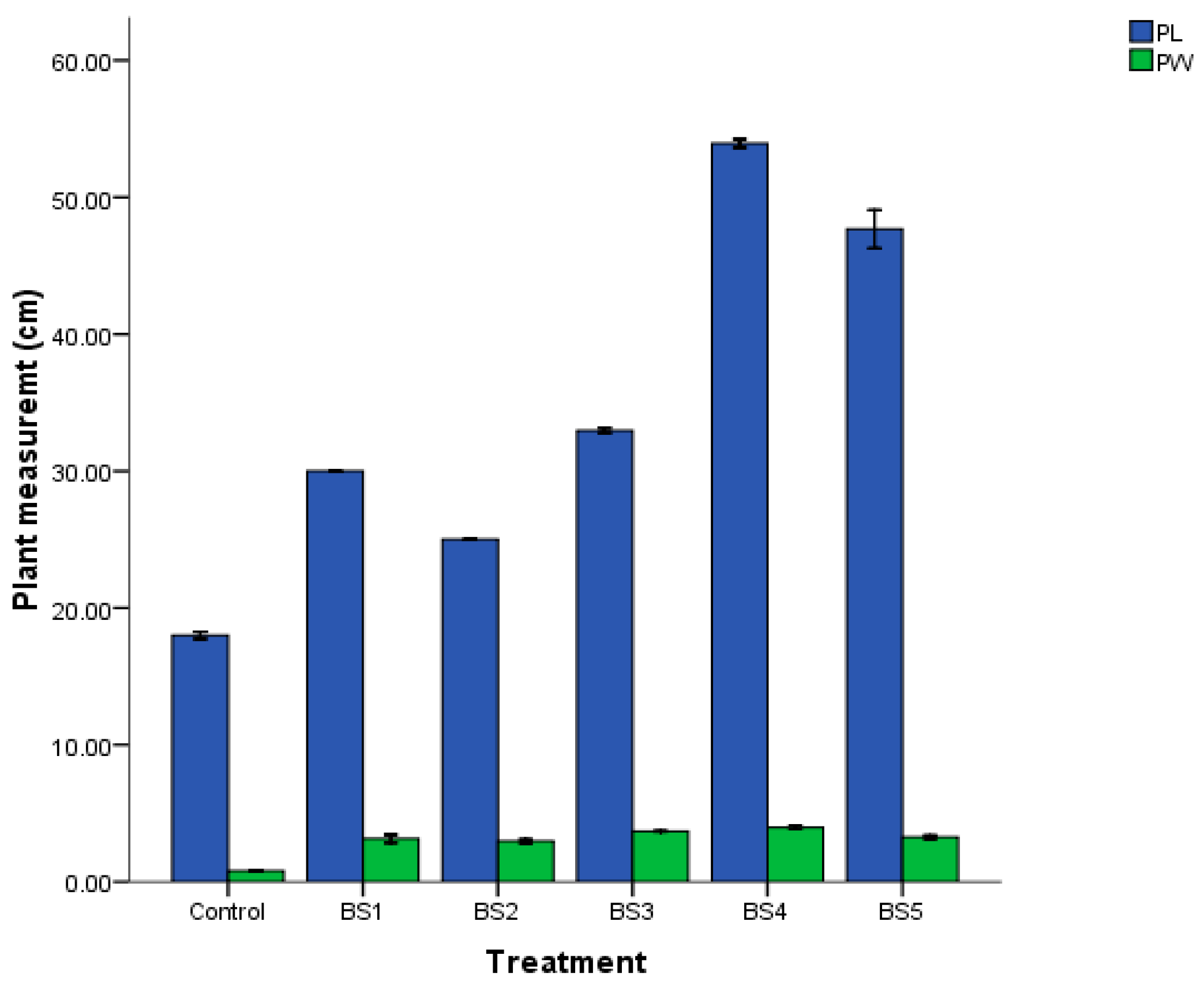
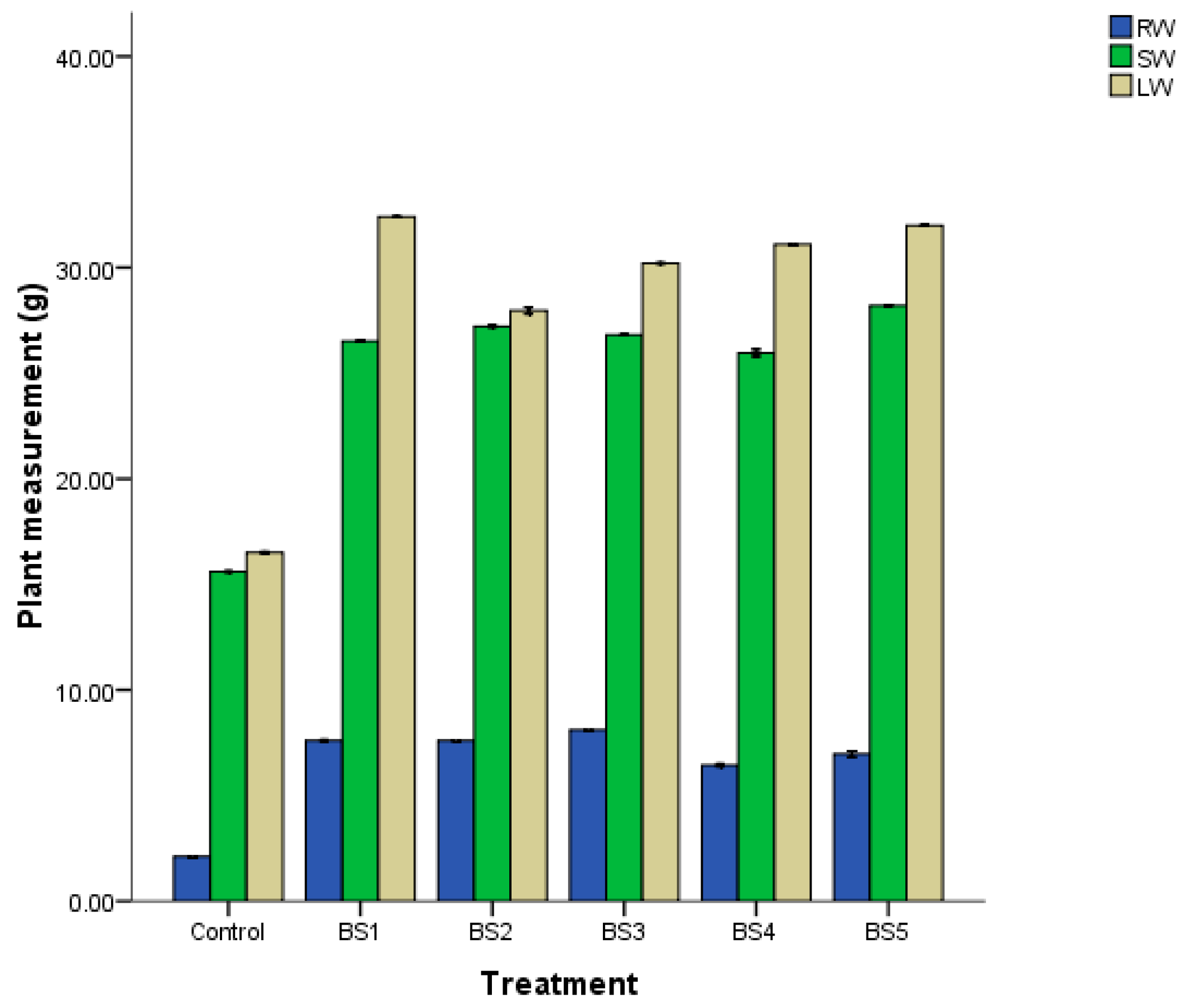
3.7. Seed and Maize Inoculation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Z.; Adeleke, B.S.; Shi, Y.; Li, C. The seed microbiomes of staple food crops. Microb. Biotechnol. 2023, 16, 2236–2249. [Google Scholar] [CrossRef]
- Adeola, A.; Van Gestel, C.A.; Doherty, V.F.; Aneyo, I.A.; Ajagbe, F.; Kasule, F. Regional variations and determinants of pesticide use among farmers in Southwestern Nigeria: Implications for sustainable agriculture. Front. Agron. 2025, 7, 1503899. [Google Scholar] [CrossRef]
- Adeleke, B.S. 16S rRNA gene sequencing data of plant growth-promoting jute-associated endophytic and rhizobacteria from coastal-environment of Ondo State, Nigeria. Data Brief 2024, 54, 110286. [Google Scholar] [CrossRef]
- Haile, D.; Tesfaye, B.; Assefa, F. Selected phosphate-solubilizing bacteria from tomato rhizosphere as plant growth promoter to improve tomato’s growth and production. Int. J. Agron. 2025, 2025, 6746599. [Google Scholar] [CrossRef]
- Rosini, B.; Bulla, A.M.; Polonio, J.C.; Polli, A.D.; da Silva, A.A.; Schoffen, R.P.; de Oliveira-Junior, V.A.; Santos, S.d.S.; Golias, H.C.; Azevedo, J.L.; et al. Isolation, identification, and bioprospection of endophytic bacteria from medicinal plant Mikania glomerata (Spreng.) and the consortium of Pseudomonas as plant growth promoters. Biocatal. Agric. Biotechnol. 2025, 64, 103530. [Google Scholar] [CrossRef]
- Ngwira, A.R.; Nyirenda, M.; Taylor, D. Toward sustainable agriculture: An evaluation of compost and inorganic fertilizer on soil nutrient status and productivity of three maize varieties across multiple sites in Malawi. Agroecol. Sustain. Food Syst. 2013, 37, 859–881. [Google Scholar] [CrossRef]
- Sattar, A.; Naveed, M.; Ali, M.; Zahir, Z.A.; Nadeem, S.M.; Yaseen, M.; Meena, V.S.; Farooq, M.; Singh, R.; Rahman, M.; et al. Perspectives of potassium solubilizing microbes in sustainable food production system: A review. Appl. Soil Ecol. 2019, 133, 146–159. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Fakoya, S. Isolation, screening and molecular identification of plant growth-promoting rhizobacteria from maize rhizosphere soil. Curr. Appl. Sci. Technol. 2025, 6, 0266217. [Google Scholar] [CrossRef]
- Dhole, A.; Shelat, H.; Vyas, R.; Jhala, Y.; Bhange, M. Endophytic occupation of legume root nodules by nifH-positive non-rhizobial bacteria, and their efficacy in the groundnut (Arachis hypogaea). Ann. Microbiol. 2016, 66, 1397–1407. [Google Scholar] [CrossRef]
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant growth-promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilisation of maize under greenhouse conditions. PLoS ONE 2016, 11, e0152478. [Google Scholar] [CrossRef] [PubMed]
- Sood, G.; Kaushal, R.; Panwar, G.; Dhiman, M. Effect of indigenous plant growth-promoting rhizobacteria on wheat (Triticum aestivum L.) productivity and soil nutrients. Commun. Soil Sci. Plant Anal. 2019, 50, 141–152. [Google Scholar] [CrossRef]
- Mukherjee, A.; Gaurav, A.K.; Patel, A.K.; Singh, S.; Chouhan, G.K.; Lepcha, A.; Pereira, A.P.d.A.; Verma, J.P. Unlocking the potential plant growth-promoting properties of chickpea (Cicer arietinum L.) seed endophytes bio-inoculants for improving soil health and crop production. Land Degrad. Dev. 2021, 32, 4362–4374. [Google Scholar] [CrossRef]
- Lebrazi, S.; Niehaus, K.; Bednarz, H.; Fadil, M.; Chraibi, M.; Fikri-Benbrahim, K. Screening and optimization of indole-3-acetic acid production and phosphate solubilization by rhizobacterial strains isolated from Acacia cyanophylla root nodules and their effects on its plant growth. J. Genet. Eng. Biotechnol. 2020, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Rawal, K.; Keharia, H. Genomics assisted functional characterization of Paenibacillus polymyxa HK4 as a biocontrol and plant growth promoting bacterium. Microbiol. Res. 2021, 248, 126734. [Google Scholar] [CrossRef]
- Bhartiya, A.; Dev, R.; Kant, L. Genetic enhancement in local food systems in North-Western Himalayan hills. Indian J. Plant Genet. Resour. 2025, 38, 11–23. [Google Scholar]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Siddika, A.; Rashid, A.A.; Khan, S.N.; Khatun, A.; Karim, M.M.; Prasad, P.V.; Hasanuzzaman, M. Harnessing plant growth-promoting rhizobacteria, Bacillus subtilis and B. aryabhattai to combat salt stress in rice: A study on the regulation of antioxidant defense, ion homeostasis, and photosynthetic parameters. Front. Plant Sci. 2024, 15, 1419764. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Jashim, A.I.I.; Islam, S.M.N.; Rahman, M.H.; Haque, M.M. Bacterial endophyte Pseudomonas mosselii PR5 improves growth, nutrient accumulation, and yield of rice (Oryza sativa L.) through various application methods. BMC Plant Biol. 2024, 24, 1030. [Google Scholar] [CrossRef]
- Alhaddad, F.; Abu-Dieyeh, M.; Jaoua, S.; Al-Ghouti, M.A.; Al-Thani, R.; Ahmed, T. Screening, diversity, and characterization of fungal endophytes isolated from the halophyte Limonium axillare and the potential of biocontrol antagonists against Fusarium oxysporum. Plant Direct 2025, 9, e70026. [Google Scholar] [CrossRef]
- Chen, Q.L.; Hu, H.W.; He, Z.Y.; Cui, L.; Zhu, Y.G.; He, J.Z. Potential of indigenous crop microbiomes for sustainable agriculture. Nat. Food 2021, 2, 233–240. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. In Vitro screening of sunflower associated endophytic bacteria with Plant growth-promoting traits. Front. Sustain. Food Syst. 2022, 6, 903114. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Amoo, A.E.; Babalola, O.O. Characterization of plant growth-promoting rhizobacterial isolates associated with food plants in South Africa. Antonie Van Leeuwenhoek 2021, 114, 1683–1708. [Google Scholar] [CrossRef] [PubMed]
- Agunbiade, V.F.; Fadiji, A.E.; Agbodjato, N.A.; Babalola, O.O. Isolation and characterization of plant-growth-promoting, drought-tolerant rhizobacteria for improved maize productivity. Plants 2024, 13, 1298. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Jansson, J.K.; McClure, R.; Egbert, R.G. Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 2023, 41, 1716–1728. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Lu, H.; Zhao, J.; Yang, T.; Wu, L.; Zou, J.; Chen, Q.; Zhang, B. Screening and identification of two novel phosphate-solubilizing Pyrenochaetopsis tabarestanensis strains and their role in enhancing phosphorus uptake in rice. Front. Microbiol. 2025, 15, 1494859. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Koskey, G.; Mburu, S.W.; Awino, R.; Njeru, E.M.; Maingi, J.M. Potential use of beneficial microorganisms for soil amelioration, phytopathogen biocontrol, and sustainable crop production in smallholder agroecosystems. Front. Sustain. Food Syst. 2021, 5, 606308. [Google Scholar] [CrossRef]
- Azeem, M.; Sultana, R.; Ahmed, N.; Abbasi, M.W.; Hasan, K.A.; Dong, R.; Alamri, S.; Alfagham, A.T. Salinity stress resilience in Sorghum bicolor through Pseudomonas-mediated modulation of growth, antioxidant system, and eco-physiological adaptations. ACS Omega 2025, 10, 940–954. [Google Scholar] [CrossRef]
- Ferreira, N.C.D.F.; Gatto, A.; Ramos, M.L.G. Co-inoculation of Trichoderma harzianum and Bradyrhizobium species augment the growth of Schizolobium parahyba var. parahyba (Vell.) blake seedlings. Microorganisms 2025, 13, 630. [Google Scholar] [CrossRef]
- da Silva Bandeira, O.N.; da Silva Bandeira, R.; de Souza, C.R.B. Systematic review and meta-analysis of the potential effects of endophytic bacteria Klebsiella on plant growth promotion and biocontrol of pathogens. World J. Microbiol. Biotechnol. 2025, 41, 89. [Google Scholar] [CrossRef]
- Hadian, S.; Smith, D.L.; Supronienė, S. Modulating the plant microbiome: Effects of seed Inoculation with endophytic bacteria on microbial diversity and growth enhancement in Pea plants. Microorganisms 2025, 13, 570. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-H.; Huang, Y.-T.; Chien, P.-R.; Huang, F.-C.; Wu, C.-L.; Chen, L.-Y.; Hung, S.-H.W.; Pan, I.-C.; Huang, C.-C. A plant endophytic bacterium Burkholderia seminalis strain 869T2 increases plant growth under salt stress by affecting several phytohormone response pathways. Bot. Stud. 2025, 66, 7. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kapoor, N.; Ohri, P. Ameliorative effect of rhizobacteria Bradyrhizobium japonicum on antioxidant enzymes, cell viability and biochemistry in tomato plant under nematode stress. Sci. Rep. 2025, 15, 8017. [Google Scholar] [CrossRef]
- Wiedenbeck, J.; Cohan, F.M. Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol. Rev. 2011, 35, 957–976. [Google Scholar] [CrossRef]
- Moussa, S.; Naor, V.; Kruh, L.I. Unraveling the ecological impact of a bacterial biocontrol agent applied to melon plants: Insights into phenotypic, biochemical, and microbiota changes affected by Frateuria defendens. Eur. J. Plant Pathol. 2025, 172, 411–421. [Google Scholar] [CrossRef]
- Sondang, Y.; Anty, K.; Siregar, R. Identification of endophytic and rhizosphere bacteria in maize (Zea mays L.) in Limapuluh Kota Region, West Sumatra, Indonesia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Manila, Philippines, 18–21 October 2018; IOP Publishing: Bristol, UK, 2019; p. 012002. [Google Scholar]
- Cavalcanti, M.I.P.; Nascimento, R.d.C.; Rodrigues, D.R.; Escobar, I.E.C.; Fraiz, A.C.R.; de Souza, A.P.; de Freitas, A.D.S.; Nóbrega, R.S.A.; Fernandes-Júnior, P.I. Maize growth and yield promoting endophytes isolated into a legume root nodule by a cross-over approach. Rhizosphere 2020, 15, 100211. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Arshad, A.; Rajput, L.; Fatima, K.; Ullah, S.; Ahmad, M.; Imran, A. Growth-stimulatory effect of quorum sensing signal molecule N-acyl-homoserine lactone-producing multi-trait Aeromonas spp. on wheat genotypes under salt stress. Front. Microbiol. 2020, 56, 2164. [Google Scholar] [CrossRef]
- Suleimanova, A.; Itkina, D.; Pudova, D.; Sharipova, M. Identification of Pantoea phytate-hydrolyzing rhizobacteria based on their phenotypic features and multilocus sequence analysis (MLSA). Microbiology 2021, 90, 87–95. [Google Scholar] [CrossRef]
- Sun, H.; Tang, Y.; Zhou, Q.; Li, M. Enhanced motility and biofilm development of Bacillus velezensis S3-1 in response to maize root sugars. Microb. Ecol. 2022, 84, 250–261. [Google Scholar]
- Wang, L.; Chen, H.; Li, Z.; Xu, X.; Zhao, J. Temporal dynamics of maize root exudate sugar composition and its influence on microbial colonization. Plant Sci. 2024, 338, 111896. [Google Scholar]
- Zhou, Y.; Li, Q.; Wang, S.; Chen, X. Functional characterization of the glucose transporter ptsG in Bacillus cereus root colonization. Microb. Ecol. 2022, 84, 123–134. [Google Scholar]
- Carril, P.; Cruz, J.; di Serio, C.; Pieraccini, G.; Ait Bessai, S.; Tenreiro, R.; Cruz, C. Modulation of the wheat seed-borne bacterial community by Herbaspirillum seropedicae RAM10 and its potential effects for tryptophan metabolism in the root endosphere. Front. Microbiol. 2021, 12, 792921. [Google Scholar] [CrossRef] [PubMed]
- Youseif, S.H. Genetic diversity of plant growth promoting rhizobacteria and their effects on the growth of maize plants under greenhouse conditions. Ann. Agric. Sci. 2018, 63, 25–35. [Google Scholar] [CrossRef]
- Qaisrani, M.M.; Zaheer, A.; Mirza, M.S.; Naqqash, T.; Qaisrani, T.B.; Hanif, M.K.; Rasool, G.; Malik, K.A.; Ullah, S.; Jamal, M.S.; et al. A comparative study of bacterial diversity based on culturable and culture-independent techniques in the rhizosphere of maize (Zea mays L.). Saudi J. Biol. Sci. 2019, 26, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, C.; Palta, J.A.; Li, Y.; Fan, X. An Enterobacter cloacae strain NG-33 that can solubilize phosphate and promote maize growth. Front. Microbiol. 2022, 13, 1047313. [Google Scholar] [CrossRef]
- Aloo, B.N.; Mbega, E.R.; Makumba, B.; Hertel, R.; Daniel, R. Molecular identification and in vitro plant growth-promoting activities of culturable potato (Solanum tuberosum L.) rhizobacteria in Tanzania. Potato Res. 2021, 64, 67–95. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernández, A.G.; Glick, B.R.; Rossi, M.J. Plant growth-promoting activities and genomic analysis of the stress-resistant Bacillus megaterium STB1, a bacterium of agricultural and biotechnological interest. Biotechnol. Rep. 2020, 25, e00406. [Google Scholar] [CrossRef]
- Bhatta, D.; Adhikari, A.; Kang, S.-M.; Kwon, E.-H.; Jan, R.; Kim, K.-M.; Lee, I.-J. Hormones and the antioxidant transduction pathway and gene expression, mediated by Serratia marcescens DB1, lessen the lethality of heavy metals (As, Ni, and Cr) in Oryza sativa L. Ecotoxicol. Environ. Saf. 2023, 263, 115377. [Google Scholar] [CrossRef]
- Thaiwong, T.; Kettler, N.M.; Lim, A.; Dirkse, H.; Kiupel, M. First report of emerging zoonotic pathogen Wohlfahrtiimonas chitiniclastica in the United States. J. Clin. Microbiol. 2014, 52, 2245–2247. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, W.; Guo, J.; Zheng, L.; Lu, G.; Hua, F.; Liu, M.; Ji, X.; Sun, Y.; Zhu, L.; et al. A Wohlfahrtiimonas chitiniclastica with a novel type of blaVEB–1-carrying plasmid isolated from a zebra in China. Front. Microbiol. 2023, 14, 1276314. [Google Scholar] [CrossRef]
- Vassileva, M.; Mocali, S.; Canfora, L.; Malusá, E.; García del Moral, L.F.; Martos, V.; Flor-Peregrin, E.; Vassilev, N. Safety level of microorganism-bearing products applied in soil-plant systems. Front. Plant Sci. 2022, 13, 862875. [Google Scholar] [CrossRef]
- Wani, A.K.; Hashem, N.M.; Akhtar, N.; Singh, R.; Madkour, M.; Prakash, A. Understanding microbial networks of farm animals through genomics, metagenomics and other meta-omic approaches for livestock wellness and sustainability–A Review. Ann. Anim. Sci. 2022, 22, 839–853. [Google Scholar] [CrossRef]
- Li, X.; Zhu, L.; Zhang, S.Y.; Li, J.; Lin, D.; Wang, M. Characterization of microbial contamination in agricultural soil: A public health perspective. Sci. Total Environ. 2024, 912, 169139. [Google Scholar] [CrossRef]
- Babalola, O.O.; Adeleke, B.S.; Ayangbenro, A.S. Whole genome sequencing of sunflower root-associated Bacillus cereus. Evol. Bioinform. 2021, 17, 11769343211038948. [Google Scholar] [CrossRef]
- Naseem, M.; Chaudhry, A.N.; Jilani, G.; Alam, T.; Naz, F.; Ullah, R.; Zahoor, M.; Zaman, S.; Sohail. Exopolysaccharide-producing bacterial cultures of Bacillus cereus and Pseudomonas aeruginosa in soil augment water retention and maize growth. Heliyon 2024, 10, e26104. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Favale, N.; Bona, E.; Novello, G.; Cesaro, P.; Massa, N.; Glick, B.R.; Orozco-Mosqueda, M.d.C.; Berta, G.; Lingua, G. Screening of bacterial endophytes able to promote plant growth and increase salinity tolerance. Appl. Sci. 2020, 10, 5767. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernandez, A.G.; Glick, B.R.; Rossi, M.J. The extreme plant-growth-promoting properties of Pantoea phytobeneficialis MSR2 revealed by functional and genomic analysis. Environ. Microbiol. 2020, 22, 1341–1355. [Google Scholar] [CrossRef]
- Rasul, M.; Yasmin, S.; Zubair, M.; Mahreen, N.; Yousaf, S.; Arif, M.; Sajid, Z.I.; Mirza, M.S. Phosphate solubilizers as antagonists for bacterial leaf blight with improved rice growth in phosphorus deficit soil. Biol. Control 2019, 136, 103997. [Google Scholar] [CrossRef]
- Hejazi Mahabadi, S.; Shorafa, M.; Motesharezadeh, B.; Etesami, H. Mitigating soil compaction and enhancing corn (Zea mays L.) growth through biological and non-biological amendments. BMC Plant Biol. 2025, 25, 74. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Guo, D.-J.; Sharma, A.; Singh, R.N.; Li, D.-P.; Malviya, M.K.; Song, X.-P.; Lakshmanan, P.; Yang, L.-T.; et al. Whole genome analysis of sugarcane root-associated endophyte Pseudomonas aeruginosa B18—A plant growth-promoting bacterium with antagonistic potential against Sporisorium scitamineum. Front. Microbiol. 2021, 12, 104. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhao, J. Sugar metabolism and its regulation in rhizosphere bacteria: Adaptations to maize root exudates. Front. Microbiol. 2023, 14, 1029345. [Google Scholar]
- Singh, R.; Jha, P. Glucose catabolism in rhizobacteria and its implications for plant stress tolerance. Environ. Microbiol. Rep. 2022, 14, 1081–1092. [Google Scholar]
- Kumar, S.; Patel, D.; Singh, R. Lactose utilization patterns in Bacillus species isolated from groundnut rhizospheres. Int. J. Soil Biol. 2020, 37, 87–96. [Google Scholar]
- Sharma, K.; Das, S.; Kumar, P. Maltose metabolism in Pseudomonas spp. associated with maize rhizosphere. Microb. Ecol. 2021, 81, 890–900. [Google Scholar]
- Fernández, M.; Rodríguez, P.; Torres, J. Fructose metabolism in Azospirillum brasilense strains isolated from maize rhizosphere. Microb. Biotechnol. 2022, 15, 112–121. [Google Scholar]
- Rao, M.; Verma, S.; Singh, A. Fructose utilization by rhizobacteria associated with groundnut: Metabolic efficiency and plant growth effects. Soil Biol. Biochem. 2019, 133, 100–109. [Google Scholar]
- Ding, Y.; Zhang, L.; Chen, H. The role of potassium nitrate in enhancing rhizobacterial growth and plant nutrient uptake. Plant Soil Microbiol. 2022, 45, 321–335. [Google Scholar]
- Ahmed, S.; Hashem, A. Nitrate utilization and nitrogen cycling by rhizobacteria associated with maize roots under varying environmental conditions. J. Microb. Ecol. 2023, 58, 115–127. [Google Scholar]
- Khan, M.; Singh, P.; Mehta, R. Nitrate utilization efficiencies of groundnut-associated rhizobacteria and their influence on plant growth. Agric. Microbiol. 2021, 62, 250–262. [Google Scholar]
- Patel, S.; Joshi, M.; Desai, A. Protease production and casein degradation by Wohlfahrtiimonas chitiniclastica isolated from groundnut roots. J. Appl. Microbiol. 2023, 135, 1150–1161. [Google Scholar]
- Gupta, R.; Sharma, V. Proteolytic enzymes from rhizobacteria: Implications for plant growth promotion and biocontrol. Appl. Microbiol. Biotechnol. 2024, 108, 405–420. [Google Scholar]
- Youssef, M.; El-Gebaly, S.; Abdel-Rahman, F. Antifungal potential of endophytic Enterobacter cloacae against phytopathogenic fungi causing leaf spot diseases. J. Plant Pathol. Microbiol. 2023, 14, 120–131. [Google Scholar]
- Kalai-Grami, L.; Mezghani-Khemakhem, M.; Nasr, C. Antifungal activity of Enterobacter cloacae strains against Fusarium species isolated from tomato plants. Afr. J. Microbiol. Res. 2011, 5, 3939–3945. [Google Scholar]
- Hnini, N.; Boulahrouf, A.; Benyoucef, M. Root-associated Enterobacter cloacae exhibits broad-spectrum antifungal activity against Pythium aphanidermatum in cucumber plants. Biocontrol Sci. Technol. 2023, 33, 451–462. [Google Scholar]
- Bjelić, D.; Marinković, J.; Tintor, B.; Mrkovački, N. Antifungal and plant growth promoting activities of indigenous rhizobacteria isolated from maize (Zea mays L.) rhizosphere. Commun. Soil Sci. Plant Anal. 2018, 49, 88–98. [Google Scholar] [CrossRef]
- Zaric, R.Z.; Jankovic, S.; Zaric, M.; Milosavljevic, M.; Stojadinovic, M.; Pejcic, A. Antimicrobial treatment of Morganella morganii invasive infections: Systematic review. Indian J. Med. Microbiol. 2021, 39, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, B.S.; Ayangbenro, A.S.; Babalola, O.O. Effect of endophytic bacterium, Stenotrophomonas maltophilia JVB5 on sunflowers. Plant Prot. Sci. 2022, 58, 185–198. [Google Scholar] [CrossRef]
- Campilan, J.R.H.; Antesa, D.A. Morganella sp.: A dual-action biocontrol agent and growth promoter against Fusarium oxysporum in banana. J. Eng. Environ. Agric. Res. 2025, 4, 5–15. [Google Scholar] [CrossRef]
- Mowafy, A.M.; Fawzy, M.M.; Gebreil, A.; Elsayed, A. Endophytic Bacillus, Enterobacter, and Klebsiella enhance the growth and yield of maize. Acta Agric. Scand. 2021, 71, 237–246. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Katsenios, N.; Chanioti, S.; Giannoglou, M.; Djordjevic, N.; Katsaros, G. Effect of foliar and soil application of plant growth promoting bacteria on growth, physiology, yield and seed quality of maize under Mediterranean conditions. Sci. Rep. 2020, 10, 21060. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Pandhi, N.; Raghav, R. Improved salt tolerance and growth parameters of groundnut (Arachis hypogaea L.) employing Halotolerant Bacillus cereus SVSCD1 isolated from Saurashtra Region, Gujarat. Gujarat. Ecol. Environ. Conserv. 2020, 26, S199–S212. [Google Scholar]
- Petrović, M.; Janakiev, T.; Grbić, M.L.; Unković, N.; Stević, T.; Vukićević, S.; Dimkić, I. Insights into endophytic and rhizospheric bacteria of five sugar beet hybrids in terms of their diversity, plant-growth promoting, and biocontrol properties. Microb. Ecol. 2024, 87, 19. [Google Scholar] [CrossRef]
- Yi, Y.; de Jong, A.; Frenzel, E.; Kuipers, O.P. Comparative transcriptomics of Bacillus mycoides strains in response to potato-root exudates reveals different genetic adaptation of endophytic and soil isolates. Front. Microbiol. 2017, 8, 1487. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Gomes, E.A.; de Sousa, S.M.; de Paula Lana, U.G.; Coelho, A.M.; Marriel, I.E.; de Oliveira-Paiva, C.A. Co-inoculation with tropical strains of Azospirillum and Bacillus is more efficient than single inoculation for improving plant growth and nutrient uptake in maize. Arch. Microbiol. 2022, 204, 143. [Google Scholar] [CrossRef]
- Jabborova, D.; Davranov, K.; Jabbarov, Z.; Bhowmik, S.N.; Ercisli, S.; Danish, S.; Datta, R. Dual inoculation of plant growth-promoting Bacillus endophyticus and Funneliformis mosseae improves plant growth and soil properties in ginger. ACS Omega 2022, 7, 34779–34788. [Google Scholar] [CrossRef]
- Preyanga, R.; Anandham, R.; Krishnamoorthy, R.; Senthilkumar, M.; Gopal, N.O.; Vellaikumar, A.; Meena, S. Groundnut (Arachis hypogaea) nodule Rhizobium and passenger endophytic bacterial cultivable diversity and their impact on plant growth promotion. Rhizosphere 2021, 17, 100309. [Google Scholar] [CrossRef]
- Prasanna, K.B.; Trimurtulu, N.; Vijaya, G.A.; Nagaraju, Y. Impact of culturable endophytic bacteria on soil aggregate formation and peanut (Arachis hypogaea L.) growth and yield under drought conditions. Curr. Microbiol. 2022, 79, 308. [Google Scholar] [CrossRef]
- Zarei, T.; Moradi, A.; Kazemeini, S.A.; Farajee, H.; Yadavi, A. Improving sweet corn (Zea mays L. var saccharata) growth and yield using Pseudomonas fluorescens inoculation under varied watering regimes. Agric. Water Manag. 2019, 226, 105757. [Google Scholar] [CrossRef]


| Entity Features | BSI | BS2 | BS3 | BS4 | BS5 |
|---|---|---|---|---|---|
| Cell Morphology | |||||
| Size | Big | Thin | Big | Big | Big |
| Gram reaction | - | + | - | - | - |
| Surface | Smooth | Smooth | Smooth | Smooth | Smooth |
| Shape | Regular | Irregular | Irregular | Circular | Circular |
| Colour | Creamy | Creamy | Creamy | Creamy | Milky |
| Cellular shape | Rod | Rod | Rod | Rod | Rod |
| Elevation | Long/Raised | Flat | Flat | Flat | Flat |
| Optical density | Opaque | Opaque | Opaque | Opaque | Opaque |
| Margin | Entire | Entire | Entire | Lobate | Entire |
| Consistency | Mucoid | Mucoid | Mucoid | Mucoid | Fibrous |
| Arrangement | Chains | Chains | Single | Chains | Chains |
| Biochemical tests | |||||
| CT | + | + | + | + | + |
| HSP | + | + | + | + | + |
| Indole | - | - | + | - | + |
| SH | + | + | + | + | + |
| Nitrate | + | + | + | + | + |
| Sugar fermentation | |||||
| Glucose | (++) | (++) | (++) | (++) | (++) |
| Fructose | (+) | (++) | (++) | (++) | (++) |
| Sucrose | (++) | (++) | (++) | (+) | (++) |
| Lactose | (+) | (++) | (++) | (+) | (++) |
| Maltose | (+) | (++) | (++) | (++) | (++) |
| Sample source | IGB | AYK | OKM | IGD | KTP |
| Isolation sources | Maize root endosphere | Groundnut rhizosphere soil | Maize root endosphere | Maize rhizosphere soil | Groundnut rhizosphere soil |
| PGP Screening | Isolates | ||||
|---|---|---|---|---|---|
| BSI | BS2 | BS3 | BS4 | BS5 | |
| IAA | + | + | + | + | + |
| EXP | + | + | + | + | + |
| HCN | + | + | + | + | + |
| AP | + | + | + | + | + |
| NF | + | + | + | + | + |
| PS | + | + | + | + | + |
| Drought | + | + | + | + | + |
| Enzymes | |||||
| Amylase | + | + | + | + | + |
| Cellulase | + | + | + | + | + |
| Mannanase | + | + | + | + | + |
| Lipase | + | + | + | + | + |
| PGP Screening | Isolates | |||||
|---|---|---|---|---|---|---|
| Control | BSI | BS2 | BS3 | BS4 | BS5 | |
| Amylase (cm) | 0.00 ± 0.00 a | 2.50 ± 0.00 c | 2.10 ± 0.00 c | 1.30 ± 0.00 b | 2.30 ± 0.00 c | 1.90 ± 0.00 c |
| Cellulase (cm) | 0.00 ± 0.00 a | 1.10 ± 0.00 c | 1.60 ± 0.00 c | 0.90 ± 0.00 b | 1.70 ± 0.00 c | 2.10 ± 0.00 c |
| Mannanase (cm) | 0.00 ± 0.00 a | 0.70 ± 0.00 b | 1.50 ± 0.00 c | 2.30 ± 0.00 c | 0.90 ± 0.00 b | 1.40 ± 0.00 c |
| Lipase (cm) | 0.00 ± 0.00 a | 0.70 ± 0.00 b | 1.80 ± 0.00 b | 1.10 ± 0.00 c | 0.90 ± 0.00 b | 0.50 ± 0.00 b |
| IAA supplemented with TRP (µg/mL) | 2.10 ± 0.01 a | 4.72 ± 0.01 b | 10.92 ± 0.02 d | 9.36 ± 0.02 c | 4.41 ± 0.02 b | 10.51 ± 0.02 d |
| IAA supplemented with TRP and 10% PEG (µg/mL) | 2.06 ± 0.02 a | 3.47 ± 0.02 b | 5.30 ± 0.00 d | 3.11 ± 0.01 b | 2.90 ± 0.00 a | 4.15 ± 0.02 c |
| PS (µg/mL) | 1.16 ± 0.01 a | 5.46 ± 0.01 c | 10.78 ± 0.02 e | 2.86 ± 0.01 b | 5.62 ± 0.01 c | 8.53 ± 0.02 d |
| HCN | 0.20 ± 0.00 a | 0.34 ± 0.02 a | 0.55 ± 0.01 a | 1.01 ± 0.01 a | 0.43 ± 0.02 a | 0.87 ± 0.03 a |
| NF | 1.93 ± 0.02 a | 2.47 ± 0.02 a | 2.22 ± 0.01 a | 2.79 ± 0.03 a | 2.50 ± 0.00 a | 2.36 ± 0.02 a |
| Isolates | AN | QC | E-Value | % SM | QL | BICS | Source |
|---|---|---|---|---|---|---|---|
| BS1 | MT263025.1 | 100% | 0.0 | 98.27 | 1500 | Enterobacter cloacae | MR-IGB |
| BS2 | KT074456.1 | 98% | 0.0 | 98.84 | 749 | Bacillus cereus | GS-AYK |
| BS3 | KR094121.1 | 100% | 0.0 | 93.10 | 1508 | Morganella morganii | MR-OKM |
| BS4 | MN636438.1 | 100% | 0.0 | 98.26 | 979 | Serratia marcescens | MS-IGD |
| BS5 | KU749295.1 | 100% | 0.0 | 98.73 | 132 | Wohlfahrtiimonas chitiniclastica | GR-KTP |
| Sugars | Concentration (g) | BS1 | BS2 | BS3 | BS4 | BS5 |
|---|---|---|---|---|---|---|
| Glucose | Control | 33.00 ± 0.00 a | 33.00 ± 0.00 a | 33.00 ± 0.00 a | 33.00 ± 0.00 a | 33.00 ± 0.00 a |
| 2.0 | 93.94 ± 0.01 d | 81.82 ± 0.02 c | 96.97 ± 0.02 d | 84.84 ± 0.02 c | 72.73 ± 0.01 d | |
| 4.0 | 30.30 ± 0.03 b | 45.45 ± 0.03 b | 57.58 ± 0.03 b | 63.64 ± 0.03 b | 51.52 ± 0.01 b | |
| 6.0 | 72.73 ± 0.01 c | 81.83 ± 0.01 c | 66.67 ± 0.02 c | 84.85 ± 0.01 c | 57.58 ± 0.02 c | |
| Lactose | Control | 24.00 ± 0.00 a | 24.00 ± 0.00 a | 24.00 ± 0.00 a | 24.00 ± 0.00 a | 24.00 ± 0.00 a |
| 2.0 | 37.50 ± 0.02 b | 83.33 ± 0.03 c | 79.17 ± 0.03 c | 91.67 ± 0.03 d | 41.67 ± 0.02 b | |
| 4.0 | 37.50 ± 0.00 b | 50.00 ± 0.00 b | 54.17 ± 0.01 b | 62.50 ± 0.02 b | 70.83 ± 0.02 c | |
| 6.0 | 100.00 ± 0.02 c | 91.67 ± 0.01 d | 83.33 ± 0.01 d | 79.17 ± 0.02 c | 91.67 ± 0.01 d | |
| Maltose | Control | 22.00 ± 0.00 a | 22.00 ± 0.00 a | 22.00 ± 0.00 a | 22.00 ± 0.00 a | 22.00 ± 0.00 a |
| 2.0 | 100.00 ± 0.00 d | 72.73 ± 0.02 d | 95.45 ± 0.03 c | 81.82 ± 0.02 c | 90.91 ± 0.02 d | |
| 4.0 | 95.451 ± 0.02 c | 68.18 ± 0.01 c | 86.36 ± 0.01 b | 90.91 ± 0.03 d | 72.73 ± 0.01 c | |
| 6.0 | 63.64 ± 0.01 b | 59.09 ± 0.03 b | 95.45 ± 0.03 c | 77.27 ± 0.01 b | 59.09 ± 0.02 b | |
| Fructose | Control | 14.00 ± 0.00 a | 14.00 ± 0.00 a | 14.00 ± 0.00 a | 14.00 ± 0.00 a | 14.00 ± 0.00 a |
| 2.0 | 78.57 ± 0.01 c | 100.00 ± 0.00 c | 85.71 ± 0.01 d | 42.86 ± 0.02 b | 85.71 ± 0.02 b | |
| 4.0 | 78.57 ± 0.03 c | 42.86 ± 0.02 b | 71.43 ± 0.02 c | 42.86 ± 0.01 b | 85.71 ± 0.02 b | |
| 6.0 | 71.42 ± 0.02 b | 100.00 ± 0.00 c | 42.86 ± 0.02 b | 78.57 ± 0.02 c | 85.71 ± 0.03 b |
| Test | Concentration (g) | BS1 | BS2 | BS3 | BS4 | BS5 |
|---|---|---|---|---|---|---|
| Control | Control | 11.00 ± 0.00 a | 11.00 ± 0.00 a | 11.00 ± 0.00 a | 11.00 ± 0.00 a | 11.00 ± 0.00 a |
| PN | 2.0 | 42.85 ± 0.01 b | 66.67 ± 0.03 b | 90.48 ± 0.00 d | 80.95 ± 0.01 c | 80.95 ± 0.01 d |
| 4.0 | 76.19 ± 0.02 c | 66.67 ± 0.01 b | 42.86 ± 0.01 b | 100.00 ± 0.00 d | 61.90 ± 0.02 c | |
| 6.0 | 95.23 ± 0.02 d | 90.48 ± 0.00 c | 76.19 ± 0.02 c | 57.14 ± 0.01 b | 52.38 ± 0.00 b | |
| Casein | Control | 20.00 ± 0.00 a | 20.00 ± 0.00 a | 20.00 ± 0.00 a | 20.00 ± 0.00 a | 20.00 ± 0.00 a |
| 2.0 | 95.00 ± 0.00 d | 100.00 ± 0.00 d | 80.00 ± 0.01 b | 80.00 ± 0.01 c | 50.00 ± 0.00 b | |
| 4.0 | 85.00 ± 0.01 b | 75.00 ± 0.01 c | 85.00 ± 0.00 c | 70.00 ± 0.00 b | 100.00 ± 0.00 c | |
| 6.0 | 90.00 ± 0.02 c | 70.00 ± 0.00 b | 60.00 ± 0.00 d | 70.00 ± 0.01 b | 50.00 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeleke, B.S.; Fakoya, S. Isolation and Molecular Characterization of Potential Plant Growth-Promoting Bacteria from Groundnut and Maize. Int. J. Plant Biol. 2025, 16, 102. https://doi.org/10.3390/ijpb16030102
Adeleke BS, Fakoya S. Isolation and Molecular Characterization of Potential Plant Growth-Promoting Bacteria from Groundnut and Maize. International Journal of Plant Biology. 2025; 16(3):102. https://doi.org/10.3390/ijpb16030102
Chicago/Turabian StyleAdeleke, Bartholomew Saanu, and Soji Fakoya. 2025. "Isolation and Molecular Characterization of Potential Plant Growth-Promoting Bacteria from Groundnut and Maize" International Journal of Plant Biology 16, no. 3: 102. https://doi.org/10.3390/ijpb16030102
APA StyleAdeleke, B. S., & Fakoya, S. (2025). Isolation and Molecular Characterization of Potential Plant Growth-Promoting Bacteria from Groundnut and Maize. International Journal of Plant Biology, 16(3), 102. https://doi.org/10.3390/ijpb16030102






