Barley Seed Germination and Seedling Growth Responses to Polyethylene Glycol (PEG)-Induced Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Trait Measurements
2.4. Statistical Analysis
3. Results
3.1. Seed Germination
3.2. Root Length
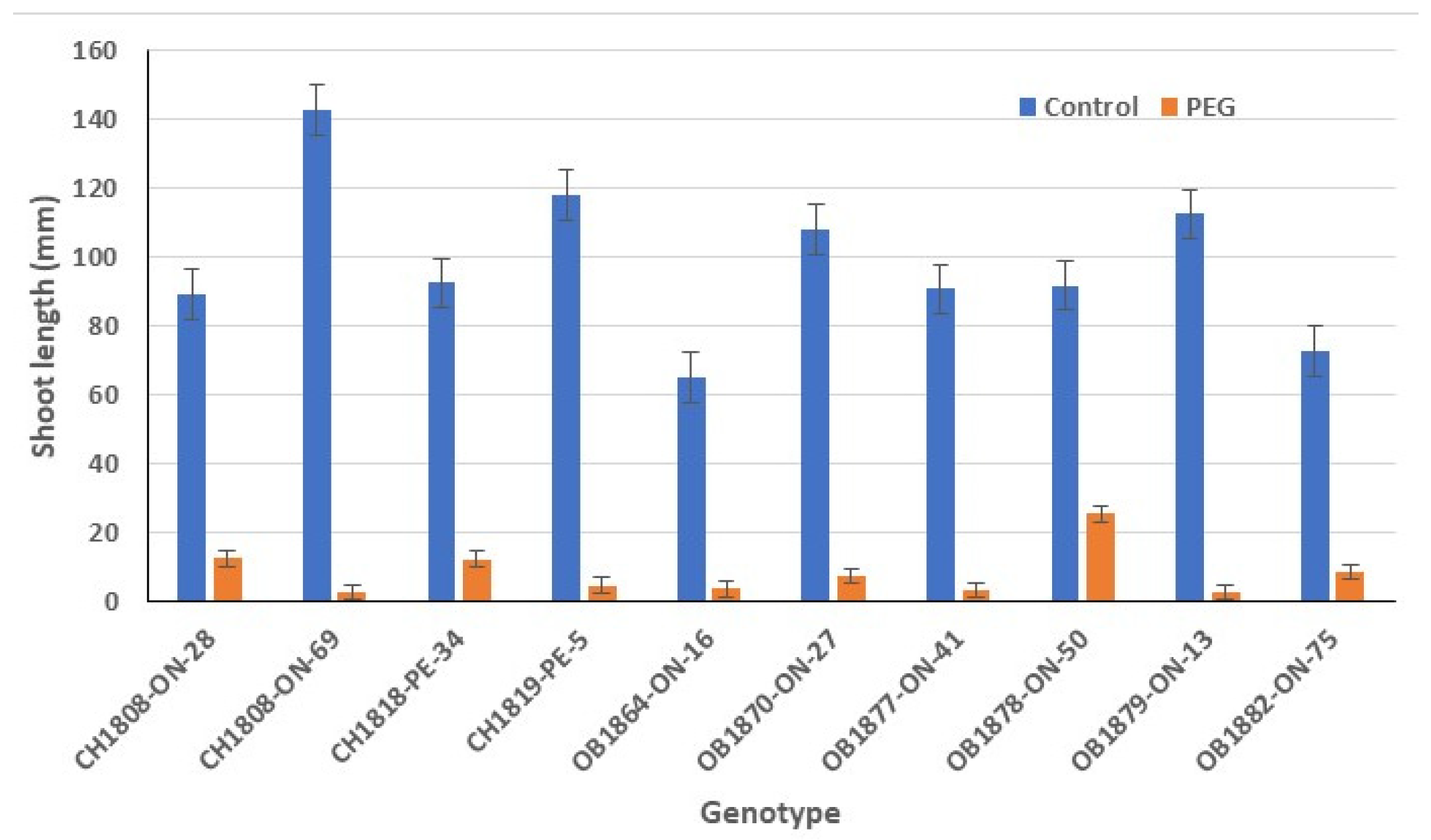
3.3. Shoot Length
3.4. Number of Roots
3.5. Germination Drought Tolerance Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Crops and Livestock Products. Food and Agriculture Organization of the United Nations. Crops and Livestock Products. 2022. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 September 2024).
- Statistics Canada. Table 32-10-0359-01-Estimated Areas, Yield, Production, Average Farm Price and Total Farm Value of Principal Field Crops, in Metric and Imperial Units. 2024. Available online: https://doi.org/10.25318/3210035901-eng (accessed on 16 September 2024).
- Sullivan, P.; Arendt, E.; Gallagher, E. The increasing use of barley and barley by-products in the production of healthier baked goods. Trends Food Sci. Technol. 2013, 29, 124–134. [Google Scholar] [CrossRef]
- Wen, G.; Liu, K.; Kubota, H.; Peng, G.; Semach, G.; Lokuruge, P.; Chau, H.W.; Khakbazan, M. Precipitation and nitrogen management are key drivers of cropping system productivity in the Canadian prairies. Can. J. Plant Sci. 2024, 1–12. [Google Scholar] [CrossRef]
- Rai, M.K.; Kalia, R.K.; Singh, R.; Gangola, M.P.; Dhawan, A. Developing stress tolerant plants through in vitro selection—An overview of the recent progress. Environ. Exp. Bot. 2011, 71, 89–98. [Google Scholar] [CrossRef]
- Basal, O.; Szabó, A.; Veres, S. PEG-induced drought stress effects on soybean germination parameters. J. Plant Nutr. 2020, 43, 1768–1779. [Google Scholar] [CrossRef]
- Hellal, F.; El-Shabrawi, H.; El-Hady, M.A.; Khatab, I.; El-Sayed, S.; Abdelly, C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2017, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lateef, D.; Mustafa, K.; Tahir, N. Screening of Iraqi barley accessions under PEG-induced drought conditions. All Life 2021, 14, 308–332. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Anastasi, U.; Santonoceto, C.; Maggio, A. Effect of PEG-induced drought stress on seed germination of four lentil genotypes. J. Plant Interact. 2014, 9, 354–363. [Google Scholar] [CrossRef]
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of peg-6000 imposed drought stress on rna content, relative water content (rwc), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar]
- Susilawati, P.N.; Tajima, R.; Giamerti, Y.; Yang, Y.; Yufdy, M.P.; Lubis, I.; Homma, K. Application of consecutive polyethylene glycol treatments for modeling the seminal root growth of rice under water stress. Sci. Rep. 2022, 12, 2096. [Google Scholar] [CrossRef]
- Badr, A.; El-Shazly, H.H.; Tarawneh, R.A.; Börner, A. Screening for Drought Tolerance in Maize (Zea mays L.) Germplasm Using Germination and Seedling Traits under Simulated Drought Conditions. Plants 2020, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Ma, B.-L.; Vanasse, A.; Caldwell, C.D.; Earl, H.J.; Smith, D.L. Machine learning-based canola yield prediction for site-specific nitrogen recommendations. Nutr. Cycl. Agroecosystems 2021, 121, 241–256. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Singh, A.K.; Ansari, M.I. Effect of Drought Stress on Crop Production. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 35–47. [Google Scholar]
- Ferioun, M.; Srhiouar, N.; Bouhraoua, S.; El Ghachtouli, N.; Louahlia, S. Physiological and biochemical changes in Moroccan barley (Hordeum vulgare L.) cultivars submitted to drought stress. Heliyon 2023, 9, e13643. [Google Scholar] [CrossRef] [PubMed]
- Partheeban, C.; Chandrasekhar, C.; Jeyakumar, P.; Ravikesavan, R.; Gnanam, R. Effect of PEG Induced Drought Stress on Seed Germination and Seedling Characters of Maize (Zea mays L.) Genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1095–1104. [Google Scholar] [CrossRef]
- Tarnawa, Á.; Kende, Z.; Sghaier, A.H.; Kovács, G.P.; Gyuricza, C.; Khaeim, H. Effect of Abiotic Stresses from Drought, Temperature, and Density on Germination and Seedling Growth of Barley (Hordeum vulgare L.). Plants 2023, 12, 1792. [Google Scholar] [CrossRef] [PubMed]
- Aghbolaghi, M.A.; Piri, R.; Fazeli-Nasab, B.; Chaleshtori, Z.S.; Dedicova, B.; Sayyed, R.; Mastinu, A. Improvement of Barley Seed Performance and Seedling Growth by Plant Growth-Promoting Bacteria. In New Perspectives on Seed Germination [Working Title]; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Rajesh, K.; Reddy, P.S.; Seiler, C.; Sreenivasulu, N. Drought Stress Tolerance Mechanisms in Barley and its Relevance to Cereals. In Biotechnological Approaches to Barley Improvement; Biotechnology in Agriculture and Forestry; Springer: Vienna, Austria, 2014; pp. 161–179. [Google Scholar] [CrossRef]
- Alsamadany, H.; Abdulbaki, A.S.; Alzahrani, Y. Unravelling drought and salinity stress responses in barley genotypes: Physiological, biochemical, and molecular insights. Front. Plant Sci. 2024, 15, 1417021. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Ma, B.; Shi, Y.; Liu, K.; Chen, W. Selection of oat (Avena sativa L.) drought-tolerant genotypes based on multiple yield-associated traits. J. Sci. Food Agric. 2023, 103, 4380–4391. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhang, Y.; Yu, Q.; Fang, W.; Chen, M.; Li, T.; Liu, Y.; Liu, Z.; Chen, L.; Yu, S.; et al. Coordination of growth and drought responses by GA-ABA signaling in rice. New Phytol. 2023, 240, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, Y.; Wang, X.; Yan, C.; Ma, C.; Liu, J.; Dong, S. Effects of Different Drought Degrees on Physiological Characteristics and Endogenous Hormones of Soybean. Plants 2022, 11, 2282. [Google Scholar] [CrossRef] [PubMed]
- Bukan, M.; Kereša, S.; Pejić, I.; Sudarić, A.; Lovrić, A.; Šarčević, H. Variability of Root and Shoot Traits under PEG-Induced Drought Stress at an Early Vegetative Growth Stage of Soybean. Agronomy 2024, 14, 1188. [Google Scholar] [CrossRef]
- Sayed, M.A.E.-A.A.E.-H. QTL Analysis for Drought Tolerance Related to Root and Shoot Traits in Barley (Hordeum vulgare L.). Ph.D. Thesis, Hohen Landwirtschaftlichen Fakultät der Rheinischen Friedrich-Wilhelms-Universität Bonn, Bonn, Germany, 2011. [Google Scholar]



| Genotype | Germination Percent | Root Length | Shoot Length | Number of Roots | GDTI | ||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Drought | Control | Drought | Control | Drought | Control | Drought | ||
| CH1808-ON-28 | 67 | 31 | 89 | 32 | 89 | 12 | 6 | 5 | 0.47 |
| CH1808-ON-69 | 69 | 16 | 111 | 13 | 143 | 3 | 6 | 3 | 0.23 |
| CH1818-PE-34 | 78 | 22 | 109 | 23 | 92 | 12 | 7 | 2 | 0.29 |
| CH1819-PE-05 | 85 | 33 | 100 | 20 | 118 | 5 | 7 | 4 | 0.39 |
| OB1864-ON-16 | 45 | 22 | 66 | 28 | 65 | 4 | 6 | 3 | 0.49 |
| OB1870-ON-27 | 58 | 38 | 73 | 37 | 108 | 7 | 6 | 5 | 0.65 |
| OB1877-ON-41 | 58 | 21 | 97 | 18 | 91 | 3 | 6 | 4 | 0.36 |
| OB1878-ON-50 | 60 | 49 | 108 | 45 | 92 | 25 | 7 | 6 | 0.81 |
| OB1879-ON-13 | 60 | 27 | 106 | 26 | 112 | 3 | 7 | 5 | 0.45 |
| OB1882-ON-75 | 24 | 16 | 62 | 32 | 73 | 8 | 6 | 2 | 0.65 |
| Range | 24–85 | 16–49 | 62–111 | 13–45 | 65–143 | 3–25 | 6–7 | 2–6 | |
| Mean | 60 | 27 | 92 | 28 | 98 | 8 | 6 | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davidson-Willis, M.; Wen, G.; Samanfar, B.; Khanal, R. Barley Seed Germination and Seedling Growth Responses to Polyethylene Glycol (PEG)-Induced Drought Stress. Int. J. Plant Biol. 2024, 15, 1353-1359. https://doi.org/10.3390/ijpb15040093
Davidson-Willis M, Wen G, Samanfar B, Khanal R. Barley Seed Germination and Seedling Growth Responses to Polyethylene Glycol (PEG)-Induced Drought Stress. International Journal of Plant Biology. 2024; 15(4):1353-1359. https://doi.org/10.3390/ijpb15040093
Chicago/Turabian StyleDavidson-Willis, Matthew, Guoqi Wen, Bahram Samanfar, and Raja Khanal. 2024. "Barley Seed Germination and Seedling Growth Responses to Polyethylene Glycol (PEG)-Induced Drought Stress" International Journal of Plant Biology 15, no. 4: 1353-1359. https://doi.org/10.3390/ijpb15040093
APA StyleDavidson-Willis, M., Wen, G., Samanfar, B., & Khanal, R. (2024). Barley Seed Germination and Seedling Growth Responses to Polyethylene Glycol (PEG)-Induced Drought Stress. International Journal of Plant Biology, 15(4), 1353-1359. https://doi.org/10.3390/ijpb15040093







