Sterol Profile in Leaves of Spring Oats (Avena sativa L.) Under Conditions of the Cryolithozone
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the A. sativa Species
2.2. Experiments on Cultivation of the First Sowing and Late Summer (Second Term) Crops of A. sativa
2.3. Collection and Storage of Samples Prior to Analysis
2.4. Extraction of Lipids and Analysis of Sterols and Triterpenoids
2.5. Activity of Sterol Methyltransferase and Acyl-Lipid Desaturase Enzymes
2.6. Data Analysis
3. Results
3.1. Growth and Development of A. sativa and the Temperature Course During the Period of Experiments
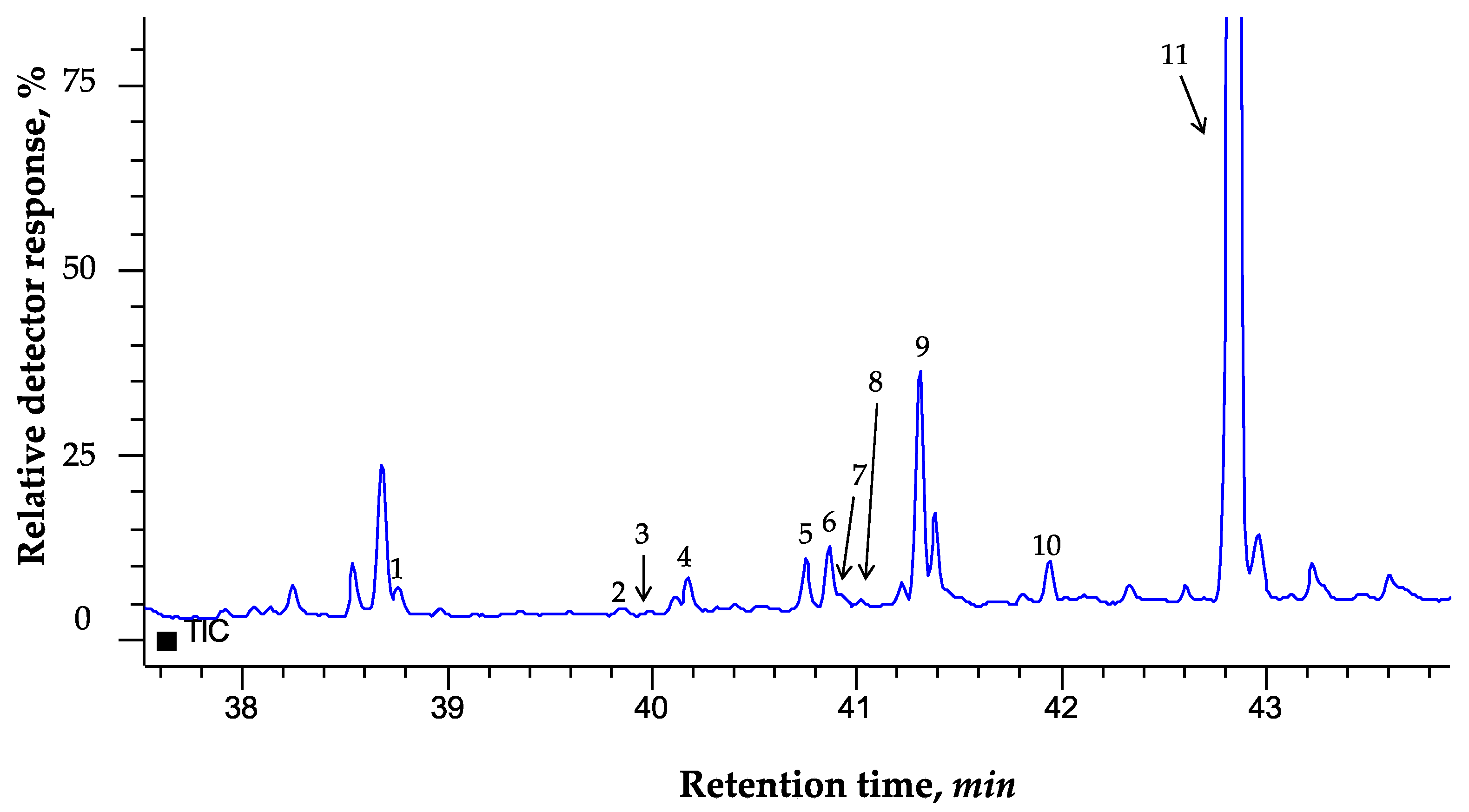
3.2. GC-MS Sterols Profile A. sativa Leaves
3.3. Dynamics of Changes in STs in A. sativa Leaves at Different Sowing Dates
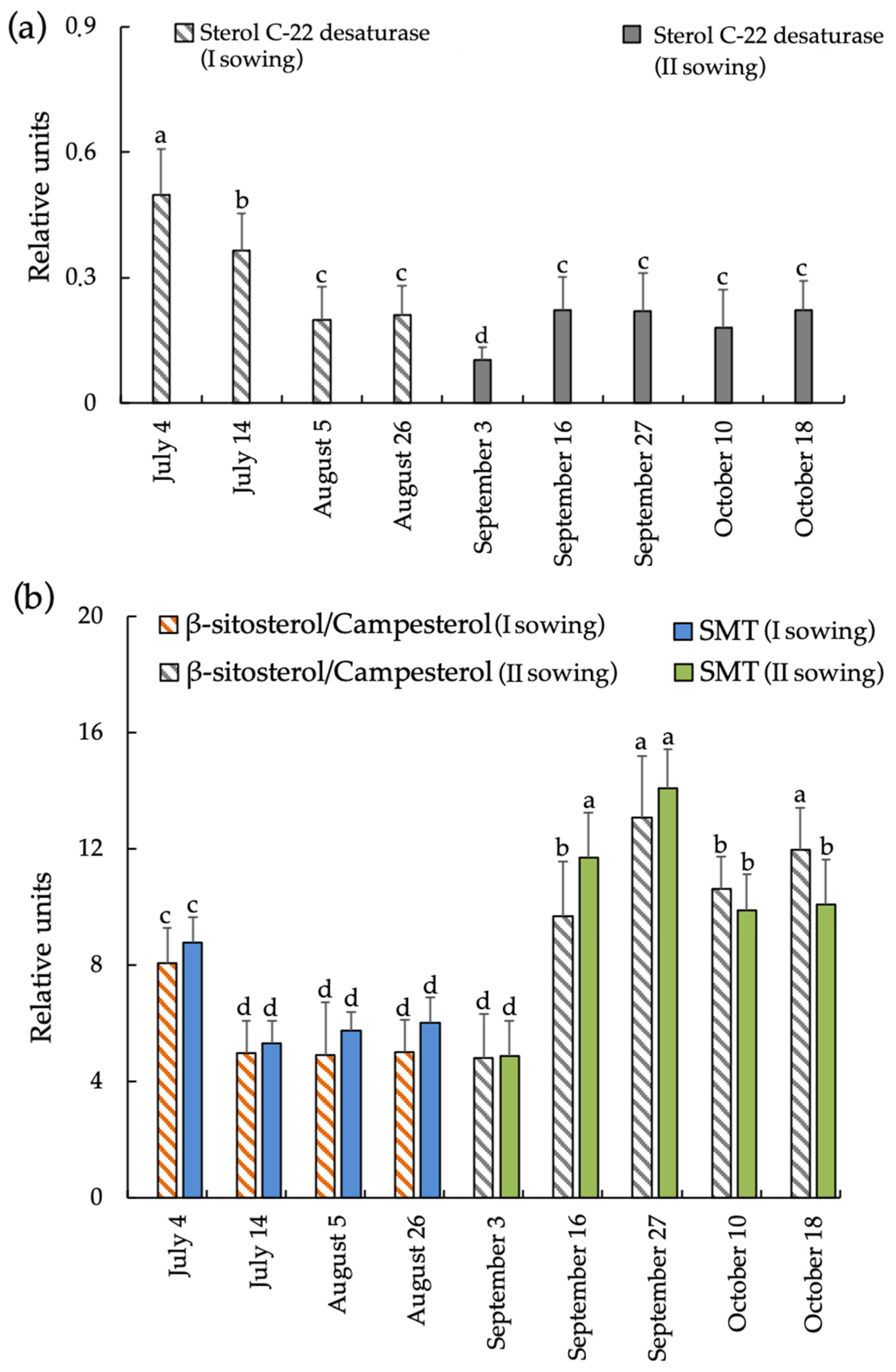
3.4. Dynamics of Changes in STs Ratios and Activity of Some Enzymes in A. sativa Leaves at Different Sowing Dates
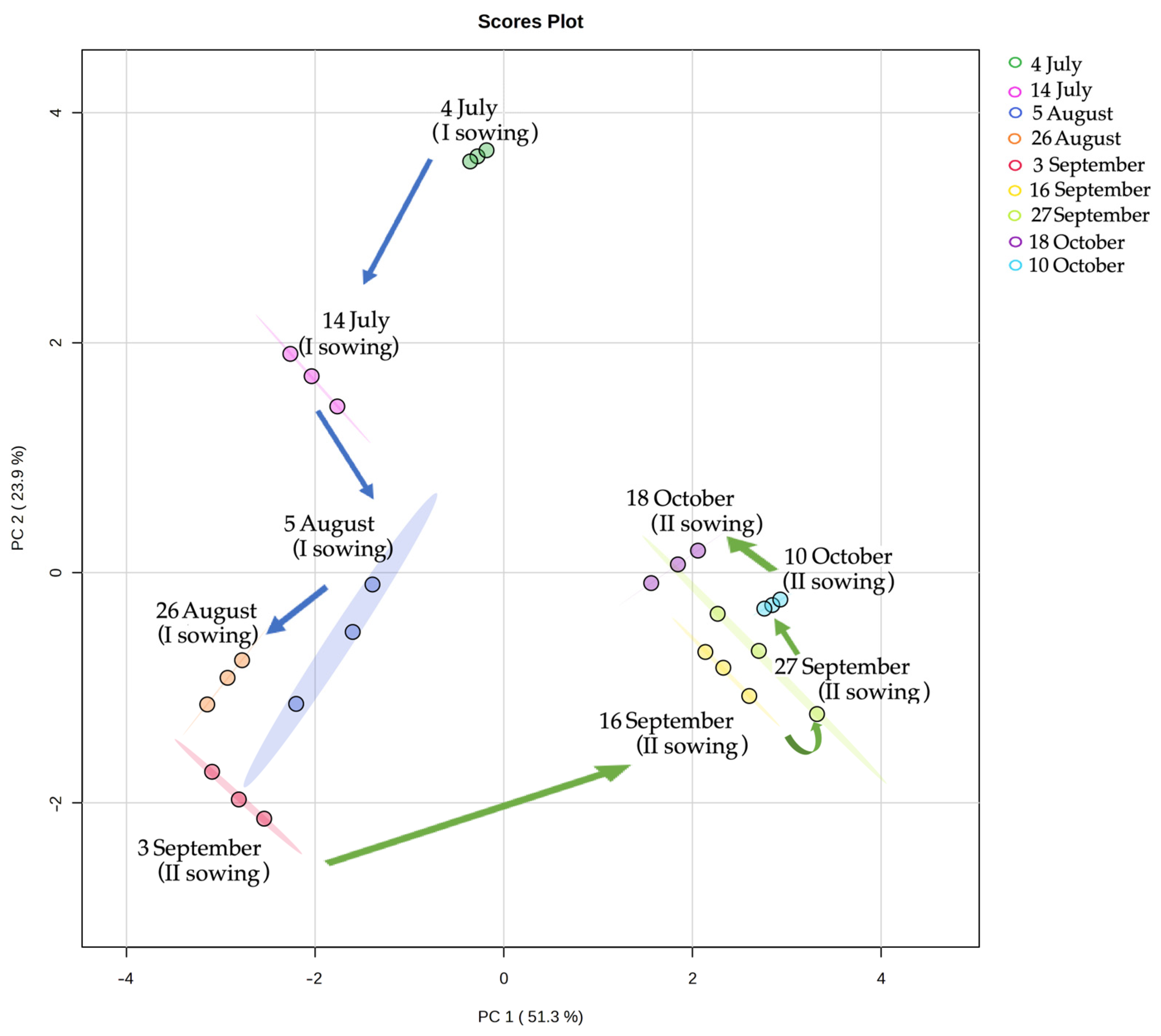
3.5. PCA of Leaves of I and II Sowing Dates of A. sativa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saini, P.; Gani, M.; Saini, P.; Bhat, J.A.; Francies, R.M.; Negi, N.; Chauhan, S.S. Molecular breeding for resistance to economically important diseases of fodder oat. In Disease Resistance in Crop Plants; Wani, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 199–239. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Li-Beisson, Y. Plant unusual fatty acids: Learning from the less common. Curr. Opin. Plant Biol. 2020, 55, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochem. Mosc. 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Schaller, H. The role of sterols in plant growth and development. Prog. Lipid Res. 2003, 42, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Behmer, S.T.; Grebenok, R.J.; Douglas, A.E. Plant sterols and host plant suitability for a phloem-feeding insect. Funct. Ecol. 2011, 25, 484–491. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Kotlova, E.R.; Bogdanova, E.S.; Nesterov, V.N.; Senik, S.V.; Shavarda, A.L. Balance of ∆5-and ∆7-sterols and stanols in halophytes in connection with salinity tolerance. Phytochemistry 2022, 198, 113156. [Google Scholar] [CrossRef]
- Hartmann, M.A. Plant sterols and the membrane environment. Trends Plant Sci. 1998, 3, 170–175. [Google Scholar] [CrossRef]
- Senthill-Kumar, M.; Wang, K.; Mysore, K.S. AtCYP710A1 gene emediated stigmasterol production plays a role in imparting temperature stress tolerance in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e23142-1. [Google Scholar] [CrossRef]
- Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An Enigmatic Plant Stress Sterol with Versatile Functions. Int. J. Mol. Sci. 2024, 25, 8122. [Google Scholar] [CrossRef]
- Bouali, I.; Trabelsi, H.; Herchi, W.; Martine, L.; Albouchi, A.; Bouzaien, G.; Sifi, S.; Boukhchina, S.; Berdeaux, O. Analysis of pecan nut (Carya illinoinensis) unsaponifiable fraction. Effect of ripening stage on phytosterols and phytostanols composition. Food Chem. 2014, 164, 309–316. [Google Scholar] [CrossRef]
- Villette, C.; Berna, A.; Compagnon, V.; Schaller, H. Plant Sterol Diversity in Pollen from Angiosperms. Lipids 2015, 50, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Mironov, K.S.; Allakhverdiev, S.I. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth. Res. 2013, 116, 489–509. [Google Scholar] [CrossRef]
- Cano-Ramirez, D.L.; Carmona-Salazar, L.; Morales-Cedillo, F.; Ramírez-Salcedo, J.; Cahoon, E.B.; Gavilanes-Ruíz, M. Plasma Membrane Fluidity: An Environment Thermal Detector in Plants. Cells 2021, 10, 2778. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Su, P.; Zhang, W. Advances in Microalgae-Derived Phytosterols for Functional Food and Pharmaceutical Applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of Stigmasterol and β-Sitosterol Alters Lipid Metabolism and Alleviates NAFLD in Mice Fed a High-Fat Western-Style Diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Petrov, K.A.; Perk, A.A.; Chepalov, V.A.; Sofronova, V.E.; Ilyin, A.N.; Ivanov, R.V. Eco-physiological and biochemical bases of the green cryo-feed forming in Yakutia. Agric. Biol. 2017, 52, 1129–1138. [Google Scholar] [CrossRef]
- Safronov, V.M.; Smetanin, R.N.; Petrov, K.A. Abundance, ecology and dispersal of wood bison (Bison bison athabascae Rhoads) in Yakutia. Arct. Subarct. Nat. Resour. 2024, 3, 470–478. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Dudareva, L.V.; Semenova, N.V.; Petrov, K.A. Study of the Effect of Mowing and Drying on the Lipid Composition of Grass Leaves in Permafrost Ecosystems. Agronomy 2023, 13, 2252. [Google Scholar] [CrossRef]
- Christie, W.W. The AOCS Lipid Library: Mass Spectra of Some Miscellaneous Lipids—Archive. Available online: https://www.lipidmaps.org/resources/lipidweb/index.php?page=ms/others/others-arch/index1.htm (accessed on 17 September 2024).
- Nes, W.D. Sterol methyltransferase: Enzymology and inhibition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1529, 63–88. [Google Scholar] [CrossRef]
- Petrova, L.V. Hozyajstvenno-biologicheskie priznaki novogo sorta ovsa yarovogo «Vilenskij» [Economic and biological characteristics of the new spring oat variety “Vilensky”]. Vestn. Sev.-Vostochnogo Fed. Univ. Im. M.K. Ammosova [Bull. North-East. Fed. Univ. Named After M. K. Ammosova] 2016, 4, 29–36. [Google Scholar]
- Petrova, L.V.; Osipova, G.M. Vliyanie vlagoobespechennosti na urozhajnost’ zerna raznyh sortov ovsa posevnogo (Avena sativa L.) v usloviyah Cen-tral’noj YAkutii [The influence of moisture availability on grain yield of different varieties of oats (Avena sativa L.) in the conditions of Central Yakutia]. Int. Agric. J. 2019, 1, 43–45. [Google Scholar] [CrossRef]
- Goyal, M.; Kaur, N. Low temperature induced oxidative stress tolerance in oats (Avena sativa L.) genotypes. Ind. J. Plant Physiol. 2018, 23, 316–324. [Google Scholar] [CrossRef]
- Rizza, F.; Pagani, D.; Stanca, A.M.; Cattivelli, L. Use of chlorophyll fluorescence to evaluate the cold acclimation and freezing tolerance of winter and spring oats. Plant Breed. 2008, 120, 389. [Google Scholar] [CrossRef]
- Uemura, M.; Steponkus, P.L. A contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiol. 1994, 104, 479–496. [Google Scholar] [CrossRef]
- Nakamoto, M.; Schmit, A.-C.; Heintz, D.; Schaller, H.; Ohta, D. Diversification of sterol methyltransferase enzymes in plants and a role for β-sitosterol in oriented cell plate formation and polarized growth. Plant J. Cell Mol. Biol. 2015, 84, 860–874. [Google Scholar] [CrossRef]
- Ozolina, N.V.; Kapustina, I.S.; Gurina, V.V.; Nurminsky, V.N. Role of tonoplast microdomains in plant cell protection against osmotic stress. Planta 2022, 255, 65. [Google Scholar] [CrossRef]
- Moreau, P.; Bessoule, J.J.; Mongrand, S.; Testet, E.; Vincent, P.; Cassagne, C. Lipid trafficking in plant cells. Prog. Lipid Res. 1998, 37, 371–391. [Google Scholar] [CrossRef]
- Sofronova, V.E.; Chepalov, V.A.; Dymova, O.V.; Golovko, T.K. Functional Condition of Photosystem II in Leaves of Spring Oats during Autumnal Decrease in Temperature. Russ. J. Plant Physiol. 2020, 67, 661–670. [Google Scholar] [CrossRef]
- Nokhsorov, V.V.; Protopopov, F.F.; Sleptsov, I.V.; Petrova, L.V.; Petrov, K.A. Metabolomic Profile and Functional State of Oat Plants (Avena sativa L.) Sown under Low-Temperature Conditions in the Cryolithozone. Plants 2024, 13, 1076. [Google Scholar] [CrossRef]
- Eichenberger, W.; Urban, B. Sterols in seeds and leaves of oats (Avena sativa L.). Plant Cell Rep. 1984, 3, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Sulkarnayeva, A.G.; Valitova, J.N.; Mukhitova, F.K.; Minibayeva, F.V. Stress-induced changes in membrane sterols in wheat roots. Dokl. Biochem. Biophys. 2014, 455, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-García, L.; Arró, M.; Altabella, T.; Ferrer, A.; Boronat, A. Structural and functional analysis of tomato sterol C22 desaturase. BMC Plant Biol. 2021, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fu, X.; Chu, Y.; Wu, P.; Liu, Y.; Ma, L.; Tian, H.; Zhu, B. Biosynthesis and the Roles of Plant Sterols in Development and Stress Responses. Int. J. Mol. Sci. 2022, 23, 2332. [Google Scholar] [CrossRef]
- Zauber, H.; Burgos, A.; Garapati, P.; Schulze, W.X. Plasma membrane lipid-protein interactions affect signaling processes in sterol-biosynthesis mutants in Arabidopsis thaliana. Front. Plant Sci. 2014, 5, 78. [Google Scholar] [CrossRef]
- Benesch, M.G.; McElhaney, R.N. A comparative calorimetric study of the effects of cholesterol and the plant sterols campesterol and brassicasterol on the thermotropic phase behavior of dipalmitoylphosphatidylcholine bilayer membranes. Biochim. Biophys. Acta 2014, 1838, 1941–1949. [Google Scholar] [CrossRef]
- Grosjean, K.; Mongrand, S.; Beney, L.; Simon-Plas, F.; Gerbeau-Pissot, P. Differential effect of plant lipids on membrane organization. J. Biol. Chem. 2015, 290, 5810–5825. [Google Scholar] [CrossRef]
- Renkova, A.G.; Valitova, J.; Schaller, H.; Minibayeva, F.V. The homoeologous genes encoding C24-sterol methyltransferase 1 in Triticum aestivum: Structural characteristics and effects of cold stress. Biol. Plant. 2019, 63, 59–69. [Google Scholar] [CrossRef]
- Griebel, T.; Zeier, J. A role for betasitosterol to stigmasterol conversion in plant–pathogen interactions. Plant J. Cell Mol. Biol. 2010, 63, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Senthil-Kumar, M.; Ryu, C.M.; Kang, L.; Mysore, K.S. Phytosterols play a key role in plant innate immunity against bacterial pathogens by regulating nutrient efflux into the apoplast. Plant Physiol. 2012, 158, 1789–1802. [Google Scholar] [CrossRef]
- Cabianca, A.; Müller, L.; Pawlowski, K.; Dahlin, P. Changes in the Plant β-Sitosterol/Stigmasterol Ratio Caused by the Plant Parasitic Nematode Meloidogyne incognita. Plants 2021, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Zhou, J.; Yuan, Y.J. Analysis of phospholipids, sterols, and fatty acids in Taxus chinensis var. mairei cells in response to shear stress. Biotechnol. Appl. Biochem. 2009, 54, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Dufourc, E.J. The role of phytosterols in plant adaptation to temperature. Plant Signal. Behav. 2008, 3, 133–134. [Google Scholar] [CrossRef]
- Yokota, T. The structure, biosynthesis and function of brassinosteroids. Trends Plant Sci. 1997, 2, 137–143. [Google Scholar] [CrossRef]
- Schaeffer, A.; Bronner, R.; Benveniste, P.; Schaller, H. The ratio of campesterol to sitosterol that modulates growth in Arabidopsis is controlled by Sterolmethyltransferase 2;1. Plant J. 2001, 25, 605–615. [Google Scholar] [CrossRef]
- Aboobucker, S.I.; Suza, W.P. Why Do Plants Convert Sitosterol to Stigmasterol? Front Plant Sci. 2019, 10, 354. [Google Scholar] [CrossRef]
- Takahashi, D.; Imai, H.; Kawamura, Y.; Uemura, M. Lipid profiles of detergent resistant fractions of the plasma membrane in oat and rye in association with cold acclimation and freezing tolerance. Cryobiology 2016, 72, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.C.; Qian, T.F.; Chu, J.P.; Zhang, X.; Liu, Y.J.; Dai, X.L.; He, M.R. Late sowing enhances lodging resistance of wheat plant via improving biosynthesis and accumulation of lignin and cellulose. J. Integr. Agric. 2022, 73, 19–24. [Google Scholar] [CrossRef]
- Aydın, N.; Mut, Z.; Mut, H.; Ayan, İ. Effect of autumn and spring sowing dates on hay yield and quality of oat (Avena sativa L.) genotypes. J. Anim. Veter-Adv. 2010, 9, 1539–1545. [Google Scholar] [CrossRef]
- Jiao, F.; Tan, Z.; Yu, Z.; Zhou, B.; Meng, L.; Shi, X. The phytochemical and pharmacological profile of taraxasterol. Front. Pharmacol. 2022, 13, 927365. [Google Scholar] [CrossRef]
- Bao, T.; Ke, Y.; Wang, Y.; Wang, W.; Li, Y.; Wang, Y.; Kui, X.; Zhou, Q.; Zhou, H.; Zhang, C.; et al. Taraxasterol suppresses the growth of human liver cancer by upregulating Hint1 expression. J. Mol. Med. 2018, 96, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Li, Y.; Song, R.; Qin, C.; Hao, W.; Wang, B.; Yang, L.; Peng, P.; Xu, F. Anti-inflammatory and anti-apoptosis activity of taraxasterol in ulcerative colitis in vitro and in vivo. Exp. Ther. Med. 2019, 18, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.W.; Chen, J.J.; Liu, S.J. Studies on the chemical constituents of rhizoma of coniogramme Japonica. J. Chin. Med. Mater. 2010, 33, 557–559. [Google Scholar] [PubMed]
- Kershengolts, B.M.; Shashurin, M.M.; Sleptcova, S.S.; Vinokurova, M.K.; Kolosova, O.N. Biological product from natural northern biological raw materials with possible use in the prevention, treatment and remission stages after bacterial and viral infections, including COVID-19. Arct. Subarct. Nat. Resour. 2021, 26, 120–135. [Google Scholar] [CrossRef]
- Sujeeth, N.; Mehterov, N.; Gupta, S.; Qureshi, M.K.; Fischer, A.; Proost, S.; Omid-bakhshfard, M.A.; Obata, T.; Benina, M.; Staykov, N.; et al. A novel seed plants gene regulates oxidative stress tolerance in Arabidopsis thaliana. Cell. Mol. Life Sci. 2020, 77, 705–718. [Google Scholar] [CrossRef]





| Timeframe for Collecting Samples | Phases of Development | Air Temperature (°C) * | Amount of Precipitation (mm) ** | Photoperiod (h) | |
|---|---|---|---|---|---|
| T Average | T Night | ||||
| I sowing date (3 June), summer period | |||||
| 4 July | Start of entering the tube | 25.4 ± 4.3 | 20.1 ± 1.9 | 13.0 | 19.5 |
| 14 July | Emergence into the tube | 14.7 ± 2.9 | 11.5 ± 0.9 | 7.6 | 18.9 |
| 5 August | Hatching | 20.5 ± 6.2 | 14.2 ± 3.1 | 32.0 | 17.1 |
| 26 August | Milk ripening | 11.2 ± 6.4 | 5.6 ± 3.2 | 26.6 | 15.0 |
| II sowing date (27 July), autumn period | |||||
| 3 September | Tillering | 6.9 ± 1.4 | 6.1 ± 0.9 | 15.5 | 14.2 |
| 16 September | Start of entering the tube | 6.8 ± 4.1 | 4.6 ± 2.7 | 23.2 | 13.0 |
| 27 September | Emergence into the tube | 1.3 ± 1.7 | 0.4 ± 1.2 | 19.4 | 11.9 |
| 10 October | Emergence into the tube | −0.3 ± 1.1 | −1.1 ± 0.5 | 10.9 | 10.6 |
| 18 October | Tubing | −2.2 ± 2.3 | −2.6 ± 2.0 | 60 *** | 9.8 |
| Sowing Date | Sterols, % of Sum | Triterpenoids, % of Sum | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesterol | Campesterol | Campestanol | Stigmasterol | β-Sitosterol | Sitostanol | Δ5-Avenasterol | Δ7-Avenasterol | Ψ-Taraxasterol | Cyclolaudenol | Betulin | |
| I (4 July) | 8.8 | 4.3 | 1.6 | 17.1 | 34.4 | 29.7 | 1.8 | 2.4 | 7.3 | 1.7 | 91.0 |
| II (3 September) | 4.2 | 13.3 | 1.1 | 6.6 | 63.9 | 9.5 | 1.3 | 0.1 | 7.1 | 1.3 | 91.6 |
| No. | Compounds | RT, min | RRT | RI | Main m/z Ratio |
|---|---|---|---|---|---|
| 1 | Cholesterol | 38.806 | 1.3020 | 3173 | 458, 443, 368, 353, 329, 255, 213, 329, 129 |
| 2 | Campesterol | 39.900 | 1.3387 | 3227 | 472, 382, 367,343, 255, 213, 129 |
| 3 | Campestanol | 40.002 | 1.3421 | 3282 | 474, 384, 369, 305, 257, 215, 75 |
| 4 | Stigmasterol | 40.198 | 1.3487 | 3301 | 484, 469, 394, 379, 355, 351, 255, 213, 129, 83 |
| 5 | β-sitosterol | 40.795 | 1.3687 | 3353 | 486, 396, 381, 357, 303, 255, 213, 129 |
| 6 | Sitostanol | 40.906 | 1.3724 | 3363 | 488, 473, 431, 398, 383, 305, 257, 215, 75 |
| 7 | Δ5-avenasterol | 40.961 | 1.3743 | 3368 | 484, 469, 394, 386, 371, 355, 343, 296, 281, 257, 255, 253, 213, 211, 129 |
| 8 | Δ7-avenasterol | 41.203 | 1.3824 | 3420 | 484, 469, 386, 371, 343, 281, 255, 253, 213 |
| 9 | Ψ-taraxasterol | 41.337 | 1.3869 | 3398 | 498, 483, 369, 297, 207, 189, 109, 72 |
| 10 | Cyclolaudenol | 41.934 | 1.4069 | 3445 | 512, 440, 422, 407, 379, 353, 300, 203, 175, 161, 147, 135, 121, 107, 95, 81, 73, 55 |
| 11 | Betulin | 42.874 | 1.4384 | 3520 | 586, 496, 483, 442, 393, 293, 279, 203, 189, 135, 109, 95, 72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokhsorov, V.V.; Sofronova, V.E.; Sleptsov, I.V.; Senik, S.V.; Petrova, L.V.; Petrov, K.A. Sterol Profile in Leaves of Spring Oats (Avena sativa L.) Under Conditions of the Cryolithozone. Int. J. Plant Biol. 2024, 15, 1304-1320. https://doi.org/10.3390/ijpb15040090
Nokhsorov VV, Sofronova VE, Sleptsov IV, Senik SV, Petrova LV, Petrov KA. Sterol Profile in Leaves of Spring Oats (Avena sativa L.) Under Conditions of the Cryolithozone. International Journal of Plant Biology. 2024; 15(4):1304-1320. https://doi.org/10.3390/ijpb15040090
Chicago/Turabian StyleNokhsorov, Vasiliy V., Valentina E. Sofronova, Igor V. Sleptsov, Svetlana V. Senik, Lidia V. Petrova, and Klim A. Petrov. 2024. "Sterol Profile in Leaves of Spring Oats (Avena sativa L.) Under Conditions of the Cryolithozone" International Journal of Plant Biology 15, no. 4: 1304-1320. https://doi.org/10.3390/ijpb15040090
APA StyleNokhsorov, V. V., Sofronova, V. E., Sleptsov, I. V., Senik, S. V., Petrova, L. V., & Petrov, K. A. (2024). Sterol Profile in Leaves of Spring Oats (Avena sativa L.) Under Conditions of the Cryolithozone. International Journal of Plant Biology, 15(4), 1304-1320. https://doi.org/10.3390/ijpb15040090







