Abstract
A prompt seed germination and emergence coupled with an excellent seedling vigor are highly desired features to ensure perfect crop establishment and subsequent vegetative growth. Seed dressing with pesticides represents the most common technology for enhancing seed performance after sowing, while little is known about biostimulant seed dressing. This practice could play a fundamental role in developing new sustainable starter fertilization for cereals. The enhancement of germination and seedling vigor of durum wheat seeds (Triticum turgidum L. subsp. durum (Desf.) Husn) was the main target of this research. The experiment took place in a germination cabinet under controlled environmental conditions, settled at the constant temperature of 10 °C and under dark conditions for 8 days. The different seed dressings, sprayed on the seeds, were composed by a combination of a fungicide and different biostimulants. Coleoptile and root length, as well as biomass, were significantly increased by the different biostimulants, compared to the control. As for germination traits, seeds treated with Codium fragile and Opuntia ficus-indica extracts, containing phytohormones and different nutrients, showed a final germination (96%) significantly higher than the one obtained with the control treatment (86%). These results show that treating seeds with a suitable dressing solution can greatly improve the germination features and seedling vigor of durum wheat. This can help the crop to withstand future stresses, especially in early stages, and possibly increase the grain yield with a reduction in agrochemicals. However, the combination of the substances used in the present study rarely showed a synergistic effect on the tested variable.
1. Introduction
High physiological seed quality is a feature greatly requested by farmers for every crop. However, the lack of close cooperation between the seed industry and scientific community is slowing down the progress in obtaining high-performance seeds [1]. A rapid and uniform field establishment is the first target for all growers, and the degree to which this target is achieved determines the success and the profitability of crops [2,3,4]. Seeds and seedlings undergo various biotic and abiotic stresses that can hinder vigorous plant establishment and, consequently, future crop production [5,6,7,8]. Seed treatment is a crop-specific and objective-oriented method, developed to protect seeds from major stressors, thus allowing plant genotypes to entirely express their potential [9]. In the past years, dressing seeds with fungicides or insecticides became a fundamental tool to ensure high agronomic performances for all major crops. This technique is considered an environmentally friendly practice, because it can provide long-term protection of the plants by using a lower dose of pesticides per hectare as compared to conventional spraying [10,11]. Moreover, it has an economic benefit and ensures higher workplace safety for farmers [12,13]. Seed dressing is a valid solution not only to face most biotic stresses but also to reduce the negative impact of abiotic stress, such as drought and salinity, through the application of nutrients, microorganisms and phytohormones [14,15,16,17,18,19].
In the Mediterranean area, wheat production is seriously threatened by severe abiotic stresses that usually result in a significant yield reduction even when the stress occurs during the initial growing stages of the crop [20,21,22,23]. Since durum wheat (Triticum turgidum L. subsp. durum (Desf.) Husn) is a fundamental crop in the Mediterranean area, methods and strategies aiming to enhance its resilience to abiotic stresses, without negatively affecting the environmental sustainability, will be the core subject to face food security in that peculiar geographic area [24].
A possible solution to help reach this fundamental goal is the use of biostimulants. These products are garnering increasing attention among researchers and farmers due to recent market trends and their capabilities to enhance crop performance, especially under abiotic stresses [25]. Seed treatment with biostimulants and/or micronutrients appears to be a sustainable and economically valid option to ensure good crop establishment in different conditions. For this reason, scholars have started to investigate the effect of soaking and/or priming seeds with various substances on germination and early development of plants [26]. In the past years, seed treatment with zinc proved to be a good practice to enhance seed germination and vigor for different crops [27]. Instead, in recent years, the use of biostimulants like seaweed or plant extracts has spread as an efficient method for seed dressing. Seaweed extracts appear to be rich in phytohormones and other compounds able to trigger the expression of several genes, connected with defense and nutrient absorption mechanisms [28]. The plant extracts play a similar role in promoting the development of different crops, by providing nutrients, amino acids and other components directly to the plant or by triggering the pathways involved in phytohormones biosynthesis, photosynthesis regulation and nitrogen assimilation [29]. Despite the overall good results obtained by these kinds of compounds on wheat under different stress conditions, especially in terms of final germination percentage, fresh biomass of the seedlings and content of chlorophyll [30,31,32,33,34,35,36], the biostimulant seed treatment is still an uncommon practice for most farmers. That is probably because seed soaking, which is the most used method in germination studies, is not viable at an industrial scale, especially for the mechanical impediment of the soaked seeds to flow inside a seeder [37]. Foreseeing the great potential of this technique, several fertilizer companies have been developing new seed dressing solutions for years. However, the limited literature describing the industrial seed application of non-microbial biostimulants evidenced contrasting results, caused by the plethora of active compounds used and the interactions among them and with the different growing conditions and crop species [38,39,40,41,42]. To better understand the effect and the efficacy of the different seed coating products on durum wheat, especially in terms of germination rate, germination speed and seedling vigor, we carried out several germination trials using different plant biostimulants. The following hypotheses were tested: (i) when compared to untreated seeds, biostimulant seed dressing significantly improves germination rate and speed as well as the seedling vigor of durum wheat; (ii) the effect is improved by combining different substances.
2. Materials and Methods
2.1. Experimental Design
To have uniform experimental conditions, durum seeds (cultivar Odisseo) were visually chosen based on their dimensions. Subsequently, they were treated with eight different solutions containing the following components:
- A liquid fungicide, commercially known as VIBRANCE GOLD by SYNGENTA, at the dose of 200 mL per 100 kg seed, which contains 4.63% Sedaxane, 2.32% Fludioxonil, 2.32% Difenoconazole;
- A liquid NPK organic fertilizer containing 3% N, 4% P2O5, 3% K2O, free amino acids (e.g., phenylalanine, methionine, tyrosine, proline, etc.), humic and fulvic acids (hereinafter referred to as NPK);
- Zinc (hereinafter referred to as Zn), derived from a commercial product containing chelated Zinc with ethylenediamine tetra-acetic acid (EDTA, 9% Zn);
- An experimental product containing a mixture of aqueous extracts from the seaweed Codium fragile (10 g of dried alga per 1 L of distilled water) and the plant species Opuntia ficus-barbarica (50 g of fresh puree of prickly pear per 1 L of distilled water). Hereinafter referred to as SWEO;
- Distilled water.
The exact formulation of each solution is shown in Table 1.

Table 1.
Active ingredients and formulations of the different dressing solutions.
Odisseo was chosen because it is a modern durum wheat cultivar extensively cultivated around the Mediterranean basin [43,44].
The eight treatments were applied at the rate of 1 L solution per 100 kg seed, to simulate the industrial dose. Seed treatment was applied by mixing 1 mL of dressing solution with 100 g of seeds and shaking them in a weighing bottle. This dosage was based on reference values found in the literature [45,46,47]. Four replicates (each consisting of 100 seeds) were used for each treatment. The treated seeds were placed into a Petri dish (120 mm diameter), with three layers of Whatman Grade 1 filter paper, soaked in distilled water, and left for 8 days in a seed germination cabinet, in dark conditions, at a constant temperature of 10 ± 1 °C [48], to simulate a possible field temperature during sowing season. The Petri dishes were randomly allocated within the cabinet.
2.2. Measured Traits
Germination was determined by radicle emergence. The germinated seeds of each replicate were counted every day to calculate
- Final germination percentage: (germinated seeds/total seeds) × 100, after 8 days;
- T50: time to achieve 50% of final germination calculated according to the following formula [49,50]:
- Germination curves.
After 8 days, all germinated seeds were analyzed, and the following traits were measured for each replicate:
- Main root’s length (cm), measured by a ruler;
- Length of the coleoptile (cm), measured by a ruler;
- Mean dry biomass of the roots (weight, mg). For each replicate, the roots were separated from the seeds, dried for 48 h at 60 °C and then the total biomass was divided by the number of germinated seeds;
- Mean dry biomass of the coleoptile (weight, mg). For each replicate, the coleoptiles were separated from the seeds, dried for 48 h at 60 °C and then the total biomass was divided by the number of germinated seeds.
2.3. Statistical Analysis:
In order to allow a better explanation of germination experiments, the data were analyzed as time-to-event data [51]. To estimate fundamental traits (e.g., T50 and final germination) and their corresponding standard errors, an event-time approach has been used by fitting a three-parameter log-logistic model to data from each germination curve separately [52]. The model equation used in this study was
where d denotes the proportion of germinated seeds (upper limit or final maximum germination), and T50 is the time (number of days) to reach 50% of final germination (median germination time). The parameter b is proportional to the slope of F at time T equal to the parameter T50.
Quantitative data indicating the seedling vigor, obtained by the analysis of germinated seeds, were evaluated with the analysis of variance (ANOVA) to test the efficacy of the different seed dressing solutions. Two means were considered different at the 95% probability level by Fisher’s protected least significant difference.
Statistical analysis was performed with the open-source environment R [53] using the add-on packages ‘drcte 1.0.30’, for event-time models, which allowed us to analyze the germination data, with a time to event curve fitting procedure, considering only three fundamental indicators for seed germination. This approach was enough to ensure a clear description of the whole-time course of events, for a simple experiment scheme [54].
3. Results
3.1. Final Germination Percentage and Median Germination Time (T50)
As reported in Table 2 and Figure 1, all treatments strongly influenced the germination of durum wheat seeds. The application of biostimulants enhanced both the final germination percentage and the median germination time (T50). SWEO was the best treatment, significantly raising the germination percentage by 11% compared to the control. The other formulations showed a germination rate that ranged from 90.5% (NPK) to 92.5% (SWEO + Zn), all significantly higher than control (86%). All treated seeds showed a significantly higher germination speed compared to control. The dressing solutions containing SWEO alone displayed the fastest germination (T50 = 1.71 days), followed by NPK and Zn (both with T50 = 1.84 days). The seed dressing solutions containing a mixture of different ingredients showed a germination speed significantly higher than that obtained with the single ones (except for SWEO + Zn) but still lower than that of the control treatment, which had a median germination time of 2.26 days.

Table 2.
Germination percentage at 8 days and median germination time (T50) for each seed treatment ± standard errors.
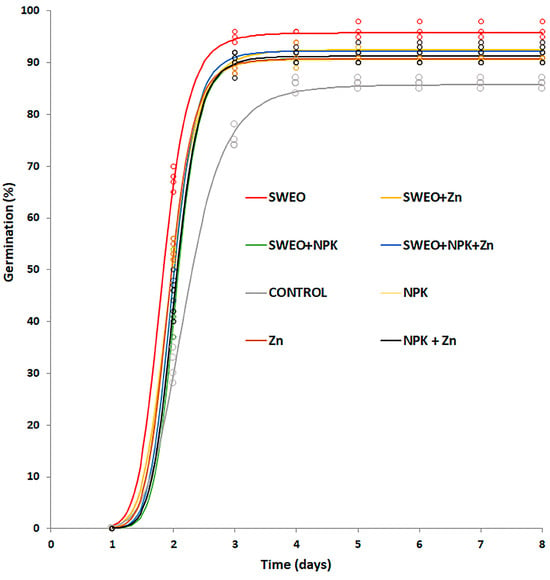
Figure 1.
Germination curves of seeds treated with different biostimulants. Curves were generated by fitting the three-parameter log-logistic model (2).
3.2. Length of the Main Root
As shown in Figure 2, the mean length of the main roots was significantly enhanced by the biostimulant seed dressing as compared to the control treatment (p < 0.001). The best result was obtained by the application of SWEO (11.4 cm), which lead to a 32% increase in root length as compared with the control (8.6 cm). All the treatments containing SWEO showed a length of the main root significantly higher than that of the other treatments (except for SWEO + NPK + Zn, with 10.3 cm). The application of zinc, NPK and their mixture had a similar effect on the root length of durum wheat seedlings (9.86 cm, 9.56 cm and 9.94 cm, respectively).

Figure 2.
Main root’s length affected by the different seed dressing solutions. The vertical bars show the standard error of the mean. Letters above the histograms are the ranking of Fisher’s protected test at p < 0.05.
3.3. Root Biomass
The mean root biomass of the single seedling was significantly influenced by the different seed treatments (p < 0.001). SWEO and SWEO + Zn were the treatments showing the highest value (6.2 mg), not significantly different to the treatment containing all the biostimulants (6.1 mg) and NPK alone (5.9 mg). As shown in Figure 3, all the other formulations produced a root biomass significantly greater, with an average increase of 8%, in comparison with the control treatment (5.3 mg).

Figure 3.
Mean dry biomass of the roots as influenced by the different seed dressing solutions. The vertical bars show the standard error of the mean. Letters above the histograms are the ranking of Fisher’s protected test at p < 0.05.
3.4. Coleoptile Length
As with the root system, the coleoptile development was significantly influenced by the different seed dressing solutions (p < 0.001). The application of biostimulants always produced a coleoptile significantly longer than that of the control treatment (Figure 4). As compared to the control, SWEO doubled the coleoptile length (6.3 cm vs. 3.2 cm), while the other treatments produced an increase ranging from 26% (NPK, 4 cm) to 81% (SWEO + NPK, 5.8 cm). Formulations containing Zn or NPK alone showed a result significantly lower than that obtained by applying their mixture (4.0 cm vs. 5.3 cm).

Figure 4.
Coleoptile length as influenced by the different seed dressing solutions. The vertical bars show the standard error of the mean. Letters above the histograms are the ranking of the Fisher’s protected test at p < 0.05.
3.5. Dry Biomass of the Coleoptile
Different seed dressings significantly influenced the biomass of the coleoptile of durum wheat seedlings (p < 0.001). Again, the SWEO treatment produced the highest biomass (4.2 mg), but the effect was less evident than that obtained for coleoptile length (Figure 5). The application of mixtures did not produce an effect significantly different from that of the single components. The Zn treatment resulted in a coleoptile biomass significantly lower than the SWEO treatment did, with a decrease of about 10%. The Zn seed dressing, however, still produced a 23% enhancement as compared to the control (3.0 mg).
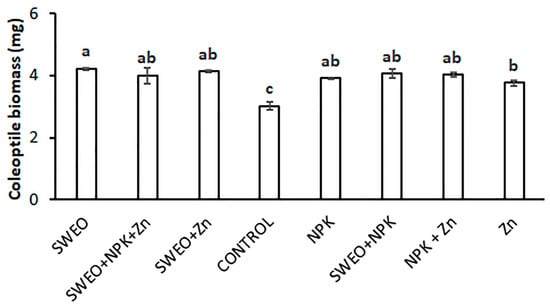
Figure 5.
Mean dry biomass of the coleoptile as influenced by the different seed dressing solutions. The vertical bars show the standard error of the mean. Letters above the histograms are the ranking of Fisher’s protected test at p < 0.05.
4. Discussion
Since the different treatments markedly improved seed germination traits and the seedling vigor of the durum wheat cultivar Odisseo, our first hypothesis was verified. Conversely, considering our findings, the second hypothesis must be rejected. Indeed, as compared to the application of single components, the combination of different biostimulants in the same seed dressing solution did not significantly enhance the performance of durum wheat, except for coleoptile length. This phenomenon, especially in the case of SWEO and NPK, could be explained by the negative effect that excessive doses of nutrients and phytohormones can have on early plant development. Similar results were found by other studies in which high doses of biostimulants or their combinations reduced the germination percentage and the seedling performance [14,55,56]. As for treatments containing Zn, the negative effect could be probably due to EDTA and its competition with other nutrients [57,58].
The Codium and Opuntia extracts showed the best results in all the analyzed traits. The effectiveness of seaweed extracts in promoting growth and the physiological traits of wheat has been reported by several studies [59,60]. Extracts from Codium fragile were recently found to enhance the germination performance and seedling vigor of durum wheat both under normal and salt stress conditions [61]. Codium spp., like all macroalgae, contain phytohormones (e.g., cytokinin, gibberellins and auxins), as well as essential nutrients and amino acids [61,62,63,64,65,66]. All these substances are positively involved in seed germination and seedling development [67,68,69]. Additionally, Opuntia spp. contain carbohydrates, proteins, vitamins, fatty acids and minerals (e.g., magnesium, potassium, calcium and zinc), as well as osmoprotective compounds, especially phenylalanine and proline [70,71,72,73,74,75]. Phenylalanine plays a fundamental role in early seedling growth and development [76,77], while proline is involved in the protective mechanism against several abiotic stresses, like water and salt stress [78]. The extremely effective action of Opuntia extracts in promoting seed germination and seedling vigor is also reported by another study, in which the final germination of tomato seeds increased from 64% with the untreated seeds to 100% with the seeds treated with the plant extracts; similar results were obtained also in terms of the fresh weight of the tomato plants [79].
The positive effect the NPK treatment had on germination traits as well as on root and coleoptile development was due to the its formulation of macronutrients as well as humic and fulvic acids. Other studies found that fulvic acids increased the germination rate and growth speed of spring cereals [80], while humic substances improved the seedling vigor of wheat [81]. As for macronutrients, the key role that phosphate fertilizer plays in promoting early growth of different crops is well referenced [82,83,84]. Similarly, nitrogen and potassium can boost seedling vigor, as reported by a study conducted on tomatoes [85]. The efficacy of NPK fertilizer in promoting wheat development was reported by another study, in which seeds of different bread wheat cultivars, soaked in different NPK solutions, showed an increase in plant height ranging from 2% to 11% [86]. In our study, seeds treated with chelated zinc performed better than those of the control for all the analyzed traits. This is consistent with other findings, in which seed priming with zinc strongly enhanced the germination percentage of wheat compared to the untreated seeds (from 22% to 38%); the dry weight of the seedlings was enhanced as well [87]. Seed dressing applied as zinc sulphate or zinc oxide was reported to be one of the most common and effective ways to increase the germination percentage and the seedling vigor of many crops [88,89,90]. While the use of chelated zinc is still little explored for seed coating, its use could be very effective in alkaline or calcareous soil, where Zn availability is limited [91,92].
5. Conclusions
Biostimulant seed dressing is a promising technique that deserves a deeper understanding to help the industry in obtaining an increasingly high-performance seed. Our study clearly demonstrated that different biostimulants delivered together with a fungicide seed dressing were great enhancers of the germination and vigor of durum wheat seeds. The application of extracts from Codium fragile and Opuntia ficus-barbarica constantly was the most effective treatment. Additionally, it never showed a synergistic action when used in a mixture with other substances. A fast and uniform emergence would result in a more resilient crop, especially in the earliest growth stages, thus leading to an important reduction in the use of agrochemicals. For these reasons, farmers should pay more attention to seed quality and to proper seed dressing. Our study, despite clearly showing the effect of different seed dressings in promoting germination and seedling vigor of durum wheat, still has strong limitations, including i) the controlled environmental conditions and ii) the relatively short duration of the experiment. Further studies are needed to reveal the mechanism of action of the different substances, especially their interactions, and to evaluate their effectiveness on diverse crops in longer studies under field conditions. Having a more complete understanding of these processes could help farmers to better face seeding uncertainties with the right seed dressing.
Author Contributions
Conceptualization, A.R. and F.R.; methodology, R.R. and F.R..; formal analysis, A.R. and R.R.; investigation, A.R. and F.R.; resources, R.R.; data curation, A.R. and R.R.; writing—original draft preparation, A.R. and R.R.; writing—review and editing, R.R.; visualization, F.R.; supervision, R.R. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted as activity of the PhD course in “Plant and Animal Production Sciences (XXXVIII cycle)” of the University of Tuscia and was co-funded by PNRR, Missione 4, componente 2 “Dalla Ricerca all’Impresa”—Investimento 3.3 and ISLA srl.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to acknowledge Fakir Mathlouthi (FBSM Nanobiology, Friedrich-Ebert-Anlage 36, 60325 Frankfurt, Germany) for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [PubMed]
- TeKrony, D.M.; Egli, D.B. Relationship of Seed Vigor to Crop Yield: A Review. Crop Sci. 1991, 31, 816–822. [Google Scholar] [CrossRef]
- Marcos-Filho, J. Seed Vigor Testing: An Overview of the Past, Present and Future Perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef]
- Costa, C.J.; Meneghello, G.E.; Jorge, M.H.A.; Costa, E. The Importance of Physiological Quality of Seeds for Agriculture. Colloq. Agrar. 2021, 17, 102–119. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Leprince, O.; Buitink, J. Molecular and Environmental Factors Regulating Seed Longevity. Biochem. J. 2020, 477, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, M.S.; Ali, H.H.; Soufan, W.; Iqbal, R.; Habib-Ur-rahman, M.; Iqbal, J.; Israr, M.; El Sabagh, A. Potential Effects of Biochar Application for Improving Wheat (Triticum aestivum L.) Growth and Soil Biochemical Properties under Drought Stress Conditions. Land 2021, 10, 1125. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Debaeke, P.; Steinberg, C.; You, M.P.; Barbetti, M.J.; Aubertot, J.-N. Abiotic and Biotic Factors Affecting Crop Seed Germination and Seedling Emergence: A Conceptual Framework. Plant Soil 2018, 432, 1–28. [Google Scholar] [CrossRef]
- Alemu, G. Review on the Effect of Seed Source and Size on Grain Yield of Bread Wheat (Tritium aestivum L.). J. Ecol. Nat. Resour. 2019, 3, 000155. [Google Scholar] [CrossRef]
- Halmer, P. Seed Technology and Seed Enhancement. Acta Hortic. 2008, 771, 17–26. [Google Scholar] [CrossRef]
- Isman, M.B.; Paluch, G. Needles in the Haystack: Exploring Chemical Diversity of Botanical Insecticides. In Green Trends in Insect Control; Lopez, O., Fernandez-Bolanos, J., Eds.; The Royal Society of Chemistry: London, UK, 2011; ISBN 978-1-84973-149-2. [Google Scholar]
- Albers, C.N.; Bollmann, U.E.; Badawi, N.; Johnsen, A.R. Leaching of 1,2,4-Triazole from Commercial Barley Seeds Coated with Tebuconazole and Prothioconazole. Chemosphere 2022, 286, 131819. [Google Scholar] [CrossRef]
- Poag, P.S.; Popp, M.; Rupe, J.; Dixon, B.; Rothrock, C.; Boger, C. Economic Evaluation of Soybean Fungicide Seed Treatments. Agron. J. 2005, 97, 1647–1657. [Google Scholar] [CrossRef]
- Makhaye, G.; Mofokeng, M.M.; Tesfay, S.; Aremu, A.O.; Van Staden, J.; Amoo, S.O. Chapter 5—Influence of Plant Biostimulant Application on Seed Germination. In Biostimulants for Crops from Seed Germination to Plant Development a Practical Approach; Academic Press: Cambridge, MA, USA, 2021; pp. 109–135. [Google Scholar]
- Badawi, M.; Seadh, S.; Emhimmid, W. Improvement Wheat Germination by Using Some Biostimulants Substances. J. Plant Prod. 2020, 11, 139–144. [Google Scholar] [CrossRef]
- Mesut Çimrin, K.; Türkmen, Ö.; Turan, M.; Tuncer, B. Phosphorus and Humic Acid Application Alleviate Salinity Stress of Pepper Seedling. Afr. J. Biotechnol. 2010, 9, 5845–5851. [Google Scholar]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Front. Plant Sci. 2019, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; Bevilacqua, V.; Logozzo, G.; Bochicchio, R.; Amato, M.; Nuzzaci, M. Seed Coating with Trichoderma harzianum T-22 of Italian Durum Wheat Increases Protection against Fusarium culmorum-Induced Crown Rot. Agriculture 2022, 12, 714. [Google Scholar] [CrossRef]
- Afzal, I.; Javed, T.; Amirkhani, M.; Taylor, A.G. Modern Seed Technology: Seed Coating Delivery Systems for Enhancing Seed and Crop Performance. Agriculture 2020, 10, 526. [Google Scholar] [CrossRef]
- Qiu, Y.; Amirkhani, M.; Mayton, H.; Chen, Z.; Taylor, A.G. Biostimulant Seed Coating Treatments to Improve Cover Crop Germination and Seedling Growth. Agronomy 2020, 10, 154. [Google Scholar] [CrossRef]
- Mehmood Rana, R.; Ur Rehman, S.; Ahmed, J.; Bilal, M. A Comprehensive Overview of Recent Advances in Drought Stress Tolerance Research in Wheat (Triticum aestivum L.). Asian J. Agric. Biol. 2013, 1, 29–37. [Google Scholar]
- Pour-Aboughadareh, A.; Mohammadi, R.; Etminan, A.; Shooshtari, L.; Maleki-Tabrizi, N.; Poczai, P. Effects of Drought Stress on Some Agronomic and Morpho-Physiological Traits in Durum Wheat Genotypes. Sustainability 2020, 12, 5610. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Alam, M.A.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Naeem, M.; Ahmad, M.; Kamran, M.; Shah, M.; Iqbal, M. Physiological Responses of Wheat (Triticum aestivum L.) to Drought Stress. Int. J. Plant Soil Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Toreti, A.; Ceglar, A.; Naumann, G.; Turco, M.; Tebaldi, C. Climate Resilience of the Top Ten Wheat Producers in the Mediterranean and the Middle East. Reg. Environ. Chang. 2020, 20, 41. [Google Scholar] [CrossRef]
- Mathlouthi, F.; Ruggeri, R.; Rossini, A.; Rossini, F. A New Fertilization Approach for Bread Wheat in the Mediterranean Environment: Effects on Yield and Grain Protein Content. Agronomy 2022, 12, 2152. [Google Scholar] [CrossRef]
- Makhaye, G.; Aremu, A.O.; Gerrano, A.S.; Tesfay, S.; Du Plooy, C.P.; Amoo, S.O. Biopriming with Seaweed Extract and Microbial-Based Commercial Biostimulants Influences Seed Germination of Five Abelmoschus esculentus Genotypes. Plants 2021, 10, 1327. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Siddique, K.H. Micronutrient application through seed treatments: A review. J. Plant Nutr. Soil Sci. 2012, 12, 125–142. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Latique, S.; Mohamed Aymen, E.; Halima, C.; Chérif, H.; Mimoun, E.K. Alleviation of salt stress in durum wheat (Triticum durum L.) seedlings through the application of liquid seaweed extracts of Fucus spiralis. Commun. Soil Sci. Plant Anal. 2017, 48, 2582–2593. [Google Scholar] [CrossRef]
- Ahmed, T.; Abou Elezz, A.; Khalid, M.F. Hydropriming with Moringa Leaf Extract Mitigates Salt Stress in Wheat Seedlings. Agriculture 2021, 11, 1254. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Farooq, M.; Nawaz, A. Seed priming with sorghum extracts and benzyl aminopurine improves the tolerance against salt stress in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2018, 24, 239–249. [Google Scholar] [CrossRef]
- Bouteraa, M.T.; Mishra, A.; Romdhane, W.B.; Hsouna, A.B.; Siddique, K.H.M.; Saad, R.B. Bio-Stimulating Effect of Natural Polysaccharides from Lobularia maritima on Durum Wheat Seedlings: Improved Plant Growth, Salt Stress Tolerance by Modulating Biochemical Responses and Ion Homeostasis. Plants 2022, 11, 1991. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Sun, S.; Zhong, K.; Li, S.; Hu, H.; Sun, C.; Zheng, Q.; Tian, Z.; Dai, T.; Sun, J. Seed Soaking with Melatonin Promotes Seed Germination under Chromium Stress via Enhancing Reserve Mobilization and Antioxidant Metabolism in Wheat. Ecotoxicol. Environ. Saf. 2021, 220, 112241. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Ali, Q.; Naseem, J.; Javed, M.T.; Kanwal, H.; Islam, W.; Aqeel, M.; Khalid, N.; Zafar, S.; Tayyeb, M.; et al. Sugar Beet Extract Acts as a Natural Bio-Stimulant for Physio-Biochemical Attributes in Water Stressed Wheat (Triticum aestivum L.). Acta Physiol. Plant. 2018, 40, 110. [Google Scholar] [CrossRef]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with Proline or an Organic Bio-Stimulant Induces Salt Tolerance in Wheat Plants by Improving Antioxidant Redox State and Enzymatic Activities and Reducing the Oxidative Stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Qiu, B.; Chen, Z. Feasibility Research on Seed Coating in Industrialized Rice Seedling Raising. Trans. Chin. Soc. Agric. Eng. 2000, 16, 96–98. [Google Scholar]
- Ferreira, L.L.; Resende, J.M.; Carvalho, I.R.; Carnevale, A.B.; Fernandes, M.S.; Carrijo dos Santos, N.S.; Batista, P.F.; Azevedo Pereira, A.I.; Silva Curvêlo, C.R.; Amaral, U.; et al. Multivariate Explanation of the Establishment of Soybean Initial Growth Pattern via Biostimulant Seed Treatment. Agron. Sci. Biotechnol. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Bezerra, A.R.G.; Silva, F.C.S.; Silva, A.F.; Sediyama, T.; Álvares, C.H.A. Effect of Biostimulants and Seed Treatment with Fungicide on the Germination and Vigor of Soybean Seedlings. Rev. Bras. Tec. Ciênc. Agrar. 2015, 8, 27–35. [Google Scholar] [CrossRef]
- Ujvári, G.; Capo, L.; Grassi, A.; Cristani, C.; Pagliarani, I.; Turrini, A.; Blandino, M.; Giovannetti, M.; Agnolucci, M. Agronomic Strategies to Enhance the Early Vigor and Yield of Maize. Part I: The Role of Seed Applied Biostimulant, Hybrid and Starter Fertilization on Rhizosphere Bacteria Profile and Diversity. Front. Plant Sci. 2023, 14, 1240310. [Google Scholar] [CrossRef] [PubMed]
- Majkowska-Gadomska, J.; Francke, A.; Dobrowolski, A.; Mikulewicz, E. The Effect of Selected Biostimulants on Seed Germination of Four Plant Species. Acta Agrophysica 2017, 24, 591–599. [Google Scholar]
- Tweddell, R.J.; Pelerin, S.; Chabot, R. A Two-Year Field Study of a Commercial Biostimulant Applied on Maize as Seed Coating. Can. J. Plant Sci. 2000, 80, 805–807. [Google Scholar] [CrossRef]
- Marino, S.; Alvino, A. Detection of Spatial and Temporal Variability of Wheat Cultivars by High-Resolution Vegetation Indices. Agronomy 2019, 9, 226. [Google Scholar] [CrossRef]
- Amoriello, T.; Belocchi, A.; Quaranta, F.; Ripa, C.; Melini, F.; Aureli, G. Behaviour of Durum Wheat Cultivars towards Deoxynivalenol Content: A Multi-Year Assay in Italy. Ital. J. Agron. 2018, 13, 12–20. [Google Scholar] [CrossRef]
- Freiberg, J.A.; Ludwig, M.P.; Avelar, S.A.G.; Girotto, E. Tratamento de Sementes e Sua Influência No Potencial Produtivo Da Cultura Do Trigo. J. Seed Sci. 2017, 39, 280–287. [Google Scholar] [CrossRef]
- Ayed, S.; Bouhaouel, I.; Jebari, H.; Hamada, W. Use of Biostimulants: Towards Sustainable Approach to Enhance Durum Wheat Performances. Plants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.G.; Fredericksen, C.; Koch, R.; Sieverding, E. Effect of Seed Treatment with Natural Products on Early Arbuscular Mycorrhizal Colonization of Wheat by Claroideoglomus Claroideum. J. Appl. Bot. Food Qual. 2014, 87, 117–123. [Google Scholar] [CrossRef]
- Ministero dell’Agricoltura e delle Foreste. Metodi Ufficiali di Analisi per le Sementi; Gazzetta Ufficiale della Repubblica Italiana: Rome, Italy, 1993. [Google Scholar]
- Scarici, E.; Ruggeri, R.; Provenzano, M.E.; Rossini, F. Germination and Performance of Seven Native Wildflowers in the Mediterranean Landscape Plantings. Ital. J. Agron. 2018, 13, 163–171. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Ahmad, N.; Hafeez, K. Thermal Hardening: A New Seed Vigor Enhancement Tool in Rice. J. Integr. Plant Biol. 2005, 47, 187–193. [Google Scholar] [CrossRef]
- Ritz, C.; Pipper, C.B.; Streibig, J.C. Analysis of Germination Data from Agricultural Experiments. Eur. J. Agron. 2013, 45, 1–6. [Google Scholar] [CrossRef]
- Rossini, F.; Ruggeri, R.; Celli, T.; Rogai, F.M.; Kuzmanović, L.; Richardson, M.D. Cool-Season Grasses for Overseeding Sport Turfs: Germination and Performance under Limiting Environmental Conditions. HortScience 2019, 54, 555–563. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing 2021. Available online: https://www.R-project.org/ (accessed on 17 September 2023).
- Onofri, A.; Mesgaran, M.B.; Ritz, C. A Unified Framework for the Analysis of Germination, Emergence, and Other Time-to-Event Data in Weed Science. Weed Sci. 2022, 70, 259–271. [Google Scholar] [CrossRef]
- Ali, Q.; Perveen, R.; El-Esawi, M.A.; Ali, S.; Hussain, S.M.; Amber, M.; Iqbal, N.; Rizwan, M.; Alyemeni, M.N.; El-Serehy, H.A.; et al. Low Doses of Cuscuta reflexa Extract Act as Natural Biostimulants to Improve the Germination Vigor, Growth, and Grain Yield of Wheat Grown under Water Stress: Photosynthetic Pigments, Antioxidative Defense Mechanisms, and Nutrient Acquisition. Biomolecules 2020, 10, 1212. [Google Scholar] [CrossRef] [PubMed]
- Layek, J.; Das, A.; Ramkrushna, G.I.; Ghosh, A.; Panwar, A.S.; Krishnappa, R.; Ngachan, S.V. Effect of Seaweed Sap on Germination, Growth and Productivity of Maize (Zea mays) in North Eastern Himalayas. Indian J. Agron. 2016, 61, 354–359. [Google Scholar] [CrossRef]
- Brown, J.C.; Tiffin, L.; Holmes, R.S. Competition between Chelating Agents and Roots as Factor Affecting Absorption of Iron and Other Ions by Plant Species. Plant Physiol. 1960, 35, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Inaba, S.; Takenaka, C. Effects of Dissolved Organic Matter on Toxicity and Bioavailability of Copper for Lettuce Sprouts. Environ. Int. 2005, 31, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Bordoloi, B.C.; Singha, D.D.; Ojha, N.J. Role of Seaweed Extract on Growth, Yield and Quality of Some Agricultural Crops: A Review. Agric. Rev. 2018, 39, 321–326. [Google Scholar] [CrossRef]
- Hamouda, M.M.; Saad-Allah, K.M.; Gad, D. Potential of Seaweed Extract on Growth, Physiological, Cytological and Biochemical Parameters of Wheat (Triticum aestivum L.) Seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 1818–1831. [Google Scholar] [CrossRef]
- Rossini, A.; Ruggeri, R.; Mzid, N.; Rossini, F.; Di Miceli, G. Codium fragile (Suringar) Hariot as Biostimulant Agent to Alleviate Salt Stress in Durum Wheat: Preliminary Results from Germination Trials. Plants 2024, 13, 283. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.M.; Abd El-Rhman, A.A.; Abdel-Raouf, N.; Ibraheem, I.B.M. Role of Marine Macroalgae in Plant Protection & Improvement for Sustainable Agriculture Technology. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 104–110. [Google Scholar] [CrossRef]
- Sadak, M.; El-Bassiouny, H.; Mahfouz, S.; El-Enany, M.; Elewa, T. Use of Thiamine, Pyridoxine and Biostimulant for Better Yield of Wheat Plants under Water Stress: Growth, Osmoregulations, Antioxidantive Defense and Protein Pattern. Egypt. J. Chem. 2022, 66, 407–424. [Google Scholar] [CrossRef]
- Stirk, W.A.; Van Staden, J. Comparison of Cytokinin-and Auxin-like Activity in Some Commercially Used Seaweed Extracts. J. Appl. Phycol. 1997, 8, 503–508. [Google Scholar] [CrossRef]
- El-Din, S.M.M. Utilization of Seaweed Extracts as Bio-Fertilizers to Stimulate the Growth of Wheat Seedlings. Egypt. Soc. Exp. Biol. 2015, 11, 31–39. [Google Scholar]
- Mzibra, A.; Aasfar, A.; Benhima, R.; Khouloud, M.; Boulif, R.; Douira, A.; Bamouh, A.; Meftah Kadmiri, I. Biostimulants Derived from Moroccan Seaweeds: Seed Germination Metabolomics and Growth Promotion of Tomato Plant. J. Plant Growth Regul. 2021, 40, 353–370. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Presowing Seed Treatment with Cytokinins and Its Effect on Growth, Photosynthetic Rate, Ionic Levels and Yield of Two Wheat Cultivars Differing in Salt Tolerance. J. Integr. Plant Biol. 2005, 47, 1315–1325. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Seed Treatment with Auxins Modulates Growth and Ion Partitioning in Salt-Stressed Wheat Plants. J. Integr. Plant Biol. 2007, 49, 1003–1015. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M.; Jamil, A. Seed Enhancement with Cytokinins: Changes in Growth and Grain Yield in Salt Stressed Wheat Plants. Plant Growth Regul. 2006, 50, 29–39. [Google Scholar] [CrossRef]
- Ventura-Aguilar, R.I.; Bosquez-Molina, E.; Bautista-Baños, S.; Rivera-Cabrera, F. Cactus Stem (Opuntia ficus-indica Mill): Anatomy, Physiology and Chemical Composition with Emphasis on Its Biofunctional Properties. J. Sci. Food Agric. 2017, 97, 5065–5073. [Google Scholar] [CrossRef]
- Cho, I.-K.; Jin, S.-W.; Kim, Y.-D. Analysis of Components in the Parts of Opuntia ficus indica from Shinan Korea. Korean J. Food Preserv. 2009, 16, 742–746. [Google Scholar]
- Hernández-Urbiola, M.I.; Pérez-Torrero, E.; Rodríguez-García, M.E. Chemical Analysis of Nutritional Content of Prickly Pads (Opuntia ficus indica) at Varied Ages in an Organic Harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia Spp. Mucilage’s: A Functional Component with Industrial Perspectives. J. Arid Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Sáenz Hernández, C.L.; Berger, H.; Rodríguez-Félix, A.; Galletti, L.; Corrales García, J.; Sepúlveda, E.; Varnero Moreno, M.T.; García de Cortázar, V.; Cuevas García, R.; Arias, E.; et al. Agro-Industrial Utilization of Cactus Pear; FAO: Rome, Italy, 2013. [Google Scholar]
- Perkowski, M.C.; Warpeha, K.M. Phenylalanine Roles in the Seed-to-Seedling Stage: Not Just an Amino Acid. Plant Sci. 2019, 289, 110223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, Y.; Mu, K.; Cai, W.; Zhao, Y.; Shen, H.; Wang, X.; Ma, H. Phenylalanine Ammonia Lyase GmPAL1.1 Promotes Seed Vigor under High-Temperature and -Humidity Stress and Enhances Seed Germination under Salt and Drought Stress in Transgenic Arabidopsis. Plants 2022, 11, 3239. [Google Scholar] [CrossRef] [PubMed]
- Ambreen, S.; Athar, H.u.R.; Khan, A.; Zafar, Z.U.; Ayyaz, A.; Kalaji, H.M. Seed Priming with Proline Improved Photosystem II Efficiency and Growth of Wheat (Triticum aestivum L.). BMC Plant Biol. 2021, 21, 502. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Santana, M.M.; Maia, R.N.; Tavares, J.; Nabti, E.; Cruz, C. Bacterial Inoculation and Extracts of Opuntia Rackets or Marine Algae Trigger Distinct Proline Balances in Tomato Salt Stress Alleviation. Agronomy 2023, 13, 2921. [Google Scholar] [CrossRef]
- Braziene, Z.; Paltanavicius, V.; Avizienytė, D. The Influence of Fulvic Acid on Spring Cereals and Sugar Beets Seed Germination and Plant Productivity. Environ. Res. 2021, 195, 110824. [Google Scholar] [CrossRef] [PubMed]
- Litvin, V.; Deriy, S.; Plakhotniuk, L. Effects of Humic Substances on Seed Germination of Wheat under the Influence of Heavy Metal. Cherkasy Univ. Bullettin Biol. Sci. Ser. 2020, 1, 42–52. [Google Scholar] [CrossRef]
- Talboys, P.J.; Healey, J.R.; Withers, P.J.A.; Roose, T.; Edwards, A.C.; Pavinato, P.S.; Jones, D.L. Combining Seed Dressing and Foliar Applications of Phosphorus Fertilizer Can Give Similar Crop Growth and Yield Benefits to Soil Applications Together With Greater Recovery Rates. Front. Agron. 2020, 2, 605655. [Google Scholar] [CrossRef]
- Izydorczyk, G.; Saeid, A.; Mironiuk, M.; Witek-Krowiak, A.; Kozioł, K.; Grzesik, R.; Chojnacka, K. Sustainable Method of Phosphorus Biowaste Management to Innovative Biofertilizers: A Solution for Circular Economy of the Future. Sustain. Chem. Pharm. 2022, 27, 100634. [Google Scholar] [CrossRef]
- Mathlouthi, F.; Ruggeri, R.; Rossini, F. Alternative Solution to Synthetic Fertilizers for the Starter Fertilization of Bread Wheat under Mediterranean Climatic Conditions. Agronomy 2022, 12, 511. [Google Scholar] [CrossRef]
- Ali, M.M.; Javed, T.; Mauro, R.P.; Shabbir, R.; Afzal, I.; Yousef, A.F. Effect of Seed Priming with Potassium Nitrate on the Performance of Tomato. Agriculture 2020, 10, 498. [Google Scholar] [CrossRef]
- Al-Obaidi, A.H.S.; Alrijabo, A.A. Soaking Technique on Bread Wheat (Triticum aestivum L.) and Its Influence on Yield and Its Components in Rain Fed Area. Indian J. Ecol. 2021, 48, 14. [Google Scholar]
- Rai-Kalal, P.; Jajoo, A. Priming with Zinc Oxide Nanoparticles Improve Germination and Photosynthetic Performance in Wheat. Plant Physiol. Biochem. 2021, 160, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Irshad, S.; Saddiq, M.S.; Bashir, S.; Khan, S.; Wahid, M.A.; Khan, R.R.; Yousra, M. Potential of Zinc Seed Treatment in Improving Stand Establishment, Phenology, Yield and Grain Biofortification of Wheat. J. Plant Nutr. 2019, 42, 1676–1692. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Wojnarowicz, J. Zinc Oxide and Zinc Oxide Nanoparticles Impact on in Vitro Germination and Seedling Growth in Allium cepa L. Materials 2020, 13, 2784. [Google Scholar] [CrossRef]
- Mendes, F.; Lourenço, S.; De Abreu Dos Santos, M.; Alves, C.Z.; Naudi, C.; Campos, S.; Carina Da, A.; Cândido, S.; De Mello Prado, R.; Barbosa, G.; et al. Effect of Two Sources of Zinc on the Physiological Quality of Seed and Nutrition of Rice Seedlings Effect of Two Sources of Zinc on the Physiological Quality of Seed and Nutrition of Rice (Oriza sativa). Rev. Fac. Cienc. Agrar. 2020, 52, 95–105. [Google Scholar]
- Zhao, A.; Yang, S.; Wang, B.; Tian, X. Effects of ZnSO4 and Zn-EDTA Applied by Broadcasting or by Banding on Soil Zn Fractions and Zn Uptake by Wheat (Triticum aestivum L.) under Greenhouse Conditions. J. Plant Nutr. Soil Sci. 2019, 182, 307–317. [Google Scholar] [CrossRef]
- Chahal, S.K.; Hettiarachchi, G.M.; Nelson, N.O.; Guttieri, M.J. Fate and Plant Uptake of Different Zinc Fertilizer Sources upon Their Application to an Alkaline Calcareous Soil. ACS Agric. Sci. Technol. 2023, 3, 725–737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).