Prospection of Nematotoxic Aqueous Seeds Extracts Derived from the Preserved Arachis (Fabaceae) Germplasm Bank
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Eggs and Second Stage Juveniles (J2) of Meloidogyne incognita from Infected Nicotiana tabacum Plants
2.2. Plant Extracts
2.3. Viability and Recovery Bioassay: Determine Nematicidal and/or Nematostatic Activity
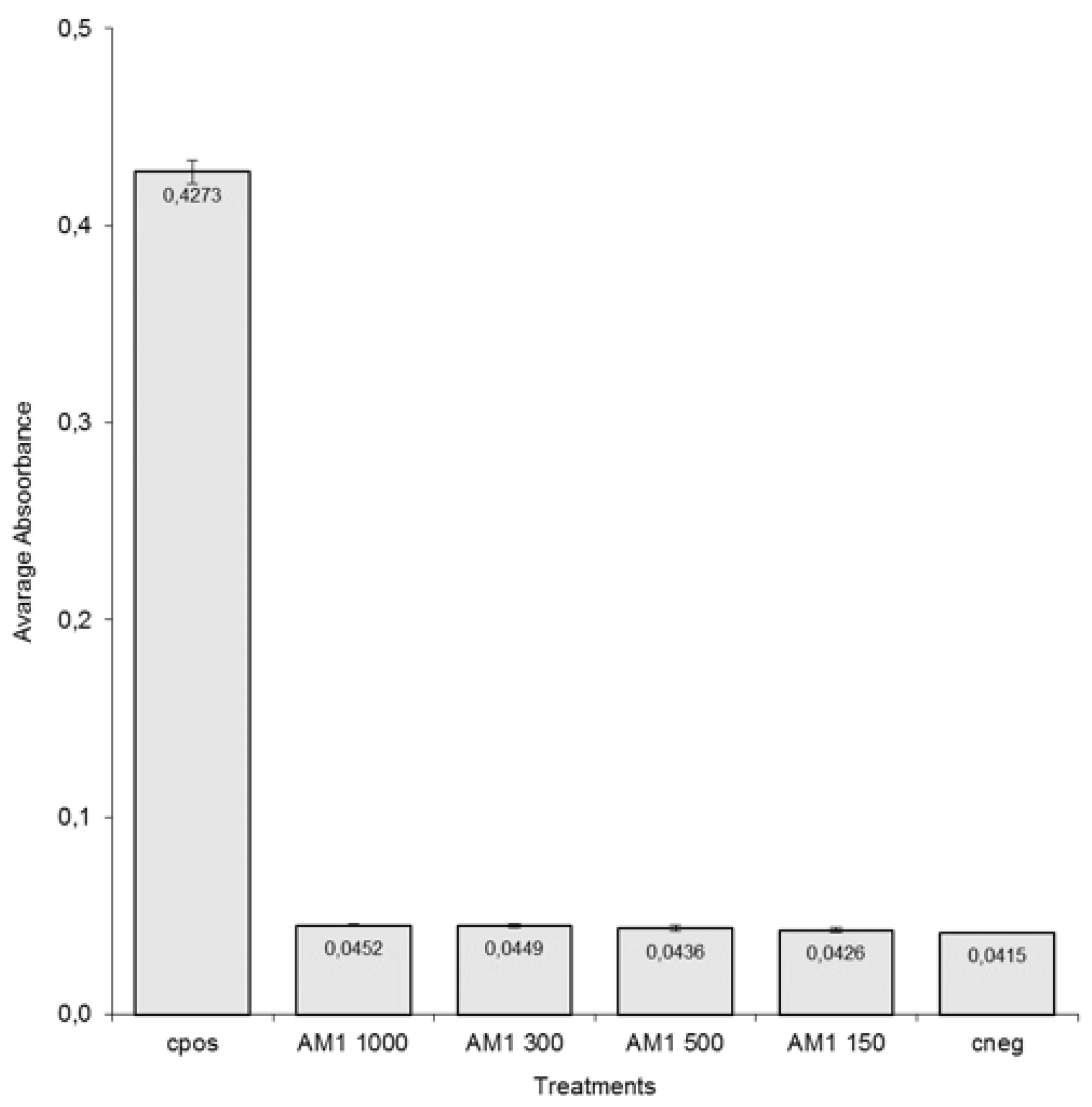
2.4. Determination of the Lowest Lethal Dose
2.5. Estimate of Arachis ACEs Thermostability
2.6. Hemolysis Assay
3. Results and Discussion
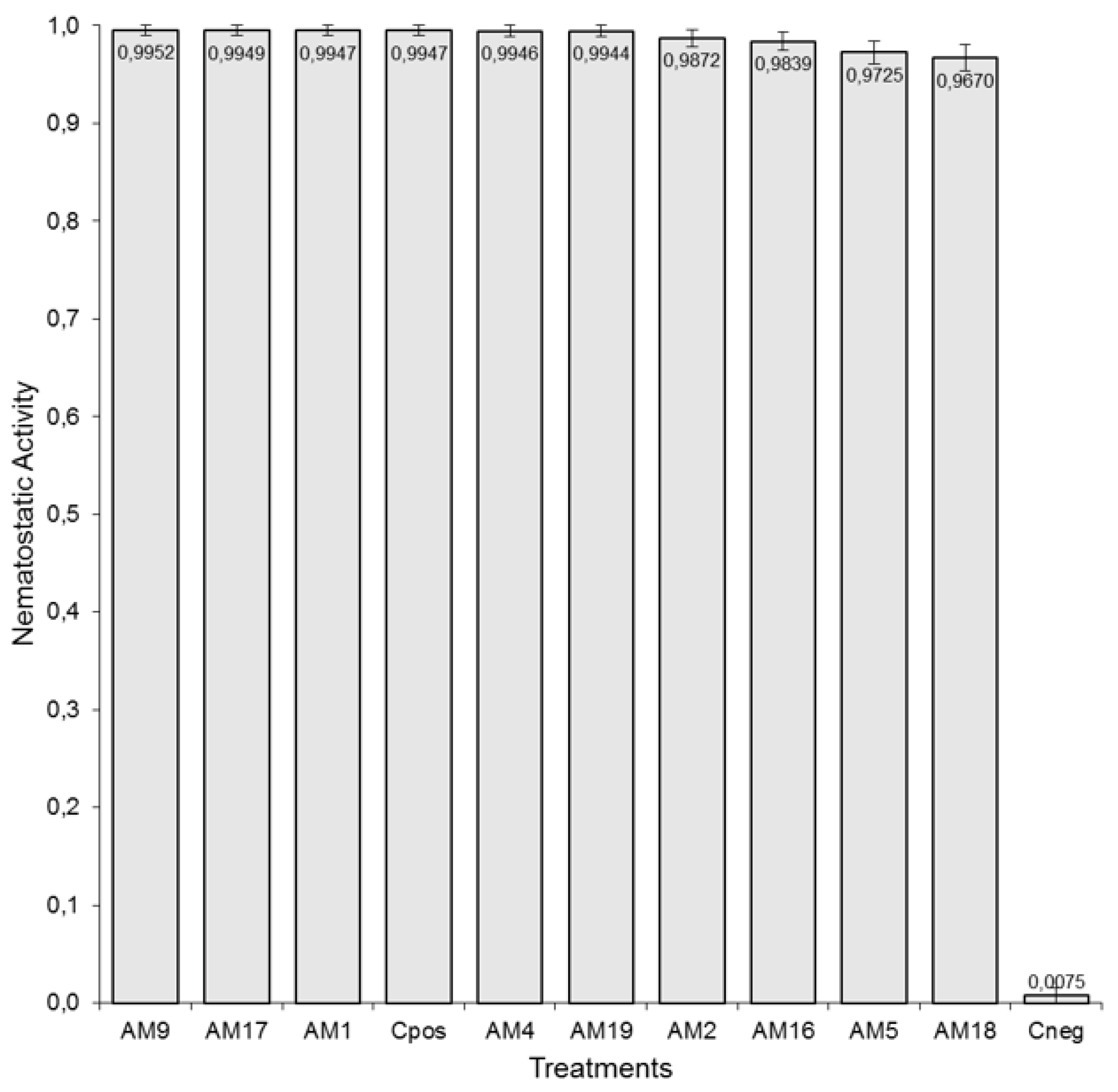
3.1. Nematotoxic In Vitro Bioassays to Evaluate the Activity of ACEs from Arachis Plant Seeds against J2 of M. incognita
3.1.1. Viability In Vitro Bioassay
3.1.2. Recovery In Vitro Bioassay
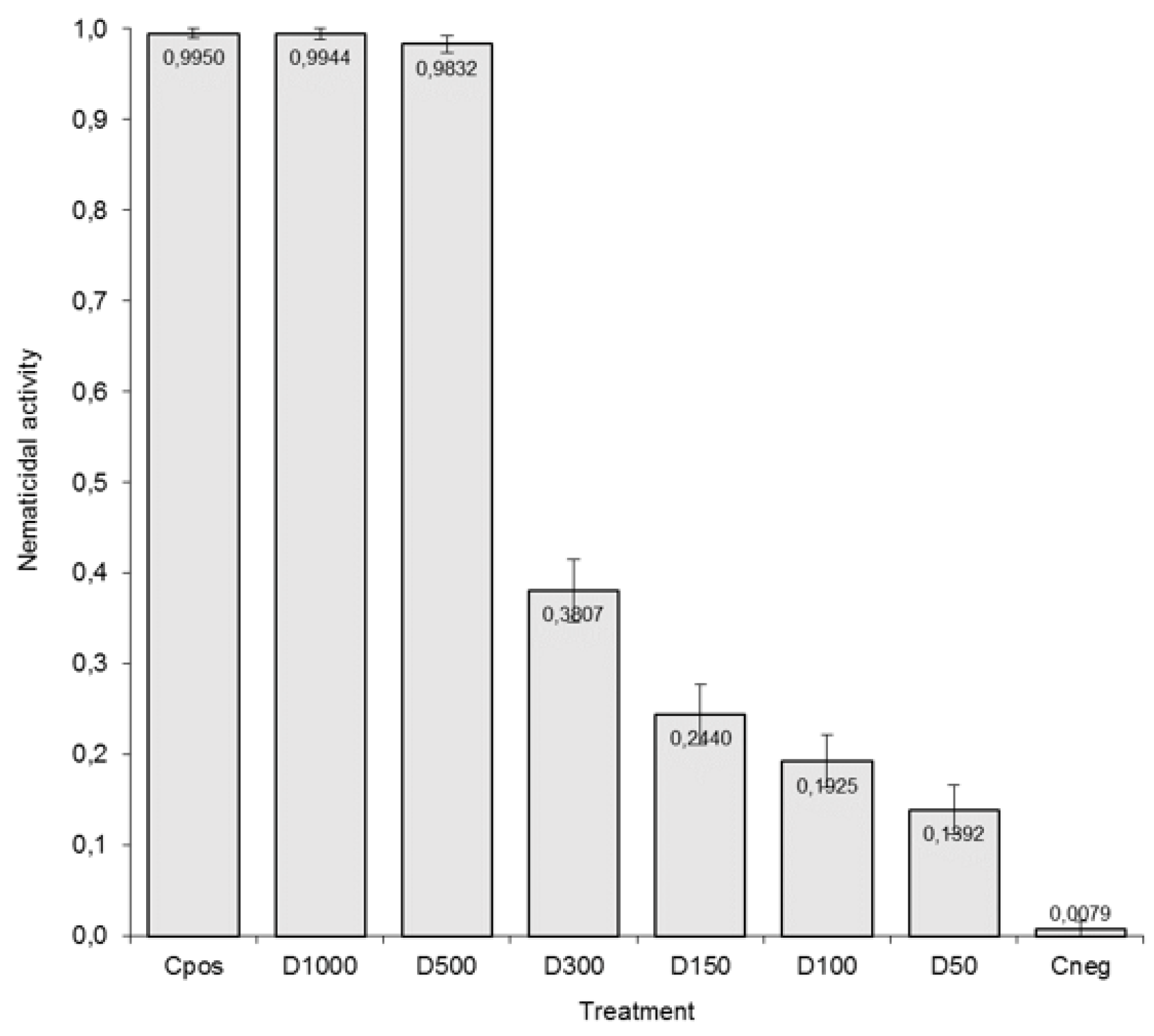
3.2. Determination of the Lowest Lethal Dose of Arachis ACEs on J2 of M. incognita
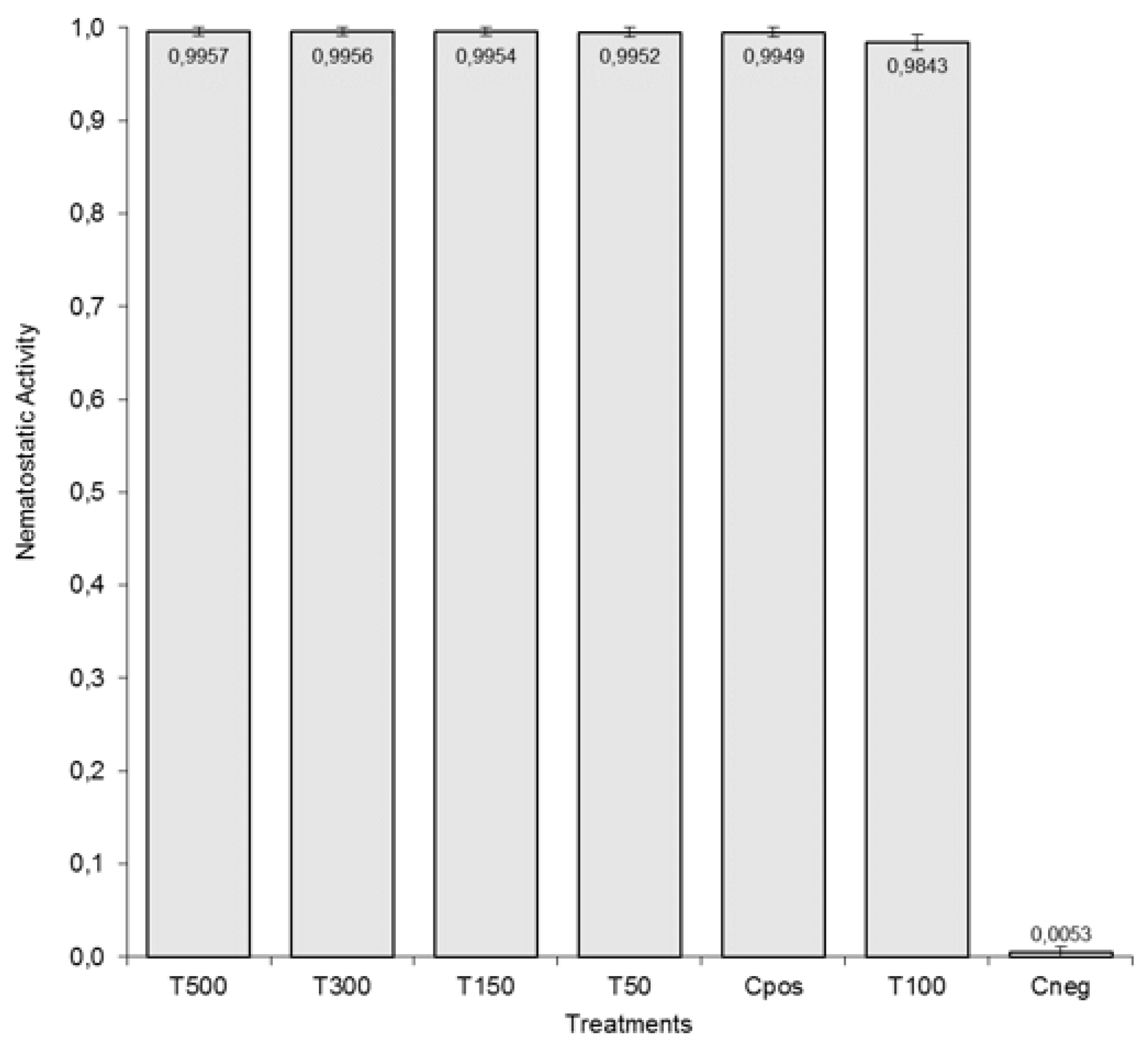
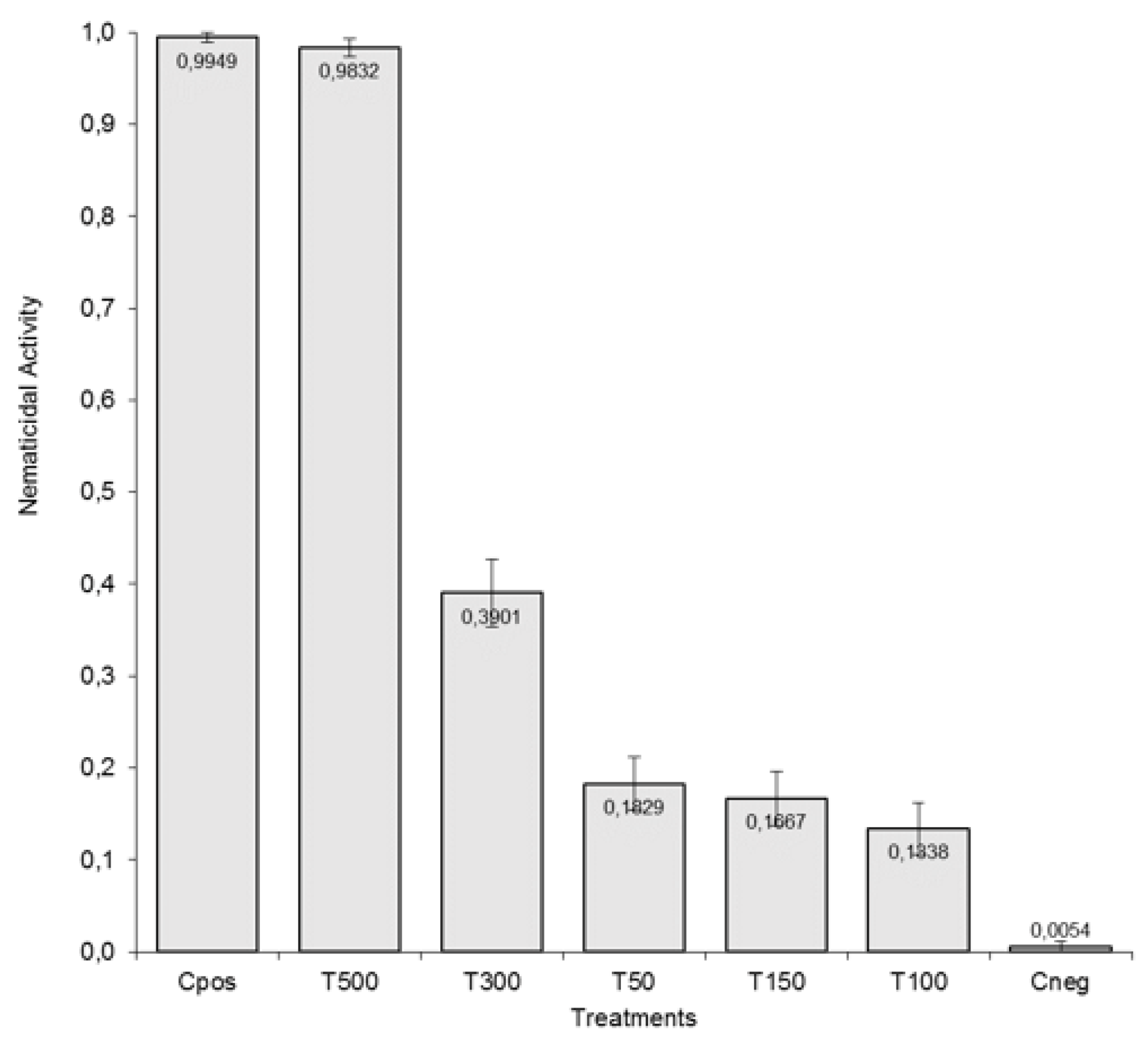
3.3. Thermostability Test: Certifying the Thermal Stability of Nematotoxic Arachis AM1 ACE against J2 of M. incognita
3.4. Cytotoxicity: Evaluating the Cytotoxicity of Nematotoxic Arachis AM1 ACE against Bovine Red Cells (Female 3379)
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abd-Elgawad, M.M.M.; Askary, T.H. Impact of phytonematodes on agriculture economy. In Biocontrol Agents of Phytonematodes; CABI: Wallingford, UK, 2015; pp. 3–49. [Google Scholar]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Nijs L den Hockland, S.; Maafi, Z.T. Current Nematode Threats to World Agriculture. Current Nematode Threats to World Agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar] [CrossRef]
- Freitas, L.G.; Oliveira, R.D.L.; Ferraz, S. Introdução à Nematologia; UFV: Viçosa, Brazil, 2001; 84p. [Google Scholar]
- Bakhetia, M.; Charlton, W.; Atkinson, H.J.; McPherson, M.J. RNA Interference of Dual Oxidase in the Plant Nematode Meloidogyne incognita. Mol. Plant-Microbe Interact. 2005, 18, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Ritzinger, C.H.S.P.; Fancelli, M. Manejo integrado de nematóides na cultura da bananeira. Rev. Bras. Frutic. 2006, 28, 331–338. [Google Scholar] [CrossRef]
- Chitwood, D.J. Phytochemical Based Strategies for Nematode Control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, J.L.; Campos, V.P. Efeito de exsudatos de colônias e de filtrados de culturas de actinomicetos na eclosão, motilidade e mortalidade de juvenis do segundo estádio de Meloidogyne javanica. Fitopatol. Bras. 2005, 30, 232–238. [Google Scholar] [CrossRef]
- Abad, P.; Williamson, V.M. Plant Nematode Interaction: A Sophisticated Dialogue. In Advances in Botanical Research; Kader, J.C., Delseny, M., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 53, pp. 147–192. Available online: https://www.sciencedirect.com/science/article/pii/S0065229610530052 (accessed on 9 December 2023).
- FAO. The Future of Food and Agriculture–Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Rocha, T.L.; Soll, C.B.; Boughton, B.A.; Silva, T.S.; Oldach, K.; Firmino, A.A.; Callahan, D.L.; Sheedy, J.; Silveira, E.R.; Carneiro, R.M.; et al. Prospection and identification of nematotoxic compounds from Canavalia ensiformis seeds effective in the control of the root knot nematode Meloidogyne incognita. Biotechnol. Res. Innov. 2017, 1, 87–100. [Google Scholar] [CrossRef]
- Pascual-Vilalobos, M.J. Plaguicidas Naturals de Origen Vegetal: Estado Actual de la Investigación; Instituto Nacional de Investigación Agrária y Alimentaria: Madrid, Spain, 1996. [Google Scholar]
- Ferraz, S.; Freitas, L.G. 19 Use of Antagonistic Plants and Natural Products. In Nematology: Advances and Perspectives; CABI Publishing: Wallingford, UK, 2004; pp. 931–977. Available online: https://books.google.com.br/books?hl=pt-BR&lr=&id=-vvi72MxhzUC&oi=fnd&pg=PA931&dq=FERRAZ,+S.+%26amp%3B+FREITAS,+L.G.+2004.+Use+of+antagonistic+plants+and+natural+products.%C2%A0Nematology-+Advances+and+Perspectives+2:+931-977.&ots=1OibsNLC6R&sig=yc3TJ23EMMWQ_utC9ArWdzDwkqk#v=onepage&q&f=false (accessed on 10 October 2023).
- De Waele, D.; Elsen, A. Challenges in Tropical Plant Nematology. Annu. Rev. Phytopathol. 2007, 45, 457–485. [Google Scholar] [CrossRef]
- Corrêa, J.C.R.; Salgado, H.R.N. Atividade inseticida das plantas e aplicações: Revisão. Rev. Bras. Plantas Med. 2011, 13, 500–506. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural Products As Sources for New Pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.H.; Alaniz, N.M.; Saleh, M.A. Nematicidal activity of terpenoids. J. Environ. Sci. Health Part B 2013, 48, 16–22. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Lee, S.J.; Byun, H.J.; Moon, C.S.; Han, B.S.; Kim, Y.H. Isolation of Nematicidal Triterpenoid Saponins from Pulsatilla koreana Root and Their Activities against Meloidogyne incognita. Molecules 2013, 18, 5306–5316. [Google Scholar] [CrossRef] [PubMed]
- Caboni, P.; Saba, M.; Oplos, C.; Aissani, N.; Maxia, A.; Menkissoglu-Spiroudi, U.; Casu, L.; Ntalli, N. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2015, 71, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the Art and Future Opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Le Dang, Q.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Cha, B.; Luu, N.H.; Kim, J.C. Nematicidal Activities of 4-Quinolone Alkaloids Isolated from the Aerial Part of Triumfetta grandidens against Meloidogyne incognita. J. Agric. Food Chem. 2015, 63, 68–74. [Google Scholar] [CrossRef]
- Tiku, A.R. Antimicrobial Compounds and Their Role in Plant Defense. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 283–307. [Google Scholar] [CrossRef]
- Khan, F.; Asif, M.; Khan, A.; Tariq, M.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: In vitro and greenhouse study. Curr. Plant Biol. 2019, 20, 100115. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of Botanical Pesticides in Agriculture as an Alternative to Synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Sithole, N.T.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Potential nematicidal properties of plant extracts against Meloidogyne incognita. S. Afr. J. Bot. 2021, 139, 409–417. [Google Scholar] [CrossRef]
- Amulu, L.U.; Oyedele, D.J.; Adekunle, O.K. Effects of Sunn hemp (Crotalaria juncea) and Mexican sunflower (Tithonia diversifolia) soil amendments on yields and quality of two indigenous vegetables grown in a nematode-infested field. Indian Phytopathol. 2021, 74, 729–737. [Google Scholar] [CrossRef]
- Bajpai, A.; Jackson, M.A.; Huang, Y.H.; Yap, K.; Du, Q.; Chau, T.C.Y.; Craik, D.J.; Gilding, E.K. Nematicidal Activity of Cyclotides: Toxicity Against Caenorhabditis elegans. J. Nat. Prod. 2023, 86, 1222–1229. [Google Scholar] [CrossRef]
- Krapovickas, A.; Gregory, W.C. Taxonomia Del Genero “Arachis (leguminosae)”. Bonplandia 1994, 8, 1–186. [Google Scholar] [CrossRef]
- Valls, J.F.M.; Simpson, C.E. Nuevas especies de Arachís (Leguminosae) de Brasil, Paraguay y Bolivia. Bonplandia 2005, 14, 35–63. [Google Scholar] [CrossRef]
- Valls, J.F.M.; Da Costa, L.C.; Custodio, A.R. A Novel Trifoliolate Species of Arachis (fabaceae) and Further Comments on the Taxonomic Section Trierectoides. Bonplandia 2013, 22, 91–97. [Google Scholar] [CrossRef]
- Moretzsohn, M.d.C.; Hopkins, M.S.; Mitchell, S.E.; Kresovich, S.; Valls, J.F.M.; Ferreira, M.E. Genetic diversity of peanut (Arachis hypogaea L.) and its wild relatives based on the analysis of hypervariable regions of the genome. BMC Plant Biol. 2004, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Hussey, R.S.; Barker, K.R. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Report. 1973, 57, 1025–1028. [Google Scholar]
- Boneti, J.I.S.; Ferraz, S. Modificação do método de Hussey & Barker para extração de ovos de Meloidogyne exigua de raízes de cafeeiro. Fitopatol. Bras. 1981, 6, 553. [Google Scholar]
- Haseeb, A.; Butool, F. Evaluation of nematicidal properties of some members of the family Solanaceae. Bioresour. Technol. 1996, 57, 95–97. [Google Scholar] [CrossRef]
- Dias, C.R.; Schwan, A.V.; Ezequiel, D.P.; Sarmento, M.C.; Ferraz, S. Effect of aqueous extracts of some medicinal plants on the survival of Meloidogyne incognita juveniles. Nematol. Bras. 2000, 24, 203–210. [Google Scholar]
- Ballén-Taborda, C.; Chu, Y.; Ozias-Akins, P.; Timper, P.; Holbrook, C.C.; Jackson, S.A.; Bertioli, D.J.; Leal-Bertioli, S.C. A new source of root-knot nematode resistance from Arachis stenosperma incorporated into allotetraploid peanut (Arachis hypogaea). Sci. Rep. 2019, 9, 17702. [Google Scholar] [CrossRef]
- Leal-Bertioli, S.C.; Moretzsohn, M.C.; Roberts, P.A.; Ballén-Taborda, C.; Borba, T.C.; Valdisser, P.A.; Vianello, R.P.; Araújo, A.C.G.; Guimarães, P.M.; Bertioli, D.J. Genetic Mapping of Resistance to Meloidogyne arenaria in Arachis stenosperma: A New Source of Nematode Resistance for Peanut. G3 Genes Genomes Genet. 2016, 6, 377–390. [Google Scholar] [CrossRef]
- Guimarães, P.M.; Brasileiro, A.C.M.; Proite, K.; de Araújo, A.C.G.; Leal-Bertioli, S.C.M.; Pic-Taylor, A.; da Silva, F.R.; Morgante, C.V.; Ribeiro, S.D.G.; Bertioli, D.J. A Study of Gene Expression in the Nematode Resistant Wild Peanut Relative, Arachis stenosperma, in Response to Challenge with Meloidogyne arenaria. Trop. Plant Biol. 2010, 3, 183–192. [Google Scholar] [CrossRef]
- Guimaraes, P.M.; Guimaraes, L.A.; Morgante, C.V.; Silva, O.B., Jr.; Araujo, A.C.G.; Martins, A.C.; Saraiva, M.A.; Oliveira, T.N.; Togawa, R.C.; Leal-Bertioli, S.C.; et al. Root Transcriptome Analysis of Wild Peanut Reveals Candidate Genes for Nematode Resistance. PLoS ONE 2015, 10, e0140937. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.P.Z.; Vidigal, B.; Danchin, E.G.; Togawa, R.C.; Leal-Bertioli, S.C.; Bertioli, D.J.; Araujo, A.C.G.; Brasileiro, A.C.M.; Guimaraes, P.M. Comparative root transcriptome of wild Arachis reveals NBS-LRR genes related to nematode resistance. BMC Plant Biol. 2018, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.M.; Agostini-Costa, T.d.S.; Gimenes, M.A.; Silveira, D. Chemical Composition and Biological Activities of Arachis Species. J. Agric. Food Chem. 2011, 59, 4321–4330. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.L.; Polez, V.L.P.; de Souza Viol, L.C.; Pimentel, R.R.; Biscaia, D.; Pinheiro, J.B. Use of Natural and Residual Resources for the Sustainable Management of Phytonematodes: Challenges and Future Trends. In Sustainable Management of Nematodes in Agriculture, Volume 1: Organic Management; Chaudhary, K.K., Meghvansi, M.K., Eds.; Sustainability in Plant and Crop Protection; Springer International Publishing: Cham, Switzerland, 2022; pp. 3–37. [Google Scholar] [CrossRef]
- Rocha, T.L.; Polez, V.L.P.; Evaristo, R.G.S.; Grossi de Sá, M.F.; Da Silva, M.C.M.; Souza, D.S.L.L. Relatório Descritivo de Patente de Invenção. Uso de Composição Nematotóxica de Efeito Sinérgico. PCT Br 2013/000598, 2013. [Google Scholar]
- Gardiano, C.G. Nematicidal Activity of Aqueous Extracts and of Plant Tinctures on Meloidogyne javanica. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2006. Available online: https://locus.ufv.br//handle/123456789/4446 (accessed on 10 November 2023).
- Amaral, D.R.; Oliveira, D.F.; Campos, V.P.; Carvalho, D.A. Effect of some plant extracts on mobility, mortality, egg hatching and pathogenicity of Meloidogyne exigua from coffee plants. Nematol. Bras. 2002, 26, 43–48. [Google Scholar]
- Inomoto, M.M.; Motta, L.C.C.; Beluti, D.B.; Machado, A.C.Z. Reação de seis adubos verdes a Meloidogyne javanica e Pratylenchus brachyurus. Nematol. Bras. 2006, 30, 44. [Google Scholar]
- Elbadri, G.A.; Lee, D.W.; Park, J.C.; Yu, H.B.; Choo, H.Y. Evaluation of various plant extracts for their nematicidal efficacies against juveniles of Meloidogyne incognita. J. Asia-Pac. Entomol. 2008, 11, 99–102. [Google Scholar] [CrossRef]
- Thoden, T.C.; Boppré, M.; Hallmann, J. Effects of pyrrolizidine alkaloids on the performance of plant-parasitic and free-living nematodes. Pest Manag. Sci. 2009, 65, 823–830. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Desmedt, W.; Mangelinckx, S.; Kyndt, T.; Vanholme, B. A Phytochemical Perspective on Plant Defense against Nematodes. Front. Plant Sci. 2020, 11, 602079. Available online: https://www.frontiersin.org/articles/10.3389/fpls.2020.602079 (accessed on 10 November 2023). [CrossRef]
- Bano, S.; Iqbal, E.Y.; Lubna Zik-ur-Rehman, S.; Fayyaz, S.; Faizi, S. Nematicidal activity of flavonoids with structure activity relationship (SAR) studies against root knot nematode Meloidogyne incognita. Eur. J. Plant Pathol. 2020, 157, 299–309. [Google Scholar] [CrossRef]
- Zhang, W.P.; Ruan, W.B.; Deng, Y.Y.; Gao, Y.B. Potential Antagonistic Effects of Nine Natural Fatty Acids Against Meloidogyne incognita. J. Agric. Food Chem. 2012, 60, 11631–11637. [Google Scholar] [CrossRef] [PubMed]
- Khurma, U.R.; Singh, A. Nematicidal potential of seed extracts: In vitro effects on juvenile mortality and egg hatch of juvenile mortality and egg hatch of Meloidogyne incognitaMeloidogyne incognita and and M. javanica. Nematol. Mediterr. 1997, 25, 49–54. [Google Scholar]
- Rosenzweig, C.; Liverman, D. Predicted effects of climate change on agriculture: A comparison of temperate and tropical regions. In Global Climate Change: Implications, Challenges, and Mitigation Measures; The Pennsylvania Academy of Sciences: East Stroudsburg, PA, USA, 1992; pp. 342–361. [Google Scholar]
- Ferris, H.; Zheng, L. Plant Sources of Chinese Herbal Remedies: Effects on Pratylenchus vulnus and Meloidogyne javanica. J. Nematol. 1999, 31, 241–263. [Google Scholar] [PubMed]
- Dawson, B.; Trapp, R.G. Basic & clinical biostatistics. In Basic & Clinical Biostatistics; Lange Medical Books/McGraw-Hill: New York, NY, USA, 2004; p. 438. Available online: https://pesquisa.bvsalud.org/portal/resource/pt/sus-24141 (accessed on 9 December 2023).

| Species Name | Code BRA | Access Number |
|---|---|---|
| Arachis Stenosperma | AM1 | V 10309 |
| Arachis triseminata | AM2 | V 13080 |
| Arachis retusa | AM4 | V 12939 |
| Arachis hassleri | AM5 | Sv 3818 |
| Arachis appressipila | AM9 | V 9060 |
| Arachis douradiana | AM16 | V 14682 |
| Arachis archeri | AM17 | V 7614 |
| Arachis gregoryi | AM18 | V 14957 |
| Arachis duranensis | AM19 | V 14167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, B.; Brauna, C.; Ferreira, P.; Melo, L.; Ferreira, P.; Rocha, T. Prospection of Nematotoxic Aqueous Seeds Extracts Derived from the Preserved Arachis (Fabaceae) Germplasm Bank. Int. J. Plant Biol. 2024, 15, 1-12. https://doi.org/10.3390/ijpb15010001
Nascimento B, Brauna C, Ferreira P, Melo L, Ferreira P, Rocha T. Prospection of Nematotoxic Aqueous Seeds Extracts Derived from the Preserved Arachis (Fabaceae) Germplasm Bank. International Journal of Plant Biology. 2024; 15(1):1-12. https://doi.org/10.3390/ijpb15010001
Chicago/Turabian StyleNascimento, Bruna, Cristiane Brauna, Paula Ferreira, Luis Melo, Paulo Ferreira, and Thales Rocha. 2024. "Prospection of Nematotoxic Aqueous Seeds Extracts Derived from the Preserved Arachis (Fabaceae) Germplasm Bank" International Journal of Plant Biology 15, no. 1: 1-12. https://doi.org/10.3390/ijpb15010001
APA StyleNascimento, B., Brauna, C., Ferreira, P., Melo, L., Ferreira, P., & Rocha, T. (2024). Prospection of Nematotoxic Aqueous Seeds Extracts Derived from the Preserved Arachis (Fabaceae) Germplasm Bank. International Journal of Plant Biology, 15(1), 1-12. https://doi.org/10.3390/ijpb15010001






