Abstract
Having started in the 1930-40s, the industrial development of the Kola North has caused elevated environmental pollution of the area’s water bodies. The pollutants contained in dust emissions, dissolved substances and waste waters require their genotoxicity to be estimated using in vivo assays. This article addresses the effect of pollutants entering the water bodies of the Murmansk region together with mining waste, which leads to a decrease in mitotic activity and an increase in chromosomal abnormalities in the roots of Allium cepa L. The evaluated waters showed an effect of reducing the mitotic index and the appearance of chromosomal aberrations; this may be associated with the presence of compounds in the water, such as mining waste.
1. Introduction
Inland water bodies are of crucial importance for the people inhabiting the Arctic region for they are not only a place where fishing and fish-farming activities can be carried out or a source of drinking, municipal and industrial water, but are also a part of the region’s culture. For the freshwater ecosystems of the Arctic and sub-Arctic, the wastes produced by the enterprises of the metallurgical and power industries are a clear and present danger, since these wastes contain huge amounts of different chemicals including heavy metals. In recent years, the anthropogenic effect on the freshwater ecosystems was aggravated by the anomalous processes in the region’s climatic system [1].
Another important point is that due to the features of atmospheric circulation in the Northern Hemisphere, a lot of pollution is brought from more southern industrial regions. The chemicals thrown into the atmosphere can travel long distances to be slowly accumulated in northern water bodies and their catchment areas [2,3].
The main sources of pollution for the water bodies of the Murmansk region are its apatite-nepheline mines that handle their wastes including waste waters causing freshwater degradation through its irrational use and outdated waste disposal techniques. The wastewater influx and dust emissions of mines and ANOF-I of JSC Apatit, which contain mainly nepheline and apatite, enrich water in Na, K, Ca, P, Al, Sr, F and other elements, which compose the apatite-nepheline ores and products of their processing [4]. These chemical compounds entering water bodies can accumulate in bottom sediments and produce a whole spectrum of hazardous effects on living organisms. In this respect, it has become necessary to carry out in vivo assays estimation of their genotoxicity and cytotoxicity for the complex assessment of their hazardous potential. The plants most often used in such assays include beans (Vicia faba L.), spiderwort (Tradescantia fluminensis Veil.), onion (Allium cepa L.), corn (Zea mays L.), etc. Classic Allium assay provides the detection of cytotoxicity and genotoxicity, and it produced important information to evaluate the mechanisms of action of chromosome aberrations and disturbances in the mitotic cycle [5]. This test is widely used in determining genotoxic and cytotoxic substances found in the water system [6,7,8].
Hence, the objective of the present study was using the Allium test to assess the toxicity of waters in the Khibiny Mountains’ water bodies that are located in the vicinity of industrial objects. The obtained data may be significant for biological monitoring of both natural and artificial water reservoirs of the Murmansk region.
2. Materials and Methods
2.1. Water Sampling
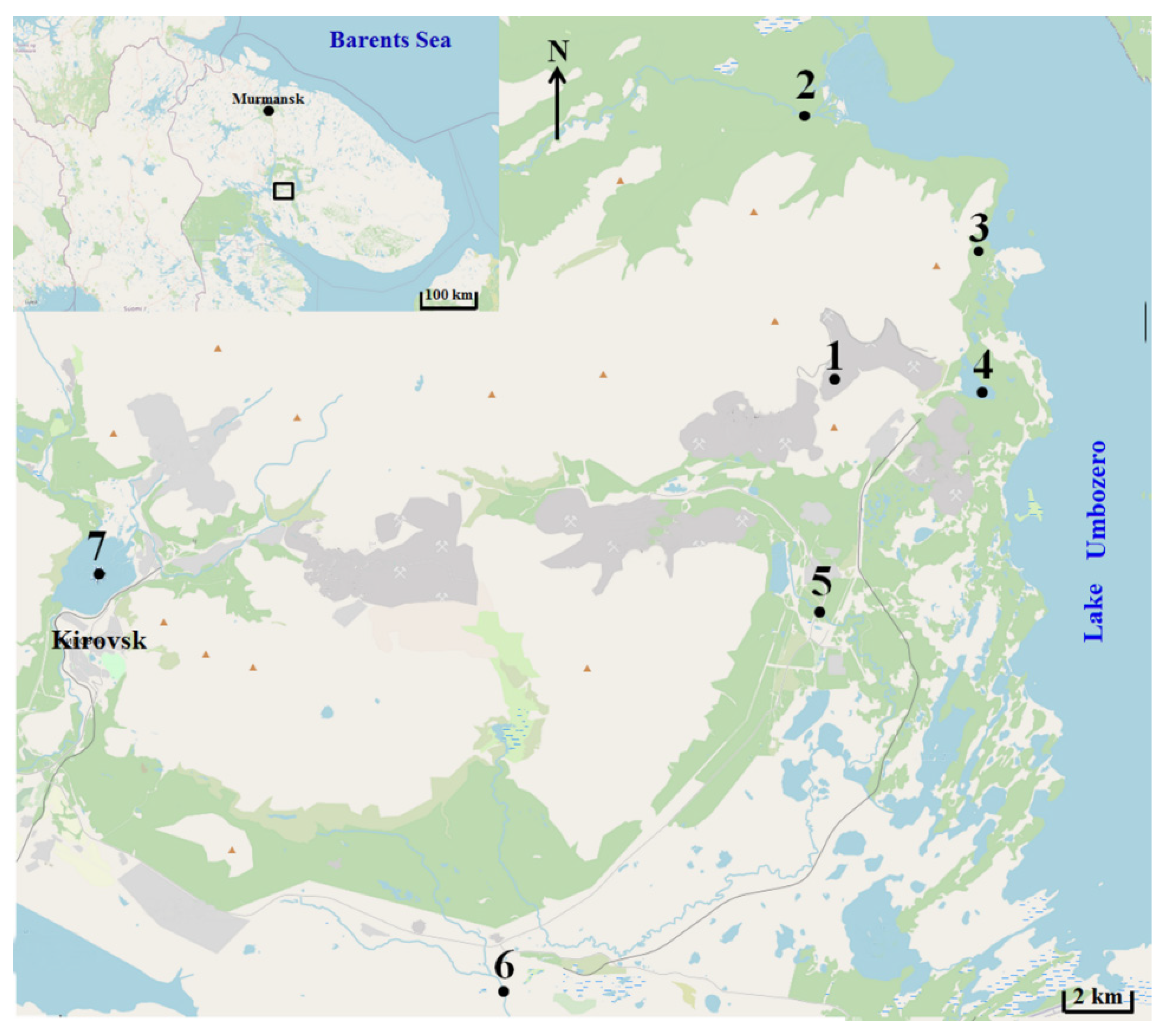
The samples were taken from the Reindeer Creek quarry belonging to a local mining and processing plant (1); the Tul’yok River (2); the Teply Creek (3); the surface waters of the Komarinoe Lake (4); the Vuonnemyok River (5); The Umbolka River (6) and the surface waters of the Bolshoy Vudjavr Lake (7). Water samples were collected by 2-L plastic bathymeters from the surface layer (1 m from the surface), with seasonal sampling by vertical with a step of 5 m up to the bottom in the central deepest part of the water area. The collection site is displayed in Figure 1. The water’s chemical composition was analyzed using professional instrumentation: pH meter 211 (HANNA Instruments, Woonsocket, RI, USA); conductometer 660 (Metrohm, Herisau, Switzerland); KFK-3-01 photometer (ZOMZ, Sergiyev Posad, Russia); HPLC detector (Waters, Milford, MA, USA); Perkin-Elmer 360 spectrometer (PerkinElmer, Waltham, MA, USA); AAS-3300 spectrometer (Thermo Scientific, Waltham, MA, USA) and ELAN 3300 spectrometer (ICP Instruments, Hsinchu, Taiwan). Measurement error control was carried out as indicated in the measurement procedures.
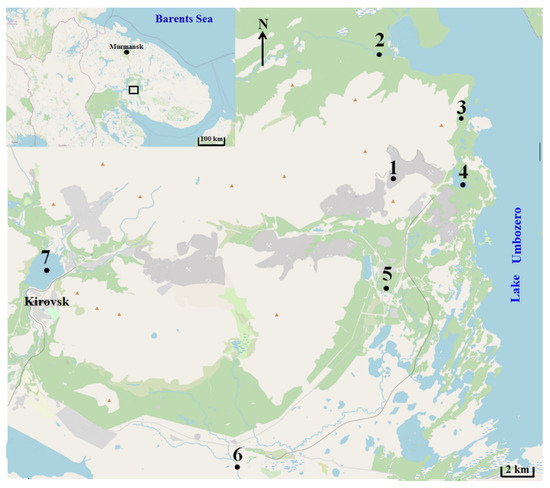
Figure 1.
Sampling collection site map. 1. The Reindeer Creek plant; 2. The Tul’yok River; 3. The Teply Creek; 4. The Komarinoe Lake; 5. The Vuonnemyok River; 6. The Umbolka River; 7. Bolshoy Vudjavr Lake.
2.2. Allium Test
The bulbs of the Stuttgarter Riesen onion (Allium cepa L., 2n = 16) were kept in a cool dark place (+4–5 °C) for 14 days to synchronize their biological functions and mitosis, then they were peeled off the dry scales and visually inspected. For each sample and control, 5 bulbs, whose roots had 4–5 mm size, were selected after a 24-h germination in distilled water. Distilled water was used as a control. The experiment lasted for 5 days. After 5 days, the roots were cut and fixed in Clark’s solution (96% of ethanol + glacial acetic acid in proportion 3:1) for a day and then rinsed three times in 80% ethanol (one hour each time) to be placed in sealable test-tubes for long-time storing. To prepare the microscopic slides, the roots were simultaneously hydrolyzed and colored in ceramic crucibles in 2% acetoorcein solution (PANECO, Moscow, Russia) boiled with a spirit lamp [9]. After cooling down, the roots were left in the dye for 24–28 h at 4 °C. The roots were placed on slides and the terminal root tips (1–2 mm) were cut off and used for further preparation. The slides were prepared from squashing the root meristems with one drop of 45% acetic acid. The number of dividing cells was determined in 1000 examined cells in the field of view, with phase and chromosome aberration marked at ×400 and ×1000 (in immersion oil) magnification of a “Micromed 1, v. 1–20” microscope (MICROMED, Moscow, Russia, 2019). The shooting was performed using a digital Toupcam 2.0 Cmos Camera (TOUPTEC, Hangzhou, China, 2019) equipped with the ToupView software for the 1/2.7” sensor of 1920 × 1080-pixel resolution. In total, more than 40,000 cells were counted. The mitotic index (MI) was calculated as a percentage ratio of the number of all dividing cells to the general number of the cells calculated in a preparation.
2.3. Statistical Analysis
The accumulated data were analyzed using the R programming language in the RStudio software environment. A Shapiro–Wilks test was performed to establish the normality of sampling distribution, and a Bartlett test—to determine variance homogeneity. The optimal sampling size was estimated considering the test power of 80% at p < 0.05. A Tukey test was carried out for multiple comparisons. The differences of total aberrations in the cells of A. cepa root meristem were calculated using the Kruskal–Wallis’s statistic and Dunn’s multiple comparison post hoc test.
3. Results
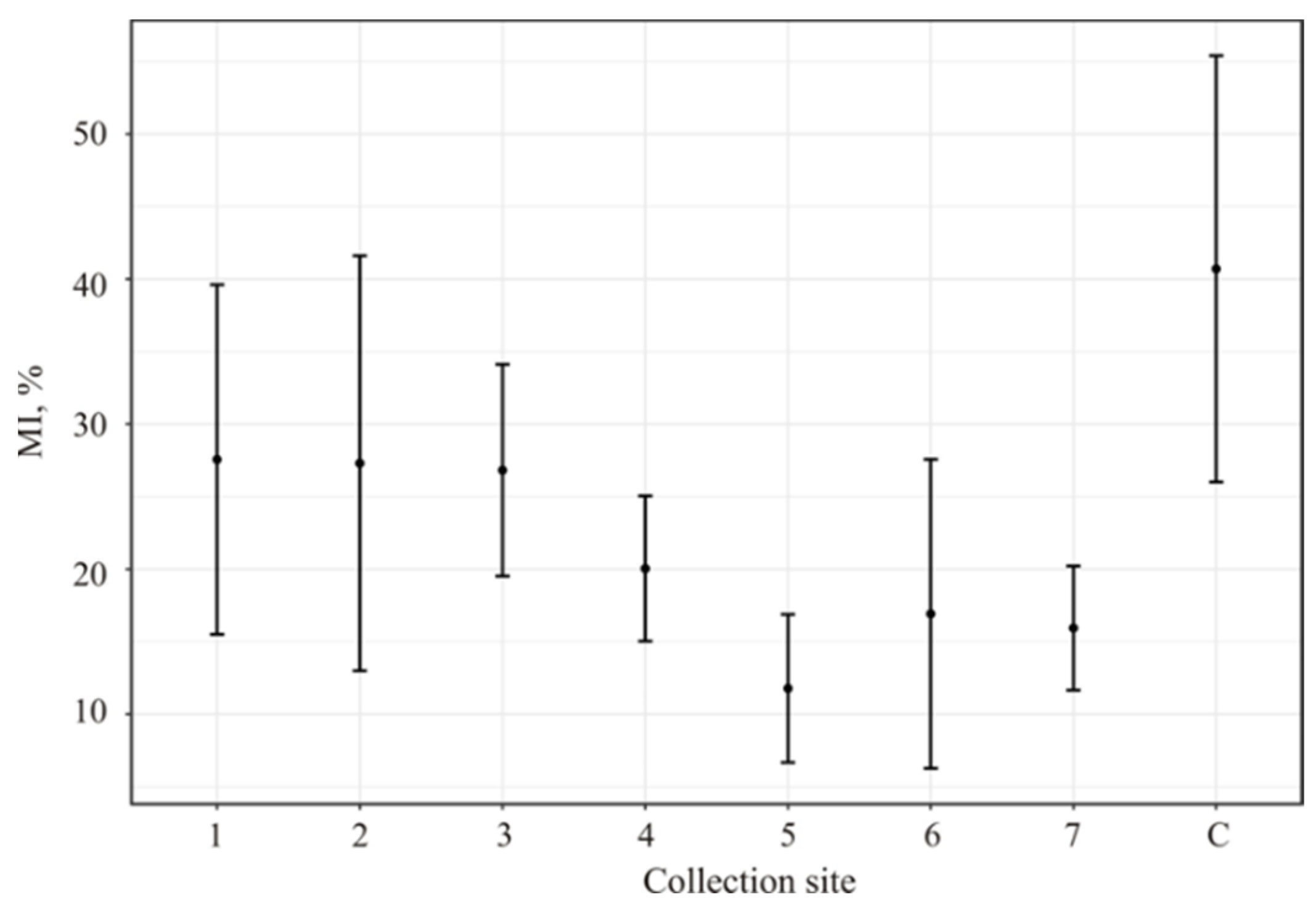
In all investigated collection sites, a significant MI reduction relative to the control was observed (Figure 2).
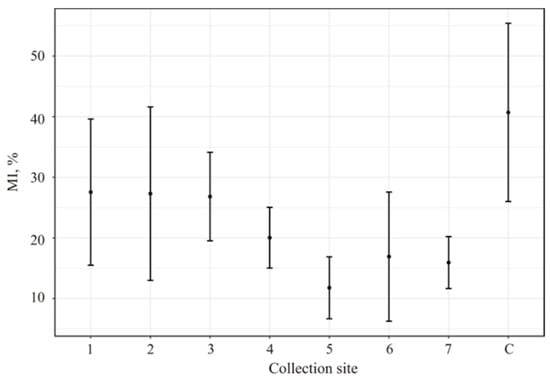
Figure 2.
A. cepa root meristem cell MIs after soaking in the water from investigated water bodies. 1. The Reindeer Creek plant; 2. The Tul’yok River; 3. The Teply Creek; 4. The Komarinoe Lake; 5. The Vuonnemyok River; 6. The Umbolka River; 7. Bolshoy Vudjavr Lake; C-control (distiller water); MIs (%)—mitotic index.
The maximum MI depression was demonstrated by the water from the Vuonnemyok River (collection site 5) that was significantly lower if compared to that from collection site 1–3 whose MI values were at the same level with the statistically insignificant increase if compared to points 4, 6 and 7.
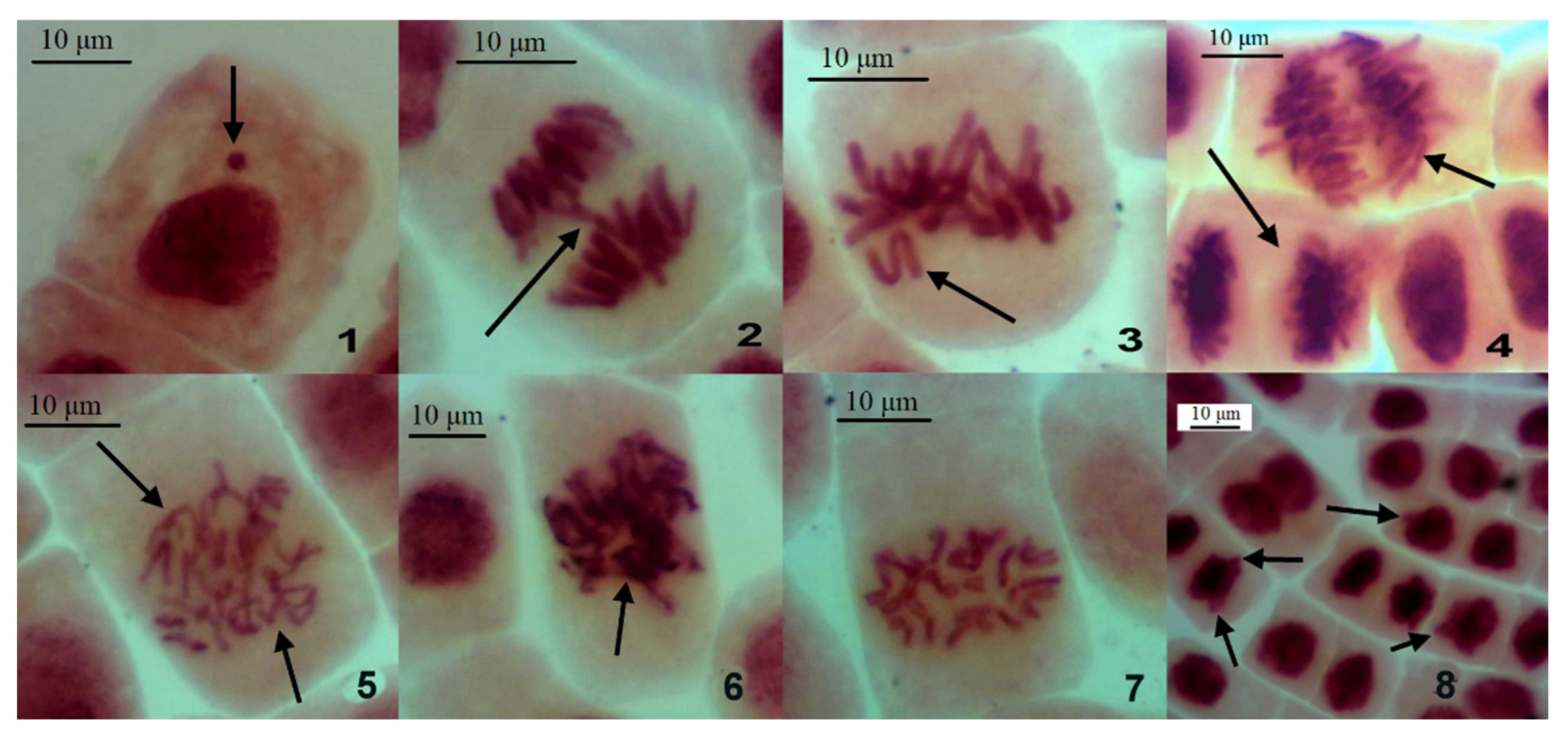
The meristematic cells developed nuclear buds, spindle apparatus abnormality, chromosome sticking, bridges and other distortions (Figure 3).
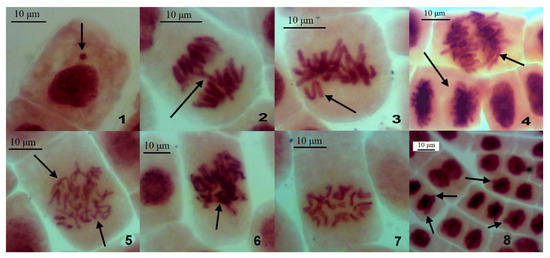
Figure 3.
Chromosomal and other aberrations in the dividing cells of A. cepa root meristem after soaking in the water from investigated water bodies. 1—interphase micronucleus; 2—anaphase bridge; 3—chromosome lagging; 4—changed chromosome number in anaphase (top) and telophase (bottom); 5—spindle apparatus abnormality in anaphase; 6—chromosome sticking in metaphase; 7—c-mitosis; 8—nuclear buds. Acetoorcein dying; magnification ×400.
The water of all investigated water bodies provoked a times increase in the number of abnormal cells, whose maxima were observed in spots 1,4 and 5 (Table 1).

Table 1.
Aberrations in the cells of A. cepa root meristem after soaking in the water from investigated water bodies (by 5000 cells in every sample).
The waters of the Kola North are characterized by the low mineralization (20–30 mg/L) and following major ions arrangement in decreasing order: HCO3− > SO42− > Cl−; Ca+ > Na > Mg2+ > K+. Ions of alkaline metals are supplied in natural waters by the alkaline rocks of the lake catchment of the Khibiny massif. Data on the chemical composition of the water samples of collection sites are presented in the Table 2.

Table 2.
Hydrochemical parameters of water of collection sites.
During the study, an analysis of the interdependence between pollutant concentrations and aberrant cell percentage (MI) was carried out. Cytogenetic analysis demonstrated the Allium test’s high sensitivity to water pollutants since almost all detected chemical parameters had high positive correlations (at p < 0.05) with aberrant cell percentage values (Table 3).

Table 3.
MI and aberrant cell percentage correlation dependencies on pollutant concentrations.
Out of 22 detected chemical characteristics, seven compounds produced a statistically significant mitodepressive effect.
4. Discussion
A feature of the water bodies considered in this paper is their high pH and mineralization levels that are due to the weathering and desalinization of the rock of the Khibiny Mountains that explains the high concentrations of Fe, Al, Cu and Sr in the Reindeer Quarry, Komarinoe Lake and the Vuonnemyok River. The quarry and the Vuonnemyok River accumulate the waters coming from the Koashva Village’s wastewater treatment facility and the drainage waters from the Vostochny Mine. According to perennial observations before and after the mining works began (2011–2019), the Komarinoe Lake contains excessive amounts of NO3−-nitrate ions that came from the nitrogen-containing explosives used to mine nepheline ores (Table 1) [10]. Mineralization, pH value, the fraction of alkali metals and in the ionic composition, the content of nitrogen and phosphorus compounds has significantly increased in the water of the Lake Bolshoy Vudjavr. Detailed hydrochemical and hydrobiological studies of Lake B. Vudjavr have been carried out by scientists from INEP of the Kola Science Centre since 1989. All investigated water bodies experienced significant transformations owing to a change of hydrological and hydrochemical conditions existing at the catchment area in response to the building of the urban territory, influx of municipal sewages, laying of underground and surface communications [11].
The observed chromosomal aberrations and changes in the mitosis of root meristem cells while tested is commonly divided in studies into groups (aneugenic and clastogenic) [12] and related to either DNA rupture and chromosome disintegration or to spindle apparatus abnormality and reduced cytokinesis, respectively. As for the aneugenic effects in our study, the assay often detected chromosome lagging and stickiness, and nuclear buds. The lagging occurred due to the spindle apparatus abnormality caused by cytoskeletal protein inhibition and tubulin polymerization [13]. There are data showing that heavy metals can affect both directly and indirectly the genetic material of cells by destroying the structure of the enzymes participating in DNA repair; damage cell walls and organelles; and disrupt cell division mechanisms through enhanced oxidation stress [14]. The decreased mitotic index treated with metal stress is due to disturbances in the cell cycle or chromatin disfunction induced by metal–DNA interaction which leads to significant reduction of the mitotic index [15,16].
Chromosome stickiness displays the toxicity degree of a chemical component. The stickiness is irreversible and frequently results in cell death. In most cases, it leads to chromosomes forming an amorphic mass (cluster) due to defected non-histone proteins responsible for organizing chromosomes while mitosis and proper chromatid segregation occurs [17]. As for nuclear buds, they occur when chemical compounds interfere with a mitotic cycle. Their formation may be due to the processes following chromosome lagging when such a chromosome is embraced by a nuclear membrane before it joins those located next to a cell pole [18]. Some sources indicate that nuclear buds are a product of polyploidization due to amplification of the genetic material removed from the nucleus but still connected to its membrane. Chromatid sticking leads to such clastogenic effects as bridges in anaphase and telophase. Additionally, the bridges can be a product of the unbalanced translocation or inversion of chromosomal segments [19].
Our study has demonstrated that the industrial waste waters entering the water bodies of the Murmansk region had a negative effect on the mitosis of root meristem cells in A. cepa, causing their cytogenic damage.
Author Contributions
Conceptualization, M.V.S. and D.B.D.; methodology, M.V.S. and D.B.D.; software, M.V.S. and D.B.D.; data curation, M.V.S.; writing—original draft preparation, M.V.S.; writing—review and editing, M.V.S.; visualization, M.V.S. Both authors have read and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out within the frameworks of Research Contracts No. FMEZ-2022-0020 and No. 1021051803677-1 (2022-2024) provided by the Ministry of Science and Higher Education of the Russian Federation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sandimirov, S.S.; Kudryavtseva, L.P.; Dauvalter, M.V.; Denisov, D.B.; Kosova, A.L.; Cherepanov, A.A.; Vandish, O.I.; Valkova, S.A.; Terentiev, P.M.; Koroleva, I.M.; et al. Methods of Ecological Research in Arctic Water Bodies; MGTU Publishing House: Murmansk, Russia, 2018; p. 186. (In Russian) [Google Scholar]
- Vinogradova, A.A.; Kotova, E.I.; Topchaya, V.Y. Atmospheric transfer of heavy metals into the Russian North Europe. Geograf. Prir. Res. 2017, 1, 108–116. (In Russian) [Google Scholar]
- Dauvalter, V.A. Geochemistry of lakes in a zone impacted by an Arctic iron-producing enterprise. Geochem. Int. 2020, 58, 933–946. [Google Scholar] [CrossRef]
- Kashulin, N.A.; Dauvalter, V.A.; Denisov, D.B.; Valkova, S.A.; Vandysh, O.I.; Terentjev, P.M.; Kashulin, A.N. Selected aspects of the current state of freshwater resources in the Murmansk region, Russia. J. Environ. Sci. Health Part A 2017, 52, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, I.A. Allium Test in Enviromental Monitoring and Health; Novas Edições Acadêmicas, OmniScriptum Publishing: Riga, Latvia, 2018; p. 94. [Google Scholar]
- Bolsunovsky, A.Y.; Trofimova, E.A.; Zueva, A.V.; Dementiev, D.V. The first results of using the Allium test in estimating the chemical and radiation toxicity of bottom sediments in the Yenisei River. Dokl. Biol. Sci. 2016, 469, 192–195. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Cabuga, J.C.C.; Abelada, J.J.Z.; Apostado, R.R.Q.; Hernando, B.J.H.; Lador, J.E.C.; Obenza, O.L.P.; Presilda, C.J.R.; Havana, H.C. Allium cepa test: An evaluation of genotoxicity. Proc. Int. Acad. Ecol. Environ. Sci. 2017, 7, 12–19. [Google Scholar]
- Artico, L.L.; Kommling, G.; Migita, N.A.; Menezes, A.P.S. Toxicological effects of surface water exposed to coal contamination on the test system Allium cepa. Water Air Soil Pollut. 2018, 229, 248. [Google Scholar] [CrossRef]
- Medvedeva, M.Y.; Bolsunovsky, A.Y.; Zotina, T.A. Cytogenetic abnormalities in aquatic plant Elodea canadensis in anthropogenic contamination zone of Yenisei River. Contemp. Probl. Ecol. 2014, 7, 422–432. (In Russian) [Google Scholar] [CrossRef]
- Dauvalter, M.V.; Dauvalter, V.A.; Denisov, D.B.; Slukovskii, Z.I. Contamination of a mountain lake by wastewaters of apatite-nepheline production. Proc. Fersman Sci. Sess. GI KSC RAS 2021, 18, 150–154. (In Russian) [Google Scholar] [CrossRef]
- Dauvalter, V.A.; Denisov, D.B.; Slukovskii, Z.I. Impact of wastewaters from apatite–nepheline production on the biogeochemical processes in an arctic mountain lake. Geochem. Int. 2022, 60, 1014–1028. [Google Scholar] [CrossRef]
- Bonciu, E.; Firbas, P.; Fontanetti, C.S.; Wusheng, J.; Karaismailoğlu, M.C.; Liu, D.; Menicucci, F.; Pesnya, D.S.; Popescu, A.; Romanovsky, A.V.; et al. An evaluation for the standardization of the Allium cepa test as cytotoxicity and genotoxicity assay. Caryologia 2018, 71, 191–209. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, A.; Soodan, R.K.; Chahal, V.; Kumar, V.; Katnoria, J.K.; Nagpal, A.K. Allium cepa Root Chromosomal Aberration Assay: A Tool to Assess Genotoxicity of Environmental Contaminants. In Evaluation of Environmental Contaminants and Natural Products: A Human Health Perspective; Bentham Science: Sharjah, United Arab Emirates, 2019; pp. 65–93. [Google Scholar] [CrossRef]
- Thomas, M.; Kozik, V.; Bąk, A.; Barbusiński, K.; Jazowiecka-Rakus, J.; Jampilek, J. Removal of heavy metal ions fromwastewaters: An application of sodium trithiocarbonate and wastewater toxicity assessment. Materials 2021, 14, 655. [Google Scholar] [CrossRef] [PubMed]
- Kopliku, D.; Mesi, A. Assessment of cytotoxic and genotoxic potency of Cr(VI)- Doped river water of Nen-Shkodra Lowland, Albania, on Allium cepa L. J. Environ. Res. Dev. 2013, 7, 1322–1332. [Google Scholar]
- Abubacker, M.N.; Sathya, C. Genotoxic effect of heavy metals Cr, Cu, Pb and Zn using Allium cepa L. Biosci. Biotechnol. Res. Asia 2017, 14, 1181–1186. [Google Scholar] [CrossRef]
- Farizan, A.; Norfatimah, M.Y.; Aili, Z.N.; Lyena, W.Z.A.; Indah, M.A. Use of cytological and molecular biological method for water pollution monitoring. Int. Natl. Symp. Aquat. Environ. Fish. IOP Conf. Ser. Earth Environ. Sci. 2021, 674, 012108. [Google Scholar] [CrossRef]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Surralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Himadri, P.; Sanjay, K. Butylated hydroxytoluene and Butylated hydroxyanisole induced cytogenotoxicity in root cells of Allium cepa L. Heliyon 2021, 7, e07055. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).