Abstract
Plants thrive in dynamic environments requiring adaptive strategies in response to environmental stressors. Furthermore, insect herbivores may be attracted or deterred by the expression of these traits. This study examines growth, physiological, and phytochemical adaptations of maple trees in response to stressors and how these stressors effect herbivore feeding behavior within an agricultural production system. Agricultural systems are unique because plants experience environmental stressors unique to production such as herbicide sprays and girdling. Using four environmental stressors commonly observed in agricultural production (control, mechanical defoliation, chemical defoliation, and girdling), applied to two cultivars of red maple (Acer rubrum, ‘Brandywine’ and ‘Franksred’), this study analyzed differentiation of expressed traits in a production system. Responses varied depending on cultivar and stress treatment but had no effect on insect herbivore behavior. Understanding the ecological interactions within these systems will provide information for better plant production and pest management recommendations.
1. Introduction
Environmental stressors are defined as any environmental conditions (biotic and abiotic) that cause a sharp reduction in fitness [1,2,3]. For plants, environmental stressors may be climatic [4,5], chemical [6,7], physical [8], biological [9,10] or a combination of stress types [11] that reduce plant fitness (i.e., growth and productivity). To minimize reductions of overall fitness, plants may utilize constitutively expressed traits (traits that are always present), or induced traits (responses that are “immune-like”) to counter environmental stressors [10,12,13]. Induced responses are often energetically costly compared to constitutive traits [9,14,15]. To reduce energetic costs, optimization of multiple metabolic pathways may be utilized to induce responses [16,17]. For example, the growth-differentiation balance hypothesis predicts that plants should re-allocate resources away from primary metabolic functions (i.e., growth) to differentiated process (i.e., defense production) [18,19,20]. Alternatively, plants may change resource acquisition strategies, such as increasing photosynthetic capacity, to meet the additional nutrients requirements of induced responses [21,22,23]. The balancing act of plants to minimize the negative effects of induced responses and maximize overall fitness often leads to the expression of a broad arsenal of induced traits [24,25]. To fully understand an induced response, analyses should include plant nutritional quality (e.g., non-structural carbohydrates), physical characteristics (e.g., leaf morphology, trichome density), regrowth capacity, direct chemical defenses (e.g., polyphenols and tannins), and indirect chemical defenses (e.g., volatile organic compounds). Thus, it is more informative to consider induced responses as a suite of traits with synergistic interactions [24,25,26].
1.1. Effects of Induced Responses on Herbivorous Insects
Induced plant-responses transcend all ecological interactions in ecosystems and strongly influence herbivore behavior [27,28]. The plasticity of induced responses changes the magnitude of expressed traits, thereby altering the nutritional quality of plant tissues [29]. Nutritional characteristics of plant tissues, including primary metabolites such as carbohydrates, may positively influence the growth and development of herbivores [30,31]. Contrastingly, anti-nutritional compounds, including secondary metabolites such as polyphenols and tannins (a subset of polyphenols), and induced volatile organic compounds (VOCs) may negatively influence herbivore growth, development, and behavior [32,33,34]. Recent advances in VOC analyses suggest that VOCs emitted in response to stress play a role in host-locating behavior of herbivores [35,36,37]. More specifically, green leaf volatiles [GLV, various C6 compounds such as limonene, (Z)-3-hexene-1-ol, (Z)-3-hexenyl acetate, and DMNT] are commonly emitted compounds by stressed plants [36,38,39,40] that often act as olfactory cues for insect herbivores [28,39,41].
1.2. Targeting Trait Complexes to Identify Stress Signals and Insect Herbivore Responses in an Agro-Ecosystem
We wanted to identify changes in red maple (Acer rubrum) growth, physiology, and phytochemistry in response to common types of injury-induced stress in nursery production. We hypothesized that stressed trees would have increased production of chemical defenses [42,43]. Additionally, to reduce the energetic costs associated with defense production, stressed trees should express changes to physiological processes (i.e., increased photosynthetic capacity and water potential) and physical characteristics (i.e., reduced leaf aspect ratio, leaf area, specific leaf area, trichome density, and growth rates) as resources are allocated to stress responses and away from primary metabolic functions [18,20]. Because pests are commonly attracted to induced stress responses [36,37], we hypothesized that stressed trees would have increased pest pressure (i.e., spider mites, broad mites, aphids and plant leafhoppers). Finally, we hypothesized that stressed trees should also express greater quantities of GLV [i.e., limonene, (Z)-3-hexene-1-ol, (Z)-3-hexenyl acetate, and DMNT] [44,45].
2. Materials and Methods
2.1. Red Maple Propagation and Treatment Applications
We used two sites in middle Tennessee, United States that have similar environmental conditions for replication of this experiment. One site was located at the Tennessee State University Otis L. Floyd Nursery Research Center, McMinnville, Tennessee, United States (NRC, 35.708867° N, −85.743953° W) (Warren Co.), using the ‘Brandywine’ cultivar. The second site was at the Moore Nursery in Irving College, Tennessee, United States (MN, 35.583889° N, 85.713056° W) (Warren Co.), using the ‘Franksred’ cultivar. Red maple cultivars have known lineages, which allow us to control for genotypical differences of expressed phenotypes [46]. Each site had a different cultivar to reproduce common nursery production methods. Mean height of trees at planting was 194.03 ± 45.71 cm, and a mean stem diameter of 23.01 ± 5.07 mm. We analyzed four stress treatments at both sites in a randomized complete block design. The stress treatments were applied in early spring, immediately after tree canopies were completely flushed. The treatments and date applied were as follows: (1) no stress (control; Figure 1a), (2) mechanical defoliation by removing all leaves (defoliated; NRC defoliated on 5 May 2021, MN defoliated 12 May 2021, Figure 1b), (3) chemical defoliation (herbicided; Scythe (Gowan, Yuma, AZ, USA) application at both sites on 11 May, 2021 with a concentration of 266 mL/3.78 L applied to runoff over the entire canopy, Figure 1c), and (4) girdling stress (girdled; a 10 cm portion (approximately 2.5 cm wide) of bark and cambium was removed 7.5 cm above the root crown; NRC girdled on 5 May 2021, MN girdled on 12 May 2021, Figure 1d). Treatments were replicated 20 times for a total of 80 trees at each site.

Figure 1.
Stress treatments applied to red maple (Acer rubrum) cultivars. Photographs are of ‘Frank’s red’ cultivars at the Moore Nursery in Irvine College, TN, USA, but ‘Brandywine’ cultivars at the Otis Floyd Nursery Research Center, McMinnville, TN, USA received the same treatments. (a) control treatment with no stress application. (b) complete mechanical defoliation of tree canopy. (c) complete chemical defoliation with a foliar contact herbicide. (d) girdling of the sapling trunk.
2.2. Analysis of Growth, Canopy, and Leaf Traits
We measured height (of apical meristem) and stem diameter growth as relative growth rate (RGR; final measurement -initial measurement/# of days) as described by [47]. This calculation avoids bias caused by initial plant size by subtracting the initial size from the final sampling size and dividing by the total number of days that elapsed (190 days in this experiment). Initial plant height (cm) and stem diameter (mm) were recorded on the date of treatment application previously mentioned and final height and stem diameter were recorded at the end of the season (18 November 2021). Height of canopy crown (cm; distance between ground and bottom of canopy) and mean canopy diameter (cm; calculated as the mean length of the widest part of the canopy and length perpendicular to the widest length) were measured on 15 September 2021, so canopies were fully flushed, but fall senescence had not begun.
After re-flush of leaves from defoliated and herbicided treatments (14 June 2021), leaf morphology was assessed for each tree. Five matured leaves from different dominant lateral branch tips of each sapling were subsampled, scanned, and analyzed using WinFOLIA™ (Regent Instruments Inc., Quebec City, Quebec, CA, USA) computer image analysis software. Key leaf morphological parameters (described in [48]) were determined as the mean measurement of the five subsamples. Parameters included total leaf area (cm2), leaf perimeter (cm), vertical length (cm), horizontal length (cm), and leaf aspect ratio (maximum leaf breadth/maximum leaf width). Specific leaf area (SLA) was calculated by dividing the total leaf area by the leaf’s dry weight (cm2/g). Trichome density was determined by using a 7 mm hole punch to remove 3 disks from each of the five leaves collected per tree. The trichomes were counted on each disk, then disks were dried and weighed. The mean number of trichomes per dry mass (g) of leaf was recorded as the trichome density (# of trichomes/g) for each sapling.
2.3. Physiological Trait Measurements
Tree physiological characteristics were also measured after re-flush of leaves. All physiological measurements were performed on four randomly selected replications of each treatment (a total of 16 saplings) from each site. For chlorophyll a, chlorophyll b, and total carotenoid (xanthophylls and carotenes) analyses, we collected three mature leaves from each replication. Three 6 mm leaf disks were punched from deveined tissues of each leaf and placed into vials with 50 µL of 2.0 mm beads into 1.5 mL locking Eppendorf tubes and 1 mL of methanol. We bullet blended the leaf tissues into a slurry consistency. Vials were then incubated at 4 °C for 24 h to extract pigment compounds. After extraction, vials were centrifuged at 4000× g rpm for 15 min then 200 µL from each sample were pipetted into a 96 well microplate. Total carotenoid (λ = 470 nm), chlorophyll a (λ = 665.2 nm), and chlorophyll b (λ = 652.4 nm) were determined using the UV-VIS Epoch microplate spectrophotometer (BioTek, Winooski, VT, USA) and conversion equation according to the Lichtenthaler and Buschmann [49] method. From the same saplings, we used a LI-6400XT Portable Photosynthesis System (LI-COR Biosciences, Lincoln, NE, USA) to measure photosynthetic rate (µmol m−2 s−1) and stomatal conductance (mol−2 s−1). The measurements were analyzed under ambient conditions (20–25 °C) with 400 µmol CO2 mol−1 CO2 concentration, a fixed flow rate (set at 300 µmol s−1) and ambient PAR with an average 1000 m−2 s−1 PPFD. For consistency of environmental variables, we conducted gas exchange measurements between 0800 and 1200 over a period of two days (14 and 15 June 2021). We used a Scholander water pressure chamber (Model 600 Classic Pressure Chamber 40 Bar, PMS Instrument, Albany, OR, USA) to measure water potential. Plant water potential is dynamic throughout the day [50,51]. Measurements were performed pre-dawn as a more reliable indicator of plant water status because the plant is assumed to be in equilibrium with soil water potential at this time [51,52] and mid-day to detect mid-day water stress [50].
2.4. Plant Phytochemistry
Plant foliar phytochemistry was analyzed by collecting five mature leaves from each sapling. We collected leaf samples after reflush (14 June 2021) and at the end of the season (30 September 2021) to observe immediate and delayed induced responses. For both collection dates, leaves were immediately oven-dried at 65 °C for 48 h, ground using a 2 mm mesh on a Wiley® Mill (product no. 3375P76, Thomas Scientific, Swedesboro, NJ, USA) and homogenized for each tree. Certain phenolic compounds are depleted using this method [52,53,54]. However, we were interested in relative concentrations and not absolute concentrations [55,56]. Polyphenols and tannins were extracted from the dried tissues using a 70% acetone solvent [57,58]. Polyphenol concentrations were analyzed using the 96-well modified Prussian blue assay for total phenols [57]. Total tannin concentrations were quantified using the radial diffusion assay [57]. There are no unique concentrations for polyphenols or tannins, so we standardized against gallic acid (gallic acid equivalents, G.A.E.) and tannic acid (tannic acid equivalents, T.A.E.), respectively [57]. Non-structural carbohydrates from foliar tissues were analyzed using the Fournier [59] method with a modification by Tomlinson et al. [60] that uses a phenol-sulfuric acid solvent for a colorimetric reaction with sugars and starches.
Green leaf volatiles were collected from 4 replicates of each treatment for each cultivar (‘Brandywine’ on 14 June 2021 and ‘Franksred’ 15 June 2021). A 15 cm branch tip was clipped from each tree. The clipped ends of the samples were placed in a 30 mL centrifuge tube filled with water to keep branch tips from desiccating during volatile collections. The branch tips with centrifuge tubes were each set in their own “collecting chamber”. The collection chambers consisted of cylindrical glass jars (custom made by Sigma Scientific, LLC, Micanopy, FL, USA) that were 31.5 cm tall with a 10 cm diameter. Each chamber had an opening at the top where air could flow in through a carbon filter to prevent external volatile contamination, and an opening at the bottom with a SuperQ filter which absorbed emitted volatiles. Volatile organic compound profiles were collected for three hours/branch sample using a pull rate of 0.5 ± 0.05 L/min. After collection of VOCs, leaves were counted from each sample, dried, weighed, and averaged to normalize emission rates per sample. The filters containing the VOCs were eluted with 250 µL of pentane into vial inserts that were placed in 2 mL glass vials. Elution vials were sealed with Teflon tape and stored in a −18 °C freezer until analyses could be performed. Eluted samples were analyzed by gas chromatography using a Shimadzu GCMS-QP2010 gas chromatograph mass spectrometer (Shimadzu Corp, Kyoto, Kyoto, Japan). One microliter of the extracts was injected into a splitless injector at 220 °C. The DB-1 column (30 m × 0.25 mm i.d. × 0.1 μm film thickness; Agilent Technologies, Santa Clara, CA, USA) was kept at 50 °C for 5 min, followed by a temperature ramp of 15 °C/min to 180 °C a and a final temperature ramp of 20 °C/min to 280 °C. Helium carrier gas flow rate was 1 mL/min (constant flow) and the transfer line temperature was 260 °C. The EI mode ion source temperature was 230 °C and 70 eV. Major plant volatiles were identified by matching compound mass spectra to the reference spectra library (NIST08) and compared to analytical standards. Green leaf volatiles were compared between samples by using the area % of peaks of each compound (i.e., limonene, (Z)-3-hexene-1-ol, (Z)-3-hexenyl acetate, and DMNT).
2.5. Quantification of Common Nursery Pests
We collected two 30.0 cm branch tips from each tree, once a month in June, July, and August. Branch tips were placed in a zip lock bag and stored at 4 °C until insect counts could be conducted. For each tree, we totaled the number of adult spider mites (Trombidiformes: Tetranychidae), broad mites (Trombidiformes: Tarsonemidae), aphids (Hemiptera: Aphididae), and thrips (Thysanoptera) counted and summed from the two branch tips collected each month. We measured plant leaf hopper damage (Hemiptera: Cicadellidae) in August as the percentage of branch tips with characteristic ‘Hopperburn’ (i.e., browning and curling of leaf tips, [61]).
2.6. Statistical Analysis
To analyze induced responses of maples to stress treatments, we used multivariate analysis of variance (MANOVA) for multiple dependent variables to minimize type I statistical error with the dplyr package [62] in R analytical software [63]. Transformations were made as necessary to meet all assumptions of normality and heteroscedasticity [64]. Due to differences in sampling techniques, we created several different models. The first model included variables growth (height and stem diameter), canopy height, canopy width, and leaf morphological traits (total leaf area, leaf perimeter, vertical length, horizontal length, leaf aspect ratio, and SLA) as the dependent variables, treatment (control, defoliated, girdled, or herbicided) and cultivar (‘Brandywine’ or ‘Franksred’) as the independent variables. We did not include site (MN or NRC) because site was confounded with cultivar type. The second model included variables that were subsampled: physiological changes (total carotenoid, chlorophyll a, and chlorophyll b concentrations, photosynthetic rate, stomatal conductance, and pre-dawn/mid-day water potential measurements) with the same independent variables as the first model. The third model included phytochemical traits (June and September measurements of polyphenol, tannin, and non-structural carbohydrate concentrations) as the dependent variables and with the same independent variables as the first model. Graphs were made using the ggplot2 package [65]. The final model included the GLV area % of peaks for each compound [limonene, (Z)-3-hexene-1-ol, (Z)-3-hexenyl acetate, and DMNT] as the dependent variables and the same independent variables as the first model.
Pearson correlations were performed between leaf morphological and phytochemical traits (using June measurements as this is when pest populations start to grow) and count data (spider mites, broad mites, and aphids) and % damage (leaf hopper burn). Phytochemical traits were categorized as nutritional (i.e., non-structural carbohydrates) and antinutritional (i.e., polyphenols and tannins).
3. Results
3.1. Growth, Canopy and Leaf Traits
Growth, canopy characteristics, and leaf morphological traits were significantly different among treatments (MANOVA: Wilk’s λ = 0.56, F(12, 2307) = 13.66, p < 0.001, Table 1) and between cultivars (MANOVA: Wilk’s λ = 0.36, F(4, 767) = 109.87, p < 0.001, Table 1). There was an interactive effect of treatment and cultivar on response variables (MANOVA: Wilk’s λ = 0.97, F(12, 2307) = 1.11, p = 0.049). Overall, ‘Brandywine’ cultivars grew much faster and had larger canopies than ‘Franksred’ cultivars (ANOVA: height RGR F = 50.94, p < 0.001, canopy height: F = 192.85, p < 0.001, canopy width: F = 12.95, p = 0.001, Table 2, Figure 2). RGR was reduced in defoliated treatments compared to controls in ‘Brandywine’ cultivars (post hoc TukeyHSD: ‘Brandywine’ p < 0.001, Figure 2a). However, ‘Brandywine’ treatments grew significantly faster than ‘Franksred” regardless of stress (ANOVA: F = 5.37, p = 0.002, Table 2, Figure 2a,b). Trichome density was greater in defoliated and herbicided treatments for both cultivars (post hoc TukeyHSD: ‘Brandywine’ p = 0.04, p = 0.021, ‘Franksred’ p = 0.031, p = 0.012), but was not different between cultivars (ANOVA: F = 0.38, p = 0.673, Table 2). ‘Brandywine’ cultivars had a larger leaf area than ‘Franksred’ cultivars (ANOVA: F = 440.28, p < 0.001, Table 2). However, control treatments had greater leaf area than defoliated treatments in both cultivars (post hoc TukeyHSD: ‘Brandywine’ p < 0.001, ‘Franksred’ p = 0.013). ‘Franksred’ cultivars had a greater specific leaf area in control treatments compared to defoliated and herbicided treatments (post hoc TukeyHSD: p < 0.001, p = 0.026, respectively).

Table 1.
MANOVA results testing the effect of treatment (control, defoliated, herbicided, and girdled) on responses of two red maple (Acer rubrum) cultivars (‘Brandywine’ and Franksred’). Significant values are in bold.

Table 2.
ANOVA results testing the effect of treatment (control, defoliated, herbicided, and girdled) on responses of two red maple (Acer rubrum) cultivars (‘Brandywine’ and Franksred’). Significant values are in bold.
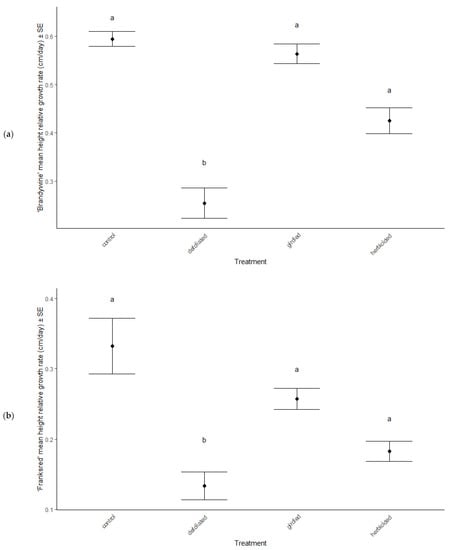
Figure 2.
Relative growth rate (RGR in cm/day) of red maple (Acer rubrum) cultivars (a) ‘Brandywine’ and (b) ‘Franksred’ in response to stress treatments. Different letters represent statistical differences between treatments according to a post hoc Tukey HSD analysis (p < 0.05).
3.2. Physiological Traits
Physiological characteristics were affected by the stress treatments (MANOVA: Wilk’s λ = 0.70, F(15, 114) = 2.29, p = 0.007), cultivar type (MANOVA: Wilk’s λ = 0.62, F(5, 36) = 11.81, p < 0.001), and the interaction between treatment and cultivar (MANOVA: Wilk’s λ = 0.66, F(15, 114) = 2.56, p = 0.012). Chlorophyll a decreased in herbicided treatments of the ‘Brandywine’ cultivars (ANOVA: F = 8.61, p < 0.001 Table 2, Figure 3a), but there were no differences between treatments in ‘Franksred’ cultivars (post hoc TukeyHSD p > 0.05, Table 2, Figure 3b) or in other pigments (post hoc TukeyHSD chlorophyll b: p > 0.05 or carotenoids: p > 0.05, Table 2). There were no changes to photosynthetic rates or stomatal conductance in response to stress treatments (ANOVA: F = 1.50, p = 0.230 and F = 0.1.28, p = 0.0.290, respectively, Table 2), but ‘Franksred’ cultivars did express a higher conductance rate compared to ‘Brandywine’ cultivars (ANOVA: F = 19.34, p < 0.001, Table 2, Figure 3a,b). Trees were not water-stressed pre-dawn (ANOVA: F = 0.072, p = 0.974), but became more water-stressed throughout the day (ANOVA: F = 31.64, p < 0.001, Table 2, Figure 4). Stressed trees became even more water-stressed midday compared to controls (post hoc TukeyHSD: all p < 0.05, Figure 4b,d).
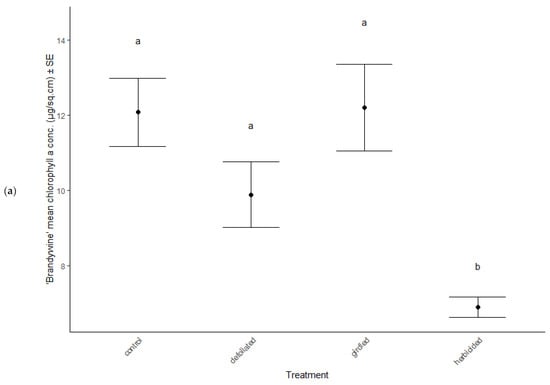
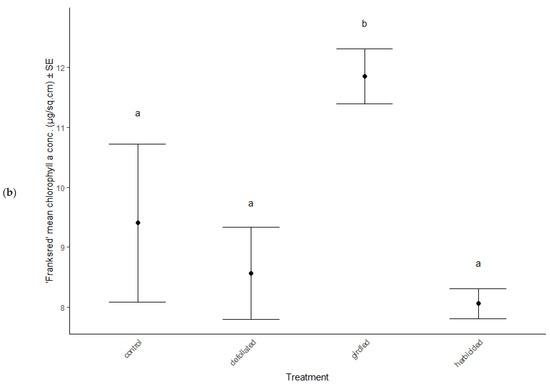
Figure 3.
Changes in chlorophyll a concentrations in response to stress treatments in red maple (Acer rubrum) cultivars (a) ‘Brandywine’ and (b) ‘Franksred’. Different letters represent statistical differences between treatments according to a post hoc Tukey HSD analysis.
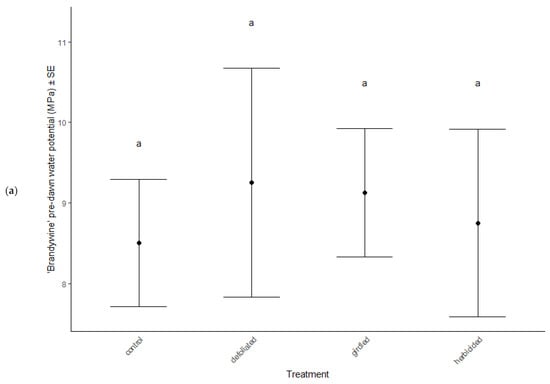
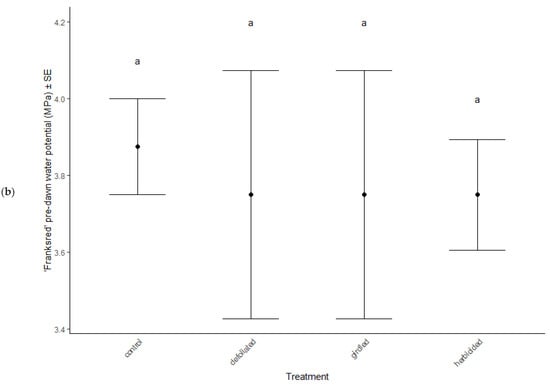
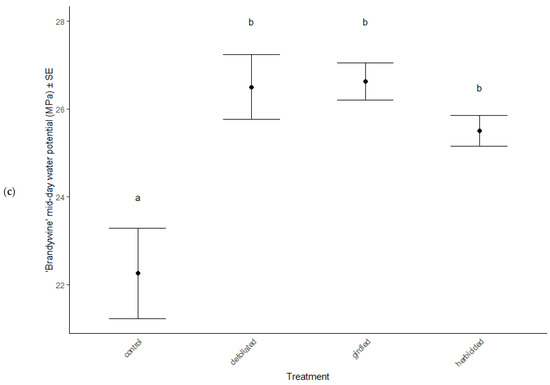
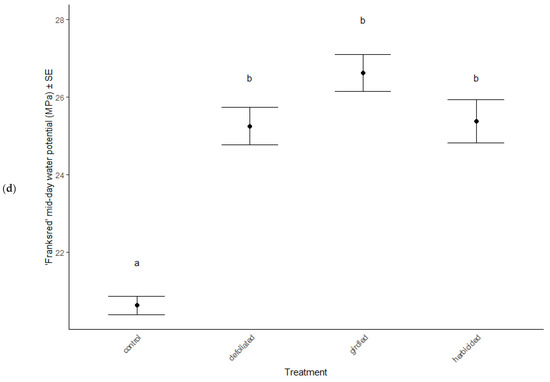
Figure 4.
Water potential (MPa) of control and stressed treatments of ‘Brandywine’ (a) pre-dawn and (b) mid-day, and ‘Franksred’ (c) pre-dawn and (d) mid-day cultivars of red maples (Acer rubrum). Different letters represent statistical differences between treatments according to a post hoc Tukey HSD analysis.
3.3. Plant Phytochemistry
Phytochemistry was significantly different among the stress treatments (MANOVA: Wilk’s λ = 1.10, F(13, 405) = 13.10, p < 0.001), between cultivars (MANOVA: Wilk’s λ = 0.45, F(6, 133) = 18.31, p < 0.001) and in the interaction between treatment and cultivar (MANOVA: Wilk’s λ = 0.33, F(18, 405) = 2.82, p < 0.001). In June, total polyphenol concentrations remained consistent across treatments (ANOVA: F = 1.70, p = 0.170) and cultivars (ANOVA: F = 0.01, p = 0.989), but total tannin concentrations significantly decreased in defoliated and herbicided treatments compared to controls of ‘Brandywine’ cultivars (post hoc TukeyHSD: p = 0.032 and p < 0.001, respectively, Figure 5a). September polyphenol concentrations also remained the same across treatments (ANOVA: F = 1.23, p = 0.384, Table 2) and cultivars (ANOVA: F = 3.58, p = 0.061, Table 2), but total tannin concentrations were elevated in defoliated and herbicided treatments compared to controls of ‘Brandywine’ cultivars (post hoc TukeyHSD: p < 0.001, p < 0.001, respectively, Figure 5b).
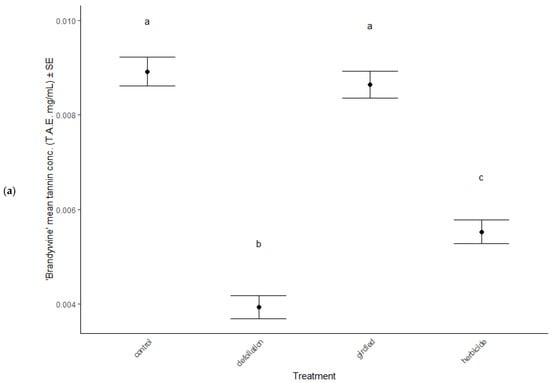
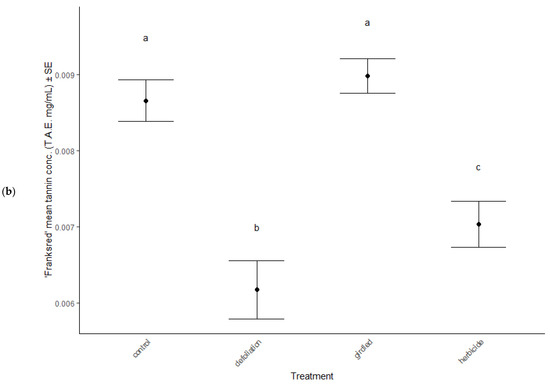
Figure 5.
Total tannin concentrations in foliage of stress treatments in June, after reflush of defoliated and herbicided treatments. (a) ‘Brandywine’ and (b) ‘Franksred’ cultivars of red maples (Acer rubrum). T.A.E. = tannic acid equivalents (see methods section for details). Different letters represent statistical differences between treatments according to a post hoc Tukey HSD analysis.
In June, total non-structural carbohydrate concentrations were elevated in defoliated and herbicided treatments compared to controls of both cultivars (post hoc TukeyHSD: all p < 0.001, Figure 6a,b), but all treatments had similar concentrations of TNC by the end of the season, in September (ANOVA: F = 0.85, p = 0.471, Table 2).
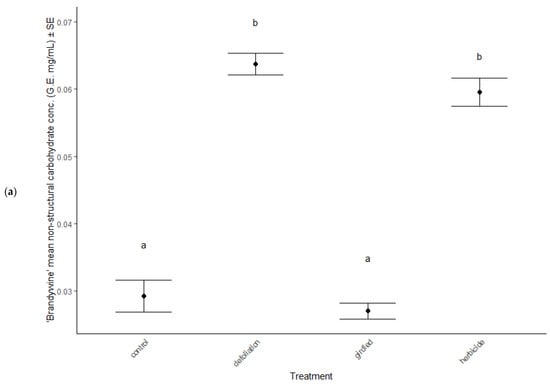
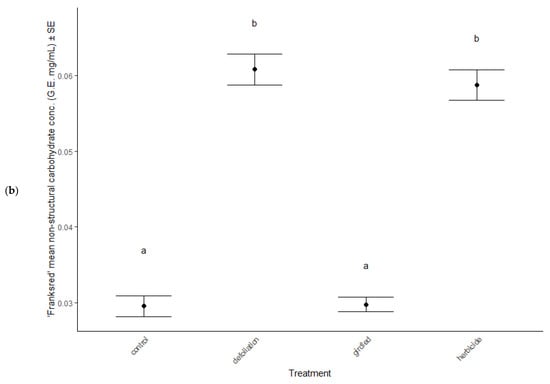
Figure 6.
Total non-structural carbohydrates (combined sugars and starches) in foliage of stress treatments in June, after reflush of defoliated and herbicided treatments. (a) ‘Brandywine’ and (b) ‘Franksred’ cultivars of red maples (Acer rubrum). G.E. = glucose equivalents (see methods for details). Different letters represent statistical differences between treatments according to a post hoc Tukey HSD analysis.
3.4. Correlations between Common Nursery Pests and induced Responses
Pest populations were not influenced by changes to foliar nutritional and antinutritional constituents due to stress treatments (see Table 3). However, aphid populations were smaller on leaves with greater trichome density (r = −0.57, p = 0.039, Table 3).

Table 3.
Pearson correlations (r) testing for relationships between leaf morphological and phytochemical traits. Significant values are in bold.
4. Discussion
Environmental stress influences induced responses of plants, including changes in developmental, physiological processes, and biochemical traits [66,67]. We found significant changes in red maple responses to different types of stress. A potential mechanism that is driving these induced responses is the expression of multiple phenotypes from a single genotype—or phenotypic plasticity [68,69]. Alternatively, environment × genotype interactions (adaptive phenotypic plasticity) may also result in multiple phenotypic expressions from a single genotype (sensu [70,71]). We used two different cultivars of red maple to explore possible genotypic limitations. However, the cultivar type was confounded by possible changes in environment between the two sites. Site limitations restrains our ability to make determinate conclusions about differences in cultivar response, but there are some overall recognizable patterns that we will discuss for further exploration.
4.1. Phenotypic Plasticity in Expression of Stress-Induced Responses
Research interests have successfully illustrated phenotypic plasticity using the concept of a reaction norm, which is a representation of trait expression in response to different environments or environmental pressures [72,73]. Different genotypes may express variation in reaction norms in response to the same environmental stress [74]. We found that water stress increased in response to our stress treatments in both cultivars. Pre-dawn measurements of water potential showed no sign of stress (Figure 4a,b). This is when plants should be experiencing the least amount of water stress because the soil and plant water potential are at equilibrium [50,51]. As temperatures warm throughout the day, we anticipate additional water stress. Interestingly, we found that the mid-day water potential measurements in treatments that had been defoliated, girdled, or herbicided suffered greater water stress than the control treatments for both sites. This suggests a confounding effect of daily water stress and additional environmental stressors [75]. Many other plant species have adapted physiological mechanisms to tolerate water stress such as reducing stomatal conductance, which lowers CO2 availability, resulting in reduced photosynthetic capabilities [75,76]. However, we did not observe any changes in stomatal conductance or photosynthetic capacity in response to stress treatment in either cultivar (Table 2). Similarly, Li et al. [76] found that several species of trees in different environmental conditions showed high plasticity in response to stress, but that stomatal conductance showed little variation. Despite the additional stress treatments we applied, both cultivars were able to compensate for changes in water potential without compromising photosynthetic capacity at two separate sites. This suggests that the adaptation to tolerate additional water stress expressed by both cultivars may result from phenotypic plasticity and not necessarily adaptive phenotypic plasticity to specific environments [71,77,78].
Further evidence of plastic traits expressed in response to stress are the non-structural carbohydrate (TNC) patterns observed (Figure 6a,b). Defoliated and herbicided treatments from both cultivars responded with elevated TNC concentrations. TNC concentrations in trees are commonly found to display high environmental and genetic plasticity [79]. Either both sites have very similar environmental conditions, or the elevated TNC concentrations in defoliated and herbicided treatments must be a plastic inducible trait.
Contrastingly, maple cultivars showed differentiated responses in growth and tannin production. Differentiation of expressed traits, combined with selective pressures dictate the evolutionary trajectory of induced responses, and may result in increased plasticity for a specific trait [80]. ‘Brandywine’ cultivar responses to growth and tannin production support the growth-differentiation balance hypothesis. In defoliated and herbicided treatments, ‘Brandywine’ individuals expressed an increase in tannin production, but an overall reduction in growth rate. According to the growth-differentiation balance hypothesis, plants should express a trade-off between growth and differentiated functions such as tannin production [18,20]. Perkovich and Ward [20] suggested that induced expression of growth-defense trade-offs is likely a result of adaptations to herbivory. However, we found differential expression between the two cultivars of maple in this experiment, and trade-offs are often scale-dependent [25]. Alternatively, differences in environmental conditions may result in differential resource acquisition and allocation which masks the presence of a trade-off [21,81]. It may be that the MN (‘Franksred’ cultivars) environment allows for greater resource acquisition, therefore no trade-off between growth and defense is observed. Unfortunately, we are unable to disentangle differences between site and cultivar in our study.
Many studies have suggested changes in the specific GLV compounds we analyzed (e.g., [39,40,82]), but we did not detect any differences between the treatments. A meta-analysis by Ameye et al. [39] found that wounding and stress increased the concentrations of GLV emitted in wooded plants. The Ameye et al. [39] meta-analysis also showed that herbivory increased GLV emissions. The interaction between stress treatment and the intensity of pest damage could mask changes in GLV emissions. That is, in our study, we only evaluated population densities and not feeding intensity. Insect pests often alter consumption rates depending on plant-host quality [31,83,84]. Increases in plant defenses and decreases in nutritional constituents of stressed plants could have reduced feeding so that feeding intensity was greater in control treatments, increasing GLV emissions at the same rate as stress in the in the treatments. The lack of changes to GLV emissions is an anomaly and more research is needed to disentangle the effects of stress, herbivory, and the interaction between stress and herbivory on GLV emissions.
4.2. Pest Correlations with Maple Characteristics (or Lack of)
Interestingly, we observed few correlations between leaf morphological or phytochemical characteristics. The only observed correlation was a negative association between aphid populations and leaf trichome density. Trichome density has been shown to be an inducible defense in several plant species [85,86], including maples [87]. We did not identify the species of aphids, but many aphids suffer negative consequences associated with trichomes [88,89]. The relationship between trichomes as a defense mechanism and their effectiveness on paid attacks is still fully understood [89]. However, we did not find an increase in trichome density in response to stress treatments, suggesting that trichome density is most likely a constitutively expressed trait, and does not necessarily act as a defense or stress response in maples. In regard to pest population correlations with defensive chemical constituents, several studies suggest that plant express high genetic variation for constitutive and induced resistance that is heterogenous across individuals [90]. The heterogeneity of defenses may have been lost when we homogenized samples and pests may be responding in spatially distributed patterns which we did not analyze [91,92].
Author Contributions
Conceptualization A.W. and K.A.; methodology, C.P., A.W., and K.A.; formal analysis, C.P.; data curation, C.P. and G.D.; writing—original draft preparation, C.P.; writing—review and editing, A.W. and K.A.; funding acquisition, A.W. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Specialty Crop Research Initiative, grant number 2020-51181-32199, from the USDA National Institute of Food and Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request. The data presented in this study (including the R code for analysis) are available upon request from the corresponding author.
Acknowledgments
The authors would like to thank Paul O’Neal and Garrett Roper for technical support. Also, thanks to Moore from Moore Nurseries and the Tennessee State University Otis L. Floyd Nursery Research Center for providing lands for this experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoffman, A.A.; Parsons, P.A. Evolutionary Genetics and Environmental Stress; Oxford University Press: Oxford, UK, 1991. [Google Scholar]
- Hoffman, A.A.; Hercus, M.J. Enviromental stress as an evolutionary force. Bioscience 2000, 50, 217–226. [Google Scholar] [CrossRef]
- He, Q.; Bertness, M.D.; Altieri, A.H. Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 2013, 16, 695–709. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Guzman-Puyol, S.; Benitez, J.J.; Anthanassiou, A.; Heredia, A.; Dominguez, E. Plant cuticle under global climate change: Biophysical implications. Glob. Chang. Biol. 2018, 24, 2749–2751. [Google Scholar] [CrossRef]
- Onyekachi, O.G.; Boniface, O.O.; Gemlack, N.F.; Nicholas, N. The effect of climate change on abiotic plant stress: A review. Abiotic Biot. Stress Plants 2019, 17, e82681. [Google Scholar]
- Dutta, S.; Mitra, M.; Agrawal, P.; Mahapatra, K.; De, S.; Sett, U.; Roy, S. Oxidative and genotoxic damages in plants in responses to heavy metal stress and maintenance of genome stability. Plant Signal. Behav. 2018, 13, e1460048. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Kumari, P.; Yadav, S.; Brestic, M.; Rastogi, A. Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 2019, 2, 739–752. [Google Scholar] [CrossRef]
- Vazquez-Hernandez, M.C.; Parola-Contreras, I.; Montoya-Gomez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-Gonzalez, R.G. Eustressors: Chemical and physical stress factors to enhance vegetables production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Cipollini, D.; Purrington, C.B.; Bergelson, J.B. Costs of induced responses in plants. Basic Appl. Ecol. 2003, 4, 79–89. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Hastings, A.P. Trade-offs constrain the evolution of an inducible defense within but not between plant species. Ecology 2019, 100, e02857. [Google Scholar] [CrossRef]
- Castagneyrol, B.; Jactel, H.; Moreira, X. Anti-herbivore defences and insect herbivory: Interactive effects of drought and tree neighbors. J. Ecol. 2018, 106, 2043–2057. [Google Scholar] [CrossRef]
- Karban, R.; Myers, J.H. Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 1989, 20, 31–348. [Google Scholar] [CrossRef]
- Haukioja, E. Induction of defenses in trees. Annu. Rev. Entomol. 1991, 36, 25–42. [Google Scholar] [CrossRef]
- Harvell, D.C. The ecology and evolution of inducible defenses. Q. Rev. Biol. 1990, 65, 323–340. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Arntz, A.M.; Berenbaum, M.R. Physiological price of an induced chemical defense: Photosynthesis, respiration, biosynthesis, and growth. Oecologia 1997, 109, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J. The cost of plant chemical defense against herbivory: A biochemical perspective. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 105–173. [Google Scholar]
- Neilson, E.H.; Goodjer, J.Q.; Woodrow, I.E.; Moller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef]
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend? Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Zust, T.; Agrawal, A.A. Trade-offs between plant growth and defense against insect herbivory: An emerging mechanistic synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef] [PubMed]
- Perkovich, C.; Ward, D. Herbivore-induced defenses are not under phylogenetic constraints in the genus Quercus (oak): Phylogenetic patterns of growth, defense, and storage. Ecol. Evol. 2021, 11, 5187–5203. [Google Scholar] [CrossRef]
- van Noordwijk, A.J.; de Jong, G. Acquisition and allocation of resources: Their influence on variation in life history tactics. Am. Nat. 1986, 128, 137–142. [Google Scholar] [CrossRef]
- Woolery, P.O.; Jacobs, D.F. Photosynthetic assimilation and carbohydrate reallocation of Quercus rubra seedlings in response to simulated herbivory. Ann. For. Sci. 2011, 68, 617–624. [Google Scholar] [CrossRef][Green Version]
- Perkovich, C.; Ward, D. Aboveground herbivory causes belowground changes in twelve oak Quercus species: A phylogenetic analysis of root biomass and non-structural carbohydrate storage. Oikos 2021, 130, 1797–1812. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Fishbein, M. Plant defense syndromes. Ecology 2006, 87, s132–s149. [Google Scholar] [CrossRef]
- Agrawal, A.A. A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology. Ecology 2020, 10, e02924. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, M.R.; Nitao, J.K.; Zangerl, A.R. Chemical barriers to adaptation by a specialist herbivore. Oecologia 1991, 80, 501–506. [Google Scholar] [CrossRef]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant defense and insect adaptation with reference to secondary metabolites. In Co-Evolution of Secondary Metabolites. Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 795–822. [Google Scholar]
- Zhou, S.; Jander, G. Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef]
- Li, Y.; Ling, L.; Xia, D.; Ji, Y.; Wang, J.; Li, C.; Meng, Y.; Fang, X.; Chen, Y. A comparative study of phenotypic plasticity of seven urban tree species in two contrasting environments. Pol. J. Environ. Sci. 2020, 30, 739–750. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Nutritional ecology and foraging theory. Curr. Opin. Insect Sci. 2018, 27, 38–45. [Google Scholar] [CrossRef]
- Perkovich, C.; Ward, D. Protein:carbohydrate ratios in the diet of gypsy moth Lymantria dispar affect its ability to tolerate tannins. J. Chem. Ecol. 2020, 46, 299–307. [Google Scholar] [CrossRef]
- Feeny, P.P. Effect of oak leaf tannin on larval growth of the winter moth Operophtera brumata. J. Insect Physiol. 1968, 14, 805–817. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Constabel, C.P. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Mezquida, E.T.; Caputo, P.; Acebes, P. Acorn crop, seed size and chemical defenses determine the performance of specialized insect predators and reproductive output in Mediterranean oak. Insects 2021, 12, e721. [Google Scholar] [CrossRef] [PubMed]
- Addesso, K.M.; McAuslane, H.J. Pepper weevil attraction to volatiles from host and nonhost plants. Environ. Entomol. 2009, 38, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Addesso, K.M.; McAuslane, H.J.; Hans, T. Attraction of pepper weevil to volatiles from damaged pepper plants. Entomol. Exp. Et Appl. 2011, 138, 1–11. [Google Scholar] [CrossRef]
- Werle, C.T.; Ranger, C.M.; Schultz, P.B.; Reding, M.E.; Addesso, K.M.; Oliver, J.B.; Sampson, B.J. Integrating repellent and attractant semiochemicals into a push-pull strategy for ambrosia beetles (Coleoptera: Curculionidae). J. Appl. Entomol. 2019, 143, 333–343. [Google Scholar] [CrossRef]
- Matsui, K.; Koeduka, T. Green leaf volatiles in plant signaling and response. In Lipids in Plant and Algae Development. Subcellular Biochemistry; Nakamura, Y., Li-Besson, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 427–443. [Google Scholar]
- Ameye, M.; Allmann, S.; Verwaeren, J.; Smagghe, G.; Haesaert, G.; Schuurink, R.C.; Audenaert, K. Green leaf volatile production by plants: A meta-analysis. New Phytol. 2018, 220, 666–683. [Google Scholar] [CrossRef]
- Failoa, C.; Taipale, D. Impact of insect herbivory on plant stress volatile emissions from trees: A synthesis of quantitative measurements and recommendations for future research. Atmos. Environ. 2020, 5, e100060. [Google Scholar] [CrossRef]
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef]
- Pigliucci, M. Phenotypic Plasticity beyond Nature and Nurture; John Hopkins University Press: Baltimore, MD, USA, 2001. [Google Scholar]
- Pigliucci, M. Evolution of phenotypic plasticity: Where are we going now? Trends Ecol. Evol. 2005, 20, 481–486. [Google Scholar] [CrossRef]
- Li, J.; Jin, Y.; Shen, Y. Changes of volatiles from drought stressed ashleaf maple (Acer negundo) in July and August. For. Stud. China 2000, 2, 27–33. [Google Scholar]
- Jardine, K.J.; Karl, T.; Lerdau, M.; Harley, P.; Guenther, A.B.; Mak, J.E. Carbon isotope analysis of acetaldehyde emitted from leaves following mechanical stress and anozia. Plant Biol. 2009, 11, 591–597. [Google Scholar] [CrossRef]
- Zwack, J.A.; Aiello, A.S.; Graves, W.R.; Townsend, A.M. Root-zone stress effects on water relations and growth of silver, red, and Freeman maples. HortScience 1996, 31, e576a. [Google Scholar] [CrossRef]
- Hoffman, W.A.; Porter, H. Avoiding bias calculations of relative growth rate. Ann. Bot. 2002, 80, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jiang, W.; Ghiassi, M.; Lee, S.; Nitin, M. Classification of Camellia (Theaceae) species using leaf architecture variations and pattern recognition techniques. PLoS ONE 2012, 7, e29704. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurements and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4-3. [Google Scholar] [CrossRef]
- Turner, N.C.; Long, M.J. Errors arising from rapid water loss in the measurement of leaf water potential by the measurement of leaf water potential by the pressure chamber technique. Aust. J. Plant Physiol. 1980, 7, 527–537. [Google Scholar]
- Knipfer, T.; Bambach, N.; Hernandez, M.I.; Bartlett, M.K.; Sinclair, G.; Duong, F.; Kluepfel, D.A.; McElrone, A.J. Predicting stomatal conductance closure and turgor loss in woody plants using predaen and midday water potential. Plant Physiol. 2020, 184, 881–894. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Sorsa, S. Testing the effects of drying methods on willow flavonoids, tannins, and salicylates. J. Chem. Ecol. 2001, 27, 779–789. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Tahvanainen, J. The effect of the sample preparation method of extractable phenolics of Salicaceae species. Planta Med. 1989, 55, 55–58. [Google Scholar] [CrossRef]
- Orians, C.M. Preserving leaves for tannin and phenolic glycoside analyses: A comparison of methods using three willow taxa. J. Chem. Ecol. 1995, 21, 1235–1243. [Google Scholar] [CrossRef]
- Ameglio, T.; Archer, P.; Cohen, M.; Valancogne, C.; Daudet, F.; Dayau, S.; Cruiziat, P. Significance and limits in the use of predawn leaf water potential for tree irrigation. Plant Soil 1999, 207, 155–167. [Google Scholar] [CrossRef]
- Mullen, W.; Stewart, A.J.; Lean, M.E.J.; Gardner, P.; Duthie, G.G.; Crozier, A. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J. Agric. Food Chem. 2011, 50, 5197–5201. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A. The Tannin Handbook. 2011. Available online: http://www.users.miami.oh.edu/hagermae/ (accessed on 15 November 2021).
- Hagerman, A. Extraction of tannin from fresh and preserved leaves. J. Chem. Ecol. 1988, 13, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E. Colorimetric quantification of carbohydrates. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–8. [Google Scholar] [CrossRef]
- Tomlinson, K.W.; van Langevelde, F.; Ward, D.; Bongers, F.; da Silva, D.A.; Prins, H.H.T.; de Bie, S.; Sterck, F. Deciduous and evergreen trees differ in juvenile biomass allometries because of differences in allocation to root storage. Ann. Bot. 2013, 112, 575–587. [Google Scholar] [CrossRef]
- Ball, E.D. The potato leafhopper and its relation to the Hopper-burn. J. Econ. Entomol. 1919, 12, 149–155. [Google Scholar] [CrossRef]
- Wickham, H.; Francois, R.; Henry, L.; Muller, K. Dplyr: A Grammar of Data Manipulation. R Package Version 1.0.7. 2021. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 4 April 2022).
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 4 April 2022).
- Salkind, N. Encyclopedia of Research and Design; SAGE Publications, Inc.: New York, NY, USA, 2010. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Laitinen, R.A.E.; Nikoloski, Z. Genetic basis of plasticity in plants. J. Exp. Bot. 2019, 70, 739–745. [Google Scholar] [CrossRef]
- Bradshaw, A.D. Evolutionary significance of phenotypic plasticity in plants. Adv. Genet. 1965, 13, 115–155. [Google Scholar]
- Bradshaw, A.D. Unraveling phenotypic plasticity- why should we bother? New Phytol. 2006, 170, 644–648. [Google Scholar] [CrossRef]
- Via, S.; Gomulkiewicz, R.; De Jong, G.; Scheiner, S.M.; Schlichting, C.D.; Van Tienderen, P.H. Adaptive phenotypic plasticity- consensus and controversy. Trends Ecol. Evol. 1995, 10, 212–217. [Google Scholar] [CrossRef]
- Ward, D.; Shrestha, M.H.; Golan-Goldhirsh, A. Evolution and ecology meet molecular genetics: Adaptive plasticity in two isolated Negev desert populations of Acacia raddiana at either end of a rainfall gradient. Ann. Bot. 2012, 109, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Stearns, S.C.; Koella, J.C. The evolution of phenotypic plasticity in life-history traits: Predictions of reaction norms for age and size at maturity. Evolution 1986, 40, 893–913. [Google Scholar] [PubMed]
- Arnold, P.A.; Kruuk, L.E.B.; Nicotra, A.B. How to analyse plant phenotypic plasticity in response to a changing climate. New Phytol. 2019, 222, 1235–1241. [Google Scholar] [CrossRef]
- Cote, G.; Perry, G.; Blier, P.; Bernatchez, L. The influence of gene-environment interactions on GHR and IGF-1 expression and their association with growth in brook charr, Salvelinus fontinalis (Mitchill). BMC Genet. 2007, 8, e87. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, e86. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Png, G.K.; Li, Y.; Jimoh, S.O.; Ding, Y.; Li, F.; Sun, S. Leaf plasticity contributes to plant anti-herbivore defenses and indicates selective foraging: Implications for sustainable grazing. Ecol. Indic. 2021, 122, e107273. [Google Scholar] [CrossRef]
- Valladares, F.; Matesanz, S.; Guilhaumon, F.; Araujo, M.B.; Balaguer, L.; Benito-Garzon, M.; Cornwell, W.; Gionaoli, E.; van Kluenen, M.; Naya, D.E.; et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 2014, 17, 1351–1364. [Google Scholar] [CrossRef]
- van Kleunen, M.; Fischer, M. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 2004, 166, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Blumstein, M.; Hopkins, R. Adaptive variation in non-structural carbohydrate storage in temperate tree species. Plant Cell Environ. 2020, 44, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Schlichting, C.D.; Pigliucci, M. Phenotypic Evolution: A Reaction Norm Perspective; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Metcalf, C. Invisible trade-offs: Van Noordwijk and de Jong and life-history evolution. Am. Nat. 2016, 187, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Erb, M. Volatiles as inducers and suppressors of plant defense and immunity—Origins, specificity, perception, and signaling. Curr. Opin. Plant Biol. 2018, 44, 117–221. [Google Scholar] [CrossRef] [PubMed]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Salgado, A.L.; Saastamoinen, M. Developmental stage-dependent response and preference for host plant quality n an insect herbivore. Anim. Behav. 2019, 150, 27–38. [Google Scholar] [CrossRef]
- Holeski, L.M.; Chase-Alone, R.; Kelly, J.K. The genetics of phenotypic plasticity in plant defense: Trichome production in Mimulus guttatus. Am. Nat. 2010, 175, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Tooker, J.; Pfeiffer, M.; Chung, S.G.; Felton, G.W. Role of trichomes in defense against herbivores: Comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 2012, 236, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Dalin, P.; Agren, J.; Bjorkman, C.; Huttunen, P.; Karkkainen, K. Leaf trichome formation and plant resistance to herbivory. In Induced Plant Resistance to Herbivory; Springer: Berlin/Heidelberg, Germany, 2008; pp. 89–105. [Google Scholar]
- Levin, D.A. The role of trichomes in plant defense. Q. Rev. Biol. 1973, 48, 3–15. [Google Scholar] [CrossRef]
- Singh, A.; Dilkes, B.; Sela, H.; Tzin, V. The effectiveness of physical and chemical defense responses of wild emmer wheat against aphids depends on the leaf position and genotype. Front. Plant Sci. 2021, 12, e667820. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.A.; Gorski, P.M.; Tallamy, D.W. Polymorphism in plant defense against herbivory: Constitutive and induced resistance in Cucumus sativus. J. Chem. Ecol. 1999, 25, 2285–2304. [Google Scholar] [CrossRef]
- Thompson, J.D.; Amiot, J.; Borron, C.; Linhart, Y.B.; Keeefover-Ring, K.; Gauthier, P. Spatial heterogeneity of gall formation in relation to chemotype distribution in Thymus vulgaris. Plant Ecol. 2019, 220, 777–778. [Google Scholar] [CrossRef]
- Zang, Z.; Wang, J.; Cui, H.L.; Yan, S. Terahertz spectral imaging based quantitative determination of spatial distribution of plant leaf constituents. Plant Methods 2019, 15, 106. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).