Abstract
The paper presents the results on resistance of Aesculus hippocastanum Linnaeus, 1753 trees to Cameraria ohridella Deschka & Dimić, 1986 (Lepidoptera, Gracillariidae) impact under conditions of a modern urban environment on the example of Dnipro city as the largest industrial city in Ukraine. Field experiments were conducted in all park areas of the city, which allowed covering the full gradient of the existing urban environment and considered the different degrees of the tree settlement by the invasive insect species. The research of the impact of C. ohridella caterpillars’ vital activity on the photosynthetic apparatus state was carried out by applying a chlorophyll fluorescence induction technique. Diagnosis of photosynthetic dysfunction of fresh Ae. hippocastanum leaves was conducted using a portable “Floratest” fluorometer manufactured in Ukraine. Interpretation of the obtained Kautsky curves showed that significant changes in their critical parameters associated with the degree of leaf damage by C. ohridella caterpillars were not detected. The influence of tree growth site conditions on the following 4 main indicators of chlorophyll fluorescence induction was established: the initial value of fluorescence induction after irradiation; the value of “plateau” fluorescence induction; the maximum value of fluorescence induction; the stationary value of fluorescence induction after light adaptation of the plant leaf. It was found that the efficiency coefficients of photochemical processes in Ae. hippocastanum trees growing in low terrain levels differed significantly, which can probably be interpreted as their response to the specific characteristics of the urban environment.
1. Introduction
The environment of an industrial city encompasses a large number of stressors for plants that inhibit their development and vital activity [1,2,3]. Among the dominant stress factors that affect living organisms in urban conditions, effects of climate changes associated with rising air temperatures and drought occurrence [4], pesticide contamination from surrounding agrocenoses [5,6], motor transport and industrial enterprises emissions are of particular interest [7,8,9,10]. Harmful effects of pollutants can manifest themselves in various functional changes in trees, including their interactions with lepidopteran phytophages [11] and predators that feed on these phytophages [12,13,14]. In an urban environment, disruption of biochemical reactions, physiological functions, morphostructure, and reduction of resistance to pests and diseases are observed in woody plants depending on concentration of the toxic substances and duration of their exposure.
In the last two decades, horse chestnut (Aesculus hippocastanum L.) introduced to the Steppe zone of Ukraine has suffered greatly from the invasive miner Cameraria ohridella Deschka & Dimić, 1986, which is manifested in damage of assimilating organs, premature defoliation and resulted in a significant reduction of reserve materials necessary for the normal plant life [15,16]. It worsens the condition and sometimes causes premature death of the trees [17,18].
Diagnostics of the influence of environmental factors for the purpose of rapid assessment of plant functional state requires the use of express and informative techniques that would allow conducting analysis both in the laboratory and in the field conditions with minimal violation of the studied object integrity. Such techniques include the method of chlorophyll fluorescence induction widely applied in modern studies of photosynthetic processes [19,20,21,22,23].
Photosynthesis is one of the processes most vulnerable to stressors, so valuable information on the state of the photosynthetic apparatus in a plant under the impact of phytophage feeding can be obtained by fluorescence analysis [24,25]. The influence of many different urbanized factors on the functional state of woody plant leaves resulted in adaptive changes of plants accompanied by certain morphological changes in the assimilation apparatus, as well as a shift in seasonal developmental rhythms [26,27].
It is known that certain sections of the chlorophyll fluorescence induction curve may be used as indicators of the corresponding physiological processes in the photosynthetic chain. Violations of its particular components caused by exo- and endogenous factors show themselves in specific changes in the corresponding sections of the curve. The photosynthetic apparatus in plants was characterized using the method in many woody plants growing in an urban environment due to its close relationship with chlorophyll fluorescence intensity [28,29,30]. White oak (Quercus alba L.) compared to red maple (Acer rubrum L.) shows more significant differences in chlorophyll fluorescence parameters under megalopolis conditions compared to these species growing in native forest conditions [31]. The results of Uhrin & Supuka [32] confirmed that the Fv/Fm (maximum efficiency of primary photosynthesis processes) parameter proved to be an effective tool for measuring the growth response of roadside sycamore maple (Acer pseudoplatanus L.) in the transformed urban environment. Analysis of the Fv/Fm, Frd (fluorescence reduction coefficient, which characterizes the quantum efficiency of photosynthesis or the viability index), and PCII (stability of light-harvesting complexes photosystem II to the influence of different strains of pathogens) parameters allowed assessing the adaptation potential of wild pear (Pyrus pyraster L.) and European mountain ash (Sorbus domestica L.) trees to water deficiency [33]. Results obtained demonstrate the possibility to use changes in certain sections of the chlorophyll fluorescence induction curve to detect deterioration in the life state of Moreton Bay fig (Ficus macrophylla Pers.), london plane (Platanus × acerifolia (Aiton) Willd.), Chinese elm (Ulmus parvifolia Jacq.) in drought conditions [34], and to assess the resistance of horse chestnut (Aesculus hippocastanum L.), small-leaved lime (Tilia cordata Mill.), and European white birch (Betula pendula Roth.) trees to soil salinization due to the use of salt as deicing agent [35]. It was found that the chlorophyll fluorescence Fv/Fm parameter of eucalyptus (Eucalyptus saligna Sm.) leaves have statistically significant association with wood density and the amount of wood decomposition in summer period [36]. The effect of heavy trimming of roadside small-leaved lime trees (Tilia cordata Mill.) on the photosynthesis process was investigated compared to neighboring non-trimmed trees [37].
Changes in chlorophyll fluorescence parameters may indicate the effect of phytophagous insects on the plant photosynthetic apparatus [38,39,40]. For example, it has been demonstrated a close relationship between the level of damage of cork oak (Quercus suber L.) and holm oak (Quercus ilex L.) trees by the great capricorn beetle and chlorophyll content in leaves depending on the age of the phytophage [41]. The effect of different residential densities of Coccus hesperidum L. (Hemiptera, Coccidae) per leaf on the plant pigments concentration (chlorophyll a, chlorophyll b, and carotenoids) and chlorophyll fluorescence parameters (maximum quantum yield of photosystem II Fv/Fm, the effectiveness of “open” reaction enters (RC) in the light Fv/Fm, and coefficient of non-photochemical of chlorophyll QN and coefficient of photochemical quenching of chlorophyll QP) was studied in lemon plants (Citrus limon var. ponderosa L.) and ferns (Nephrolepis biserrata (Swartz) Schott.). The effect of the degree of infestation with C. hesperidum on the pigments loss in plants and changes in the photosynthetic productivity of host plants was characterized [42].
The goal of our study was to establish the effect of C. ohridella feeding on the critical parameters of the Kautsky curve of A. hippocastanum in different conditions of the urban environment.
2. Material and Methods
The research was conducted during the 2019 growing season in Dnipro city (Ukrainian North Steppe subzone). The city is situated in temperate zone with a relatively active atmospheric circulation (the atmospheric circulation is predominantly from east to west). The climate is temperate continental [15,43,44]. One of the climate features in the territory is the wide fluctuations in weather conditions from year to year. Moderately wet years alternate with sharply dry ones, and hot dry winds occurrs fairly common. In general, the climate is characterized by rather cool winters and hot summers.
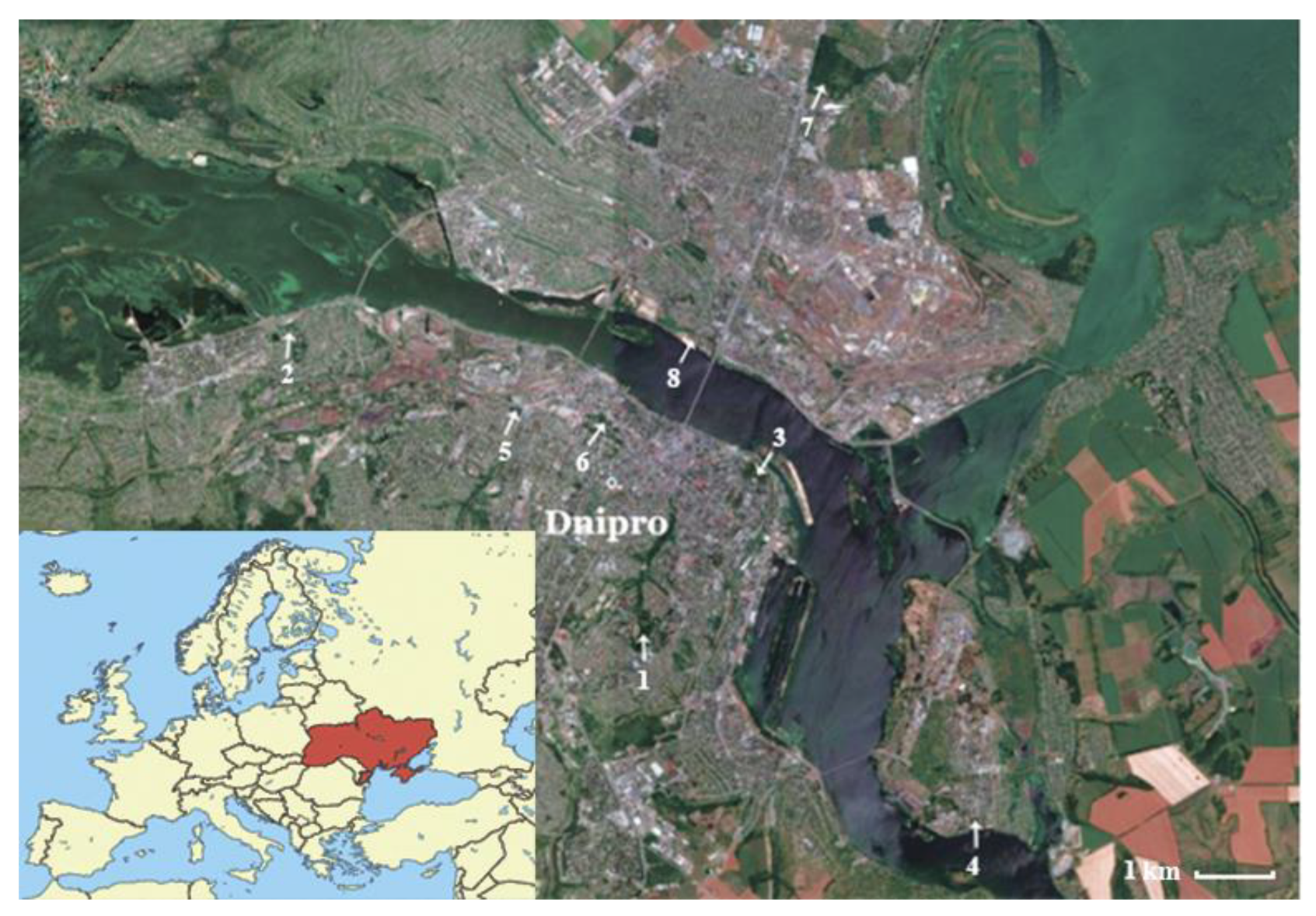
Within Dnipro city territory, we selected eight park ecosystems (Figure 1, Table 1) that have different conditions of horse chestnut growth (Table 2). Four trees of Ae. hippocastanum were selected on the territory of each park area with similar morphological and taxational characteristics (trunk diameter 132–151 cm; height 17–21 m).
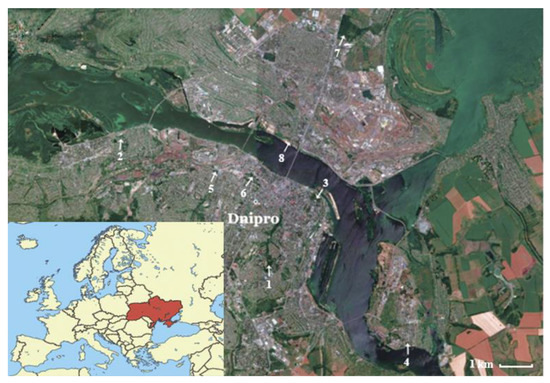
Figure 1.
Map of the sampling units within the territory of Dnipro city (Ukraine).

Table 1.
Characteristics of research areas.

Table 2.
Characteristics of the location of parks within the gradient of landscape and soil-climatic conditions.
To study the effect of C. ohridella caterpillars feeding on the photosynthesis processes in Ae. hippocastanum plants, leaves of medium formation were selected at 5 pcs. from the illuminated crown exposition (which was mostly infected by a miner). Model leaves were selected on annual vegetative growth from the lower third in dry weather. Each examined leaf was marked individually.
The research was conducted on 14 June 2019, which corresponded to the development of the 5th-age caterpillars of C. ohridella second generation, which begins to give an irruption in this generation in the conditions of Dnipro city. The age of the caterpillars was determined with the visual parameters of the mines made by their. The damage degree of the horse chestnut leaf blades by C. ohridella was assessed visually with a previously self-developed scale [15]. Light intensity measurements were conducted with RCE-174 luxometer (PCE Instruments, Meschede, Germany, 2018). Temperature and relative humidity measurements were conducted with HE-173 thermohygrometer (Huato Electronic Co.LTD, Shenzhen, China, 2018).
Portable fluorometer “Floratest” was used for the diagnosis of native chlorophyll disorders in fresh Ae. hippocastanum leaves. Portable fluorometer “Floratest” comprises a base unit with a graphic liquid crystal display, control buttons, a remote optoelectronic sensor, connecting cable to the USB port of a personal computer, and a network adapter. The remote optoelectronic sensor includes an LED that has a maximum radiation intensity of λ = 470 ± 20 nm. Irradiation indicators in the sensor were the following: irradiation wavelength 470 ± 15 nm; irradiated spot area not less than 15 mm2; light intensity within the spot at least 2.4 W/m2. Signal reception indicators in an optoelectronic sensor: the spectral range of fluorescence intensity measurement was 670–800 nm; receiving window area 9 mm2; photodetector sensitivity at λ = 650 nm was 0.45 A/W.
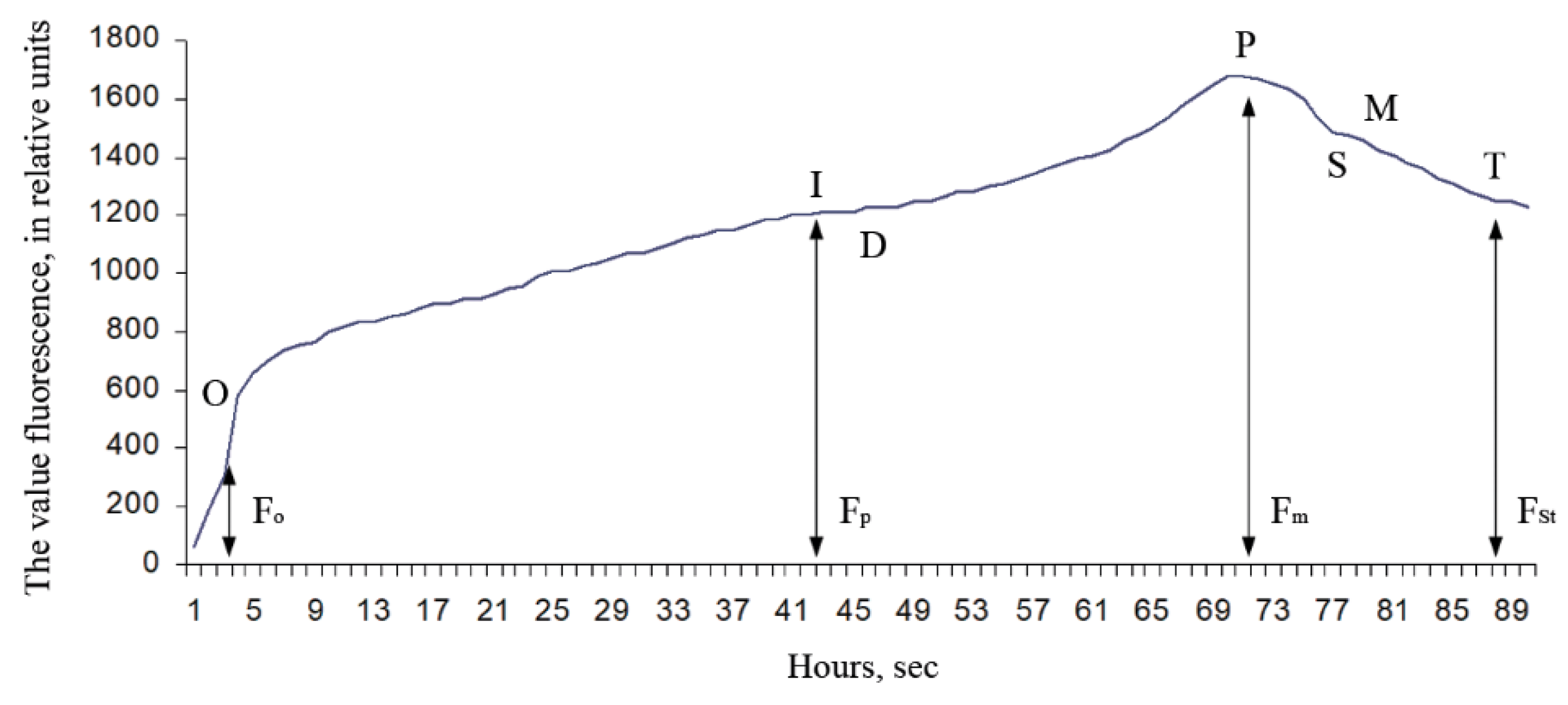
Observations were made using fresh Ae. hippocastanum leaves. After the start of light exposure, the intensity of chlorophyll fluorescence (fluorescence induction or fluorescence induced (caused) by light) begins to change significantly over time. The time dependence of the chlorophyll fluorescence intensity has the characteristic form of a curve having one or more maximum, and it is called the chlorophyll fluorescence induction curve (the Kautsky curve) (Figure 2).
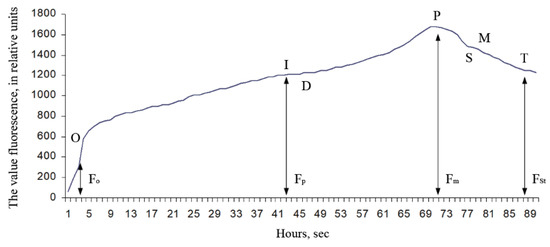
Figure 2.
Typical chlorophyll fluorescence induction curve [45]: F0 is the initial value of fluorescence induction after irradiation is turned on; Fp is the “plateau” fluorescence induction value; Fm is the maximum value of fluorescence induction; FSt is the stationary value of fluorescence induction after light adaptation of a plant leaf.
The shape of this curve is quite sensitive to changes that occurred in the photosynthetic apparatus in plants when adapting to different environmental conditions, which has become the basis for the widespread use of the Kautsky effect in the study of photosynthesis. To interpretation the Kautsky curve [45], we used its known critical parameters: F0 means the initial value of fluorescence induction after irradiation is turned on; Fp means the value of “plateau” fluorescence induction; Fm means the maximum value of fluorescence induction; FSt means the stationary value of fluorescence induction after light adaptation of a plant leaf In addition to the critical parameters of the Kautsky curve, we used calculated parameters as variable chlorophyll fluorescence (Fv = Fm − F0); maximum efficiency of primary photosynthesis processes (Ef = Fv/Fm), and coefficient of photochemical processes efficiency (E = (Fm − FSt)/FSt).
The data were analyzed using Statistica 8.0 program (StatSoft Inc., USA). The Table 1 demonstrate the results as x ± SD (mean ± standard deviation). The experimental groups were determined using ANOVA and the Tukey test, where the differences were considered significant at p < 0.05. In the Figures small square is median, the upper and lower line of the rectangle is 75% and 25% quartiles, the upper line is minimum and maximum values, circles and asterisks is outliers. The empirical correlation coefficient (r) and its error (Sr) were calculated to assess the association between the Kautsky curve parameters and the degree of leaf blade damage; the r/Sr ratio was compared with the threshold value of Student’s t-test for a given sample size.
3. Results
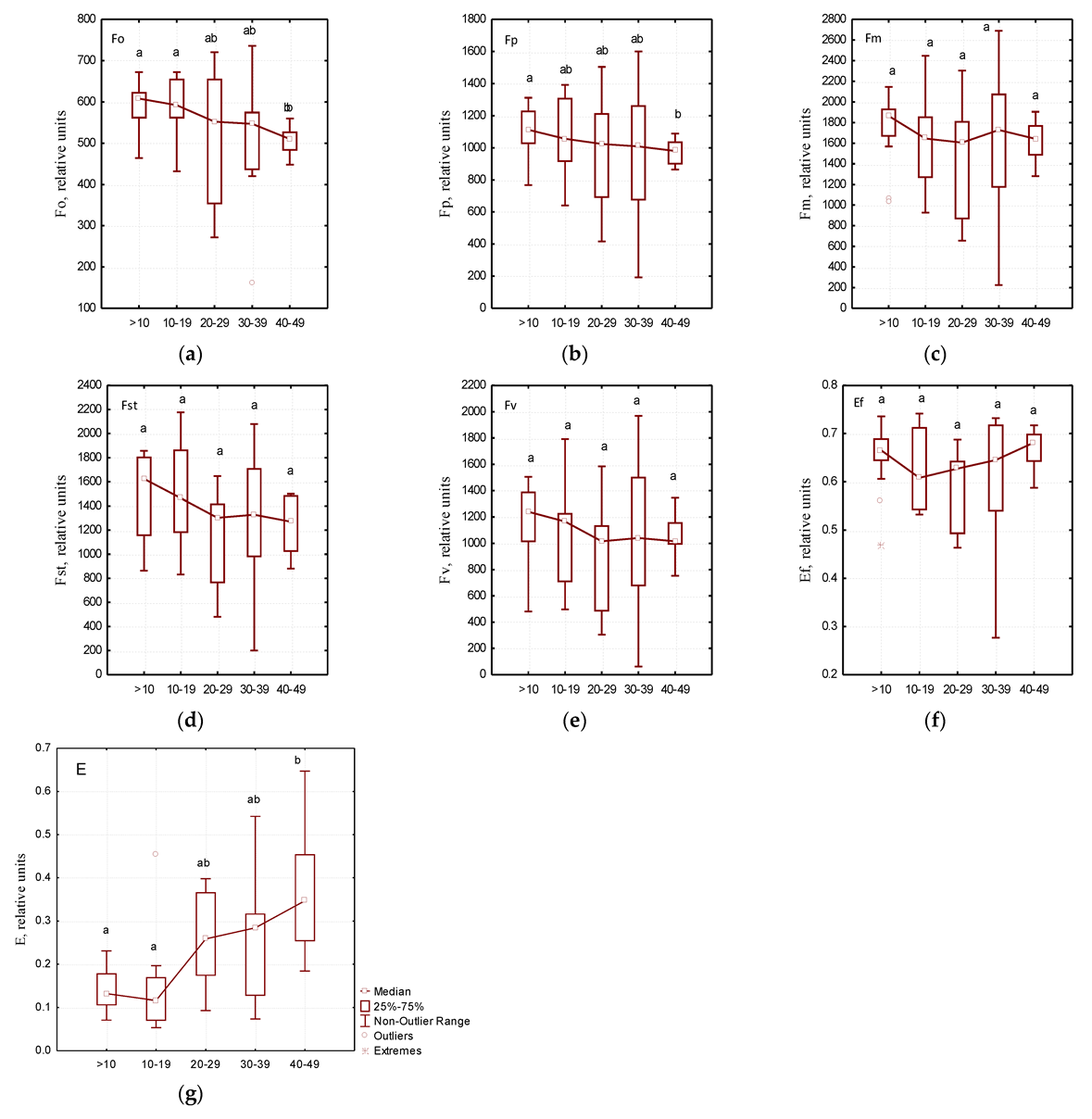
As a result of the phytosanitary monitoring of park areas in Dnipro city, it was found that the trees of Ae. hippocastanum were not equally infected with C. ohridella (Table 3). The analysis of chlorophyll fluorescence induction variability showed specific patterns of changes in a number of physiological parameters of photosynthesis. Significant changes in the critical parameters of the Kautsky curve depending on the degree of leaf blade damage by C. ohridella caterpillars were not detected (Figure 3).

Table 3.
Average damage level of Ae. hippocastanum leaf blades by C. ohridella miner (x ± SD, n = 40).
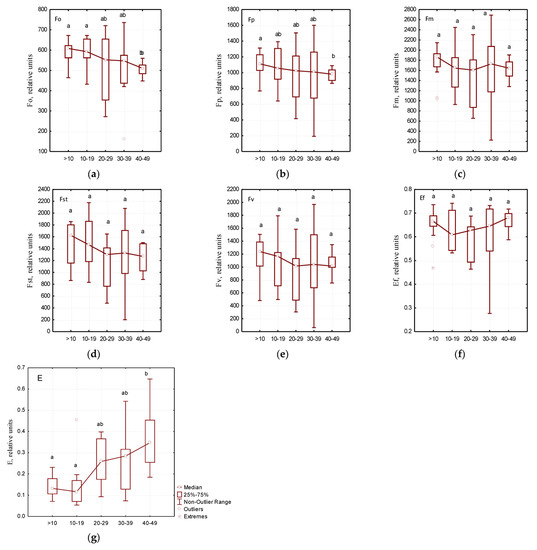
Figure 3.
Measurements of physiological parameters of photosynthesis depending on the leaf damage degree: (a)—F0—the initial value of fluorescence induction after switching on irradiation, (b)—Fp—the value of fluorescence induction “plateau”, (c)—Fm—the maximum value of fluorescence induction, (d)—FSt—the steady value of fluorescence induction after light adaptation of the leaf of the plant, (e)—FV—variable chlorophyll fluorescence, (f)—Ef—the maximum efficiency of primary photosynthesis processes, (g)—E—efficiency coefficients of photochemical processes; different letters within each figure indicate significant differences between the groups (p < 0.05) according to the results of Tukey test, n = 7.
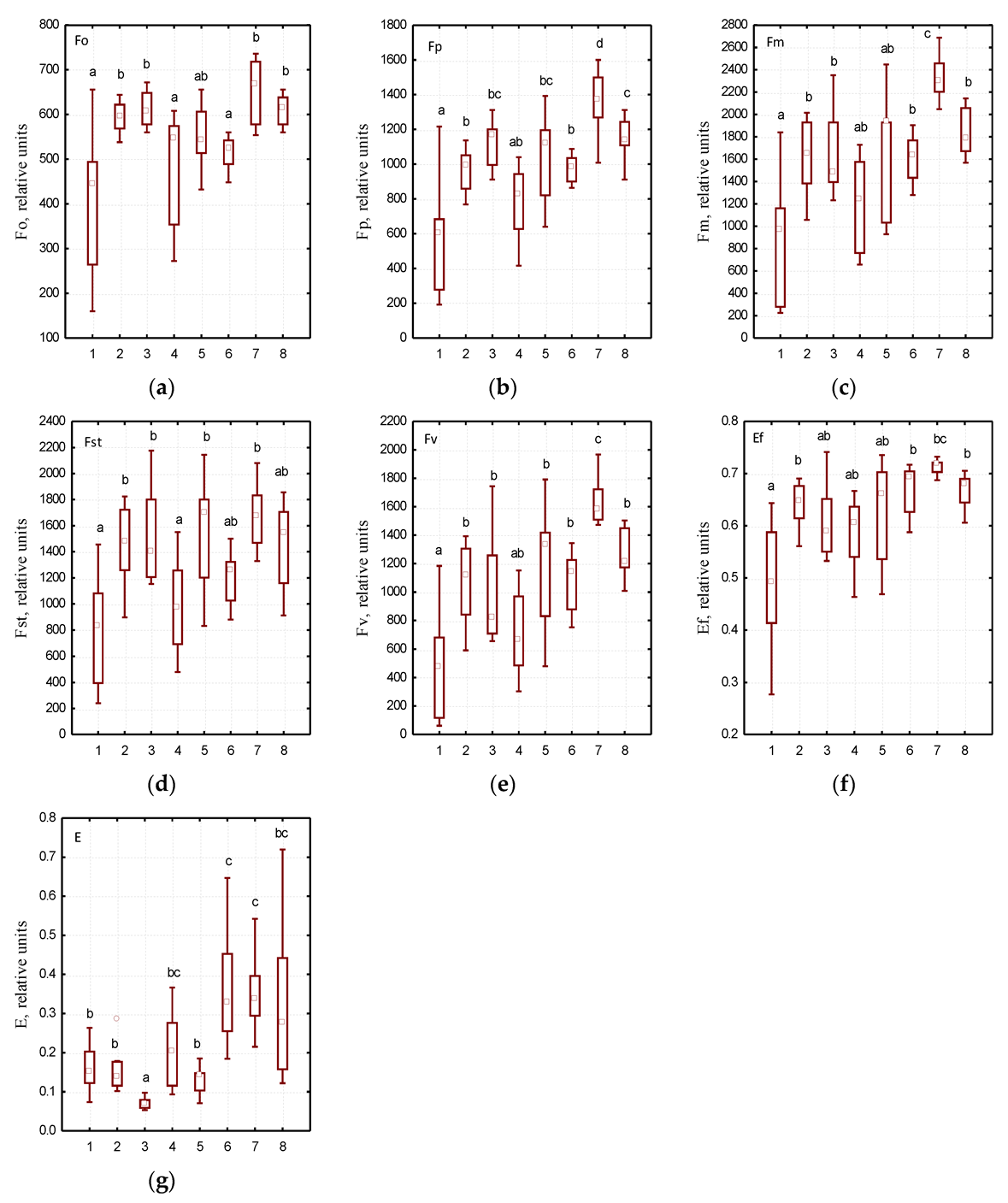
The use of the fluorescence analysis method allowed the determination the effect of growing site conditions on individual indicators of chlorophyll fluorescence induction in the leaves of the studied Ae. hippocastanum trees (Figure 3). High plasticity was established in the structure of chloroplasts in horse chestnut leaves which was characterized with F0, Fv, Fp, and FSt parameters. We found significant differences in the above parameters in Ae. hippocastanum leaves sampled from Botanical Garden of DNU compared to the leaves of the studied plant species sampled from different parks in Dnipro city. It was noted that the efficiency coefficients of photochemical processes (E) in Ae. hippocastanum trees in the parks located at low terrain levels were grouped separately (Figure 4c). The lowest E values were recorded in horse chestnut trees in Botanical Garden of DNU, Novokodatskyi Park, Taras G. Shevchenko Park, and Metallurgists Square; this may indicate a decrease in the intensity of photochemical reactions.
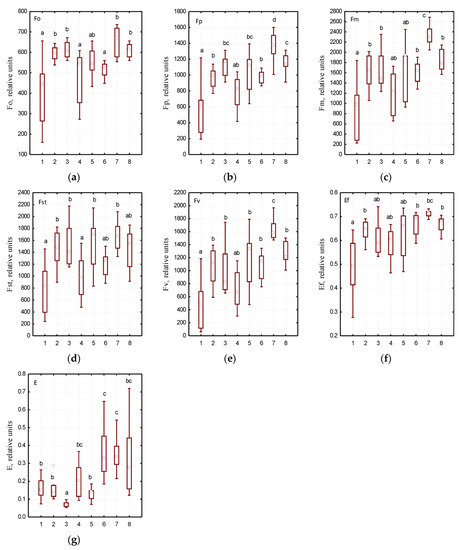
Figure 4.
Variability of photosynthesis characteristics in various urban parks of Dnipro city: (a)—F0—the initial value of fluorescence induction after switching on irradiation, (b)—Fp—the value of fluorescence induction “plateau”, (c)—Fm—the maximum value of fluorescence induction, (d)—FSt—the steady value of fluorescence induction after light adaptation of the leaf of the plant, (e)—FV—variable chlorophyll fluorescence, (f)—Ef—the maximum efficiency of primary photosynthesis processes, (g)—E—efficiency coefficients of photochemical processes; at the x-axis: 1—Botanical Garden of DNU, 2—Novokodatskyi Park, 3—Taras G. Shevchenko Park, 4—Pridneprovsky Park, 5—Metallurgists Square, 6—Lazaria Hloby Park, 7—Druzhby narodiv Forest Park, 8—Park Sahaydak; at the y-axis: the values of parameters indicated in Table 1; please see Figure 3 for an explanation of statistical processing.
Of all the parameters of the Kautsky curve (Table 4), only E values correlated with the degree of leaf blade damage by phytophages (p < 0.05). At the same time, a very high degree of correlation was found between the remaining parameters of the Kautsky curve: Fp, Fm, Fst, Fv, and Ef (p < 0.001).

Table 4.
Correlations between the Kautsky curve parameters and the degree of leaf damage by phytophages (n = 40).
4. Discussion
The analysis of chlorophyll fluorescence induction variability allowed determining specific patterns of changes in series of photosynthesis physiological parameters. The background fluorescence level (F0) depends on the loss of excitation energy during migration along pigment matrix, as well as on the content of chlorophyll molecules that do not functionally associated with reaction centers [46,47]. The maximum values of F0 parameter were recorded in the Ae. hippocastanum leaves sampled from Park Sahaydak and Novokodatskyi Park, and the lowest values were observed in leaves sampled from Lazaria Hloby Park. This is due to the fact that a structural change in the pigment complex associated with the loss of green leaf tissues under the phytophage influence. As the number of antenna chlorophylls decreases, the initial level of fluorescence decreases, and vice versa.
The Fp parameter characterizes the highest level of fluorescence, i.e., it means the maximum value on the induction curve. It has the most variable pattern characterized by adaptive changes [48]. In the structure of the pigment complex in the studied Ae. hippocastanum plants, the lowest Fp values were found in trees having the highest degree of leaf damage by C. ohridella larvae (Botanical Garden of DNU, Lazaria Hloby Park, and Pridneprovsky Park). It was caused by a decrease in the number of both light-harvesting and antenna chlorophylls. The obtained data are supported by the tendency to decrease the variable fluorescence of chlorophyll (Fv) with an increase in the damage degree of horse chestnut leaves by mining moth larvae. The calculated Fv parameter is expressed as the difference between the highest level of fluorescence and background fluorescence indicating the amplitude value of changes in the Kautsky curve [49,50].
The value of the steady-state fluorescence level (FSt) also decreased with an increasing degree of damage of Ae. hippocastanum leaves. This parameter is characterized by a dynamic equilibrium between the processes that cause an increase in fluorescence and the processes that lead to its decrease [51]. As shown in Figure 3, there is a significant difference in the values of all key parameters of chlorophyll fluorescence induction. We have shown that an increase in the number of C. ohridella mines reduces the values of all major indicators (F0, Fv, Fp and FSt), which affects the overall physiological state of Ae. hippocastanum leaves. In general, plant defense responses to insect attacks are very often associated with a decrease in the rate of photosynthesis [52]. Genotypes that are capable to maintain the rate of photosynthesis under these conditions probably show greater resistance, i.e., the plant’s ability to grow, develop, and bear fruit satisfactorily even at a certain level of herbivorous insect infestation [53].
Another important indicator to assess the functional state of the leaves is the efficiency of dark photochemical reactions, E. This parameter reflects the relative number of electrons transported along the electron transport chain. Increasing the efficiency of dark photochemical reactions in the leaves of Ae. hippocastanum sampled in Lazaria Hloby Park, Druzhby narodiv Forest Park, Park Sahaydak shows that electronic transport on FSII and FSI is more efficient, while some decrease in E parameter may indicate inhibition of dark photochemical reactions. In addition, the reduction of E coefficient in the leaves Ae. hippocastanum sampled in Botanical Garden of DNU, Novokodatskyi Park, Taras G. Shevchenko Park and Metallurgists Square (especially against the background of Fo increasing), may indicate not only a decrease in the intensity of photochemical reactions, but also destructive changes in the photosynthetic apparatus [54,55].
The value of the efficiency coefficient of dark photochemical processes (E) reflects the activity of ribulose bisphosphate carboxylase as the main enzyme in the Calvin cycle. Apparently, ribulose bisphosphate carboxylase has not only carboxylase activity, but also oxygenase activity; thereby, an increase in this enzyme activity can be associated with an increase in the process competitive to photosynthesis, photorespiration. The photorespiration can account for up to 50% of the enzyme activity. Our correlation analysis confirmed the current data of numerous researchers [56,57] about the fact that photorespiration process has a protective effect on the photosynthetic apparatus in plants, and its intensity significantly increases under the action of stress factors.
5. Conclusions
Effect of C. ohridella caterpillars on the photosynthetic apparatus in Ae. hippocastanum allowed determining specific patterns of changes in critical parameters of chlorophyll fluorescence induction. Significant changes in the critical parameters of the Kautsky curve depending on the degree of leaf blade damage by C. ohridella caterpillars were not identified. The use of the fluorescence analysis method allowed determining the effect of growing site conditions on individual indicators of chlorophyll fluorescence induction in the leaves of the studied Ae. hippocastanum trees. High plasticity was established in the structure of chloroplasts in horse chestnut leaves which was characterized with Fo, Fv, Fp, and FSt parameters. We found significant differences in the above parameters in Ae. hippocastanum leaves sampled from Botanical Garden of DNU compared to the leaves of the studied plant species sampled from different parks in Dnipro city. It was noted that the efficiency coefficients of photochemical processes (E) in Ae. hippocastanum trees in parks located at low terrain levels were differed markedly. The lowest efficiency coefficients of dark photochemical reactions were recorded in horse chestnut trees from the Botanical Garden of DNU, Novokodatskyi Park, Taras G. Shevchenko Park, and Metallurgists Square, which can probably be associated with the urban environment characteristics.
Author Contributions
Conceptualization, V.B., K.H. and O.P.; methodology, O.S. and K.H.; analysis, I.L., I.I. and V.B.; investigation, S.S. and O.P.; data curation, V.L., V.B. and Y.G.; writing---original draft preparation, K.H., V.B. and A.A.; writing---review and editing, all authors; visualization, M.S., V.B. and L.B.; funding acquisition, V.L. and Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the grants 0122U001226 Innovative concept of management of ecological functions of introduced tree species in the conditions of urban ecosystems (Ministry of Education and Science of Ukraine).
Institutional Review Board Statement
The study was conducted according to the regulations for the protection of terrestrial wild animals.
Informed Consent Statement
Not applicable.
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Kosova, K.; Vıtamvas, P.; Urban, M.O.; Prasil, I.T.; Renaut, J. Plant abiotic stress proteomics: The major factors determining alterations in cellular proteome. Front. Plant Sci. 2018, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagon, D.; Arbona, V.; Gomez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 62, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Avtaeva, T.A.; Sukhodolskaya, R.A.; Brygadyrenko, V.V. Modeling the bioclimating range of Pterostichus melanarius (Coleoptera, Carabidae) in conditions of global climate change. Biosyst. Divers. 2021, 29, 140–150. [Google Scholar] [CrossRef]
- Komlyk, V.O.; Brygadyrenko, V.V. Morphological variability of Bembidion minimum (Coleoptera, Carabidae) populations under the influence of natural and anthropogenic factors. Biosyst. Divers. 2019, 27, 250–269. [Google Scholar] [CrossRef]
- Kozak, V.M.; Romanenko, E.R.; Brygadyrenko, V.V. Influence of herbicides, insecticides and fungicides on food consumption and body weight of Rossiulus kessleri (Diplopoda, Julidae). Biosyst. Divers. 2020, 28, 272–280. [Google Scholar] [CrossRef]
- Shulman, M.V.; Pakhomov, O.Y.; Brygadyrenko, V.V. Effect of lead and cadmium ions upon the pupariation and morphological changes in Calliphora vicina (Diptera, Calliphoridae). Folia Oecol. 2017, 44, 28–37. [Google Scholar] [CrossRef]
- Langraf, V.; Petrovičová, K.; David, S.; Kanská, M.; Nozdrovická, J.; Schlarmannová, J. Change phenotypic traits in ground beetles (Carabidae) reflects biotope disturbance in Central Europe. J. Entomol. Res. Soc. 2018, 20, 119–129. [Google Scholar]
- Langraf, V.; Petrovičová, K.; David, S.; Nozdrovická, J.; Petrovič, F.; Schlarmannová, J. The bioindication evaluation of ground beetles (Coleoptera: Carabidae) in three forest biotopes in the southern part of Central Slovakia. Ekológia 2019, 38, 25–36. [Google Scholar] [CrossRef]
- Putchkov, A.V.; Brygadyrenko, V.V.; Nikolenko, N.Y. Ecological-faunistic analysis of ground beetles and tiger beetles (Coleoptera: Carabidae, Cicindelidae) of metropolises of Ukraine. Biosyst. Divers. 2020, 28, 163–174. [Google Scholar] [CrossRef]
- Ruchin, A.B. Seasonal dynamics and spatial distribution of lepidopterans in selected locations in Mordovia, Russia. Biodiversitas 2021, 22, 2569–2575. [Google Scholar] [CrossRef]
- Faly, L.I.; Kolombar, T.M.; Prokopenko, E.V.; Pakhomov, O.Y.; Brygadyrenko, V.V. Structure of litter macrofauna communities in poplar plantations in an urban ecosystem in Ukraine. Biosyst. Divers. 2017, 25, 29–38. [Google Scholar] [CrossRef]
- Chaplygina, A.B.; Savynska, N.O.; Brygadyrenko, V.V. Trophic links of the spotted flycatcher, Muscicapa striata, in transformed forest ecosystems of North-Eastern Ukraine. Balt. For. 2018, 24, 304–312. [Google Scholar]
- Putchkov, A.V.; Brygadyrenko, V.V.; Markina, T.Y. Ground beetles of the tribe Carabini (Coleoptra, Carabidae) in the main megapolises of Ukraine. Vestn. Zool. 2019, 53, 3–12. [Google Scholar] [CrossRef]
- Shupranova, L.V.; Holoborodko, K.K.; Seliutina, O.V.; Pakhomov, O.Y. The influence of Cameraria ohridella (Lepidoptera, Gracillariidae) on the activity of the enzymatic antioxidant system of protection of the assimilating organs of Aesculus hippocastanum in an urbogenic environment. Biosyst. Divers. 2019, 27, 238–243. [Google Scholar] [CrossRef]
- Seliutina, O.V.; Shupranova, L.V.; Holoborodko, K.K.; Shulman, M.V.; Bobylev, Y.P. Effect of Cameraria ohridella on accumulation of proteins, peroxidase activity and composition in Aesculus hippocastanum leaves. Regul. Mech. Biosyst. 2020, 11, 299–304. [Google Scholar] [CrossRef]
- Sett, R. Responses in plants exposed to dust pollution. Hortic. Int. J. 2017, 1, 53–56. [Google Scholar] [CrossRef]
- Dimitrijevic, M.V.; Mitic, V.D.; Rankovic, G.Z.; Miladinovic, D.L. Survey of antioxidant properties of barberry: A chemical and chemometric approach. Anal. Lett. 2019, 53, 671–682. [Google Scholar] [CrossRef]
- Martínez-Ferri, E.; Zumaquero, A.; Ariza, M.T.; Barceló, A.; Pliego, C. Nondestructive detection of white root rot disease in avocado rootstocks by leaf chlorophyll fluorescence. Plant Dis. 2016, 100, 49–58. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, Y.; Zhang, M.; Hong, A.; Yang, H.; Liu, Y. Shade effects on growth, photosynthesis and chlorophyll fluorescence parameters of three Paeonia species. PeerJ 2020, 8, e9316. [Google Scholar] [CrossRef] [PubMed]
- Berner, J.M.; Cloete, H.; Shuuya, T. A baseline assessment of the photosynthetic potential of Welwitschia mirabilis using the JIP-test for monitoring and conservation purposes. Bothalia 2021, 51, a9. [Google Scholar] [CrossRef]
- Holoborodko, K.K.; Seliutina, O.V.; Ivanko, I.A.; Alexeyeva, A.A.; Shulman, M.V.; Pakhomov, O.Y. Effect of Cameraria ohridella feeding on Aesculus hippocastanum photosynthesis. Regul. Mech. Biosyst. 2021, 12, 346–352. [Google Scholar] [CrossRef]
- Baghbani, F.; Lotfi, R.; Moharramnejad, S.; Bandehagh, A.; Roostaei, M.; Rastogi, A.; KalajiImpact, M.H. Impact of Fusarium verticillioides on chlorophyll fluorescence parameters of two maize lines. Eur. J. Plant Pathol. 2019, 154, 337–346. [Google Scholar] [CrossRef]
- Koski, T.M.; Lindstedt, C.; Klemola, T.; Troscianko, J.; Mäntylä, E.; Tyystjärvi, E.; Stevens, M.; Helander, M.; Laaksonen, T. Insect herbivory may cause changes in the visual properties of leaves and affect the camouflage of herbivores to avian predators. Behav. Ecol. Sociobiol. 2017, 71, 97. [Google Scholar] [CrossRef]
- Uhrin, P.; Supuka, J.; Billiková, M. Growth adaptability of Norway maple (Acer platanoides L.) to urban environment. Folia Oecol. 2018, 45, 33–45. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, Z.; Zhou, S.; Peng, Y.; Zhang, L. Relationships between leaf physiognomy and sensitivity of photosynthetic processes to freezing for subtropical evergreen woody plants. iForest 2019, 12, 551–557. [Google Scholar] [CrossRef]
- Li, P.; Feng, Z.; Catalayud, V.; Yuan, X.; Yansen, X.; Paoletti, E. A meta-analysis on growth, physiological, and biochemical responses of woody species to ground-level ozone highlights the role of plant functional types. Plant Cell Environ. 2017, 40, 2369–2380. [Google Scholar] [CrossRef]
- Lin, K.H.; Wu, C.W.; Chang, Y.S. Applying Dickson quality index, chlorophyll fluorescence, and leaf area index for assessing plant quality of Pentas lanceolata. Not. Bot. Horti. Agrobot. Cluj Napoca 2019, 47, 169–176. [Google Scholar] [CrossRef]
- Castillo-Campohermoso, M.A.; Broetto, F.; Rodríguez-Hernández, A.M.; Soriano-Melgar, L.d.A.A.; Mounzer, O.; Sánchez-Blanco, M.J. Disponibilidad de agua, variaciones en el diámetro del tallo, fluorescencia de la clorofila y contenido iónico en Pistacia lentiscus bajo estrés salino. Terra Latinoam. 2020, 38, 103–111. [Google Scholar] [CrossRef]
- Sonti, N.F.; Hallett, R.A.; Griffin, K.L.; Trammell, T.L.E.; Sullivan, J.H. Chlorophyll fluorescence parameters, leaf traits and foliar chemistry of white oak and red maple trees in urban forest patches. Tree Physiol. 2020, 41, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Uhrin, P.; Supuka, J. Quality assessment of urban trees using growth visual and chlorophyll fluorescence indicators. Ekológia 2016, 35, 160–172. [Google Scholar] [CrossRef][Green Version]
- Šajbidorová, V.; Lichtnerová, H.; Paganová, V. The impact of different water regime on chlorophyll fluorescence of Pyrus pyraster L. and Sorbus domestica L. Acta Univ. Agric. Silvic. Mendel. Brun. 2015, 63, 1575–1579. [Google Scholar] [CrossRef]
- Sepúlveda, P.; Johnstone, D.M. Novel way of assessing plant vitality in urban trees. Forests 2019, 10, 2. [Google Scholar] [CrossRef]
- Swoczyna, T.; Latocha, P. Monitoring seasonal damage of photosynthetic apparatus in mature street trees exposed to road-side salinity caused by heavy traffic. Photosynthetica 2020, 58, 573–584. [Google Scholar] [CrossRef]
- Johnstone, D.; Tausz, M.; Moore, G.; Nicolas, M. Bark and leaf chlorophyll fluorescence are linked to wood structural changes in Eucalyptus saligna. AoB Plants 2014, 6, plt057. [Google Scholar] [CrossRef] [PubMed]
- Suchocka, M.; Swoczyna, T.; Kosno-Jończy, J.; Kalaji, H.M. Impact of heavy pruning on development and photosynthesis of Tilia cordata Mill. trees. PLoS ONE 2021, 16, e0256465. [Google Scholar] [CrossRef]
- de Almeida, K.E.C.; da Silva, J.G.S.; de Araujo Silva, I.M.; da Costa, A.L.; de Laia, M.L. de A. Ecophysiological analysis of Eucalyptus amaldulensis (Dehnh.) submitted to attack from Thaumastocoris peregrinus (Carpintero & Dellape). Rev. Árvore 2018, 42, e420120. [Google Scholar] [CrossRef]
- Ullah, M.I.; Arshad, M.; Ali, S.; Mehmood, N.; Khalid, S.; Afzal, M. Physiological characteristics of Citrus plants infested with citrus leafminer, Phyllocnistis citrella (Lepidoptera: Gracillariidae). Int. J. Fruit Sci. 2020, 20 (Suppl. S2), S871–S883. [Google Scholar] [CrossRef]
- Moustaka, J.; Meyling, N.V.; Hauser, T.P. Induction of a compensatory photosynthetic response mechanism in tomato leaves upon short time feeding by the chewing insect Spodoptera exigua. Insects 2021, 12, 562. [Google Scholar] [CrossRef]
- Cárdenas, A.M.; Gallardo, P. Relationship between insect damage and chlorophyll content in mediterranean oak species. Appl. Ecol. Environ. Res. 2016, 14, 477–491. [Google Scholar] [CrossRef]
- Golan, K.; Rubinowska, K.; Kmieć, K.; Kot, I.; Górska-Drabik, E.; Łagowska, B.; Michałek, W. Impact of scale insect infestation on the content of photosynthetic pigments and chlorophyll fluorescence in two host plant species. Arthropod-Plant Interact. 2015, 9, 55–65. [Google Scholar] [CrossRef]
- Baranovski, B.A.; Ivanko, I.A.; Gasso, V.J.; Ponomarenko, O.L.; Dubyna, D.V.; Roshchyna, N.O.; Karmyzova, L.O.; Poleva, J.L.; Nikolaieva, V.V. Biodiversity of the Regional Landscape Park Samara Plavni within the first large reservoir in Europe. Biosyst. Divers. 2021, 29, 160–179. [Google Scholar] [CrossRef]
- Baranovski, B.A.; Karmyzova, L.A.; Roshchyna, N.O.; Ivanko, I.A.; Karas, O.G. Ecological-climatic characteristics of the flora of a floodplain landscape in Southeastern Europe. Biosyst. Divers. 2020, 28, 98–112. [Google Scholar] [CrossRef]
- Kautsky, H.; Hirsch, A. Neue Versuche zur Kohlensäureassimilation. Naturwissenschaften 1931, 19, 964. [Google Scholar] [CrossRef]
- Antal, T.; Konyukhov, I.; Volgusheva, A.; Plyusnina, T.; Rubin, A. Chlorophyll fuorescence induction and relaxation system for the continuous monitoring of photosynthetic capacity in photobioreactors. Physiol. Plant 2018, 165, 476–486. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Li, B.; Zhang, H.; Hu, J.; Zhao, J. Photosynthetic rate prediction model of newborn leaves verified by core fluorescence parameters. Sci. Rep. 2020, 10, 3013. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, W.; Liu, Y.; Wang, L. Responses of growth and photosynthetic fluorescent characteristics in Ottelia acuminata to a water-depth gradient. J. Freshw. Ecol. 2018, 33, 285–297. [Google Scholar] [CrossRef]
- Alonso, L.; Van Wittenberghe, S.; Amorós-López, J.; Vila-Francés, J.; Gómez-Chova, L.; Moreno, J. Diurnal cycle relationships between passive fluorescence, PRI and NPQ of vegetation in a controlled stress experiment. Remote Sens. 2017, 9, 770. [Google Scholar] [CrossRef]
- Ayyaz, A.; Amir, M.; Umer, S.; Iqbal, M.; Bano, H.; Gul, H.S.; Noor, Y.; Kanwal, A.; Khalid, A.; Javed, M.; et al. Melatonin induced changes in photosynthetic efficiency as probed by OJIP associated with improved chromium stress tolerance in canola (Brassica napus L.). Heliyon 2020, 6, e04364. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Balabanova, D.; Paunov, M.; Goltsev, V.; Cuypers, A.; Vangronsveld, J.; Vassilev, A. Photosynthetic performance of the imidazolinone resistant sunflower exposed to single and combined treatment by the herbicide imazamox and an amino acid extract. Front. Plant Sci. 2016, 7, 1559. [Google Scholar] [CrossRef] [PubMed]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Vasylenko, O.; Kondratenko, T.; Havryliuk, O.; Andrusyk, Y.; Kutovenko, V.; Dmytrenko, Y.; Grevtseva, N.; Marchyshyna, Y. The study of the productivity potential of grape varieties according to the indicators of functional activity of leaves. Potravin. Slovak J. Food Sci. 2021, 15, 639–647. [Google Scholar] [CrossRef]
- Stefanov, M.A.; Rashkov, G.D.; Apostolova, E.L. Assessment of the Photosynthetic Apparatus Functions by Chlorophyll Fluorescence and P700 Absorbance in C3 and C4 Plants under Physiological Conditions and under Salt Stress. Int. J. Mol. Sci. 2022, 23, 3768. [Google Scholar] [CrossRef]
- Chen, X.; Mo, X.; Hu, S.; Liu, S. Relationship between fluorescence yield and photochemical yield under water stress and intermediate light conditions. J. Exp. Bot. 2019, 70, 301–313. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Antonacci, A.; Arduini, F.; Moscone, D.; Campos, V.R.E.; Fraceto, L.F.; Palleschi, G. An eco-designed paper-based algal biosensor for nanoformulated herbicide optical detection. J. Hazard. Mater. 2019, 373, 483–492. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).