Arbuscular Mycorrhiza Extraradical Mycelium Promotes Si and Mn Subcellular Redistribution in Wheat Grown under Mn Toxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Chlorophyll a Fluorescence Parameters
2.3. Activity of Antioxidant Enzymes in Wheat Shoots
2.4. Wheat Shoots Subcellular Partitioning
2.5. Multi-Element Analysis of the Shoots and Respective Fractions
2.5.1. Plant Sample Acidic Digestion
2.5.2. Elemental Analysis
2.6. Statistical Analysis
3. Results
3.1. Wheat Shoot Growth and Chlorophyll a Fluorescence
3.2. Activity of Antioxidant Enzymes
3.3. Element Contents
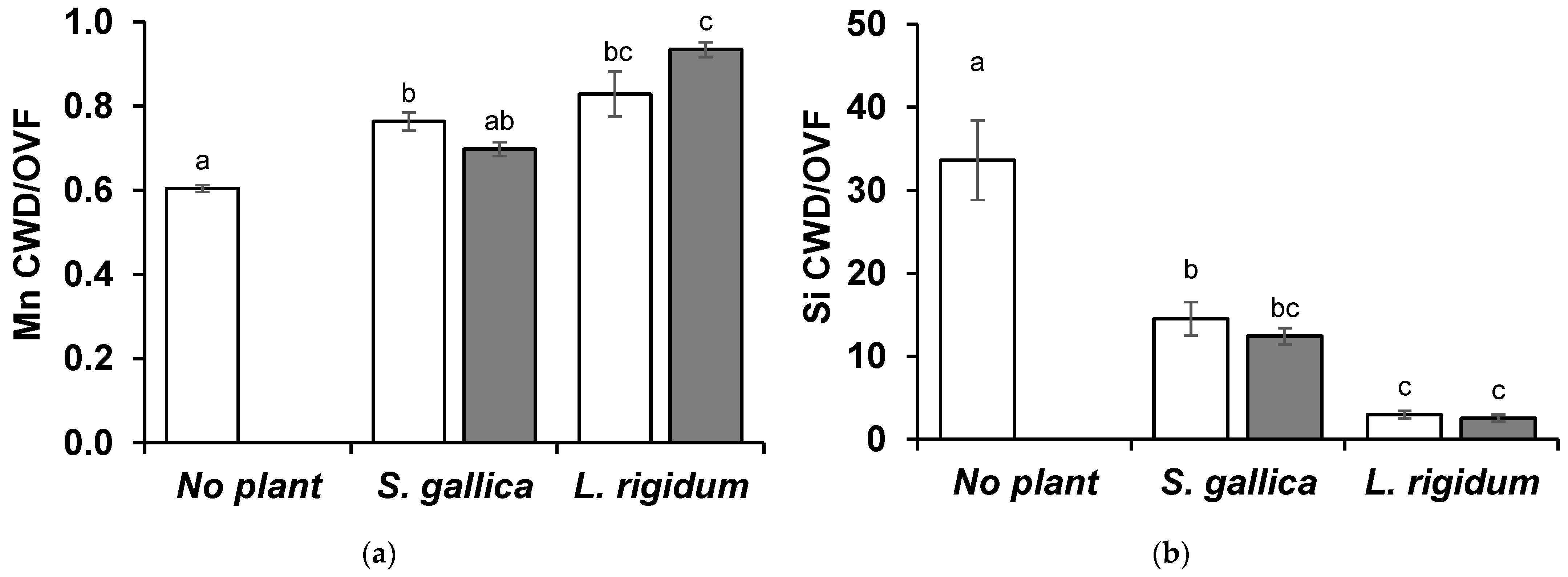
3.4. Subcellular Element Distribution
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manag. 2016, 32, 390–399. [Google Scholar] [CrossRef]
- Goss, M.J.; Carvalho, M.J.G.P.R. Manganese toxicity: The significance of magnesium for the sensitivity of wheat plants. Plant Soil 1992, 139, 91–98. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. Aluminium, Iron and Silicon Subcellular Redistribution in Wheat Induced by Manganese Toxicity. Appl. Sci. 2021, 11, 8745. [Google Scholar] [CrossRef]
- Keisling, T.C.; Thompson, L.F.; Slabaugh, W.R. Visual symptoms and tissue manganese concentrations associated with manganese toxicity in wheat. Commun. Soil Sci. Plant Anal. 1984, 15, 537–540. [Google Scholar] [CrossRef]
- Le Bot, J.; Goss, M.J.; Carvalho, M.J.G.P.R.; Van Beusichem, M.L.; Kirkby, E.A. The significance of the magnesium to manganese ratio in plant tissues for growth and alleviation of manganese toxicity in tomato (Lycopersicon esculentum) and wheat (Triticum aestivum) plants. Plant Soil 1990, 124, 205–210. [Google Scholar] [CrossRef]
- Carvalho, M.; Goss, M.J.; Teixeira, D. Manganese toxicity in Portuguese Cambisols derived from granitic rocks: Causes, limitations of soil analyses and possible solutions. Rev. Ciênc. Agrár. 2015, 38, 518–527. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 476–494. [Google Scholar] [CrossRef] [Green Version]
- Lidon, F.C.; Teixeira, M.G. Oxy radicals production and control in the chloroplast of Mn-treated rice. Plant Sci. 2000, 152, 7–15. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Alho, L.; Carvalho, M. Toxic levels of manganese in an acidic Cambisol alters antioxidant enzymes activity, element uptake and subcellular distribution in Triticum aestivum. Ecotoxicol. Environ. Saf. 2020, 193, 110355. [Google Scholar] [CrossRef]
- de Vargas, J.P.R.; dos Santos, D.R.; Bastos, M.C.; Schaefer, G.; Parisi, P.B. Application forms and types of soil acidity corrective: Changes in depth chemical attributes in long term period experiment. Soil Tillage Res. 2019, 185, 47–60. [Google Scholar] [CrossRef]
- Che, J.; Yamaji, N.; Shao, J.F.; Ma, J.F.; Shen, R.F. Silicon decreases both uptake and root-to-shoot translocation of manganese in rice. J. Exp. Bot. 2016, 67, 1535–1544. [Google Scholar] [CrossRef] [Green Version]
- Doncheva, S.; Poschenrieder, C.; Stoyanova, Z.; Georgieva, K.; Velichkova, M.; Barceló, J. Silicon amelioration of manganese toxicity in Mn-sensitive and Mn-tolerant maize varieties. Environ. Exp. Bot. 2009, 65, 189–197. [Google Scholar] [CrossRef]
- Li, P.; Song, A.; Li, Z.; Fan, F.; Liang, Y. Silicon ameliorates manganese toxicity by regulating manganese transport and antioxidant reactions in rice (Oryza sativa L.). Plant Soil 2012, 354, 407–419. [Google Scholar] [CrossRef]
- Alam, S.; Rahman, M.H.; Kamei, S.; Kawai, S. Alleviation of manganese toxicity and manganese-induced iron deficiency in barley by additional potassium supply in nutrient solution. Soil Sci. Plant Nutr. 2002, 48, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.; Kamei, S.; Kawai, S. Amelioration of manganese toxicity in young rice seedlings with potassium. J. Plant Nutr. 2003, 26, 1301–1314. [Google Scholar] [CrossRef]
- Alam, S.; Akiha, F.; Kamei, S.; Huq, S.M.I.; Kawai, S. Mechanism of potassium alleviation of manganese phytotoxicity in barley. J. Plant Nutr. 2005, 28, 889–901. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R. Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol. Environ. Saf. 2018, 147, 881–896. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Kuşvuran, A.; Alharby, H.F.; Kuşvuran, S.; Rady, M.M. The defensive role of silicon in wheat against stress conditions induced by drought, salinity or cadmium. Ecotoxicol. Environ. Saf. 2018, 154, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant—Fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.G. Role of soil microbes in the rhizospheres of plants growing on trace metal contaminated soils in phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef]
- Cabral, L.; Soares, C.R.F.S.; Giachini, A.J.; Siqueira, J.O. Arbuscular mycorrhizal fungi in phytoremediation of contaminated areas by trace elements: Mechanisms and major benefits of their applications. World J. Microbiol. Biotechnol. 2015, 31, 1655–1664. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 68, 139–146. [Google Scholar] [CrossRef]
- Corrêa, A.; Cruz, C.; Pérez-Tienda, J.; Ferrol, N. Shedding light onto nutrient responses of arbuscular mycorrhizal plants: Nutrient interactions may lead to unpredicted outcomes of the symbiosis. Plant Sci. 2014, 221–222, 29–41. [Google Scholar] [CrossRef]
- Cicatelli, A.; Todeschini, V.; Lingua, G.; Biondi, S.; Torrigiani, P.; Castiglione, S. Epigenetic control of heavy metal stress response in mycorrhizal versus non-mycorrhizal poplar plants. Environ. Sci. Pollut. Res. 2014, 21, 1723–1737. [Google Scholar] [CrossRef]
- Alho, L.; Carvalho, M.; Brito, I.; Goss, M.J. The effect of arbuscular mycorrhiza fungal propagules on the growth of subterranean clover (Trifolium subterraneum L.) under Mn toxicity in ex situ experiments. Soil Use Manag. 2015, 31, 337–344. [Google Scholar] [CrossRef]
- Brito, I.; Carvalho, M.; Alho, L.; Goss, M.J. Managing arbuscular mycorrhizal fungi for bioprotection: Mn toxicity. Soil Biol. Biochem. 2014, 68, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Brígido, C.; van Tuinen, D.; Brito, I.; Alho, L.; Goss, M.J.; Carvalho, M. Management of the biological diversity of AM fungi by combination of host plant succession and integrity of extraradical mycelium. Soil Biol. Biochem. 2017, 112, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.; Nobre, T.; Goss, M.J.; Faria, J.; Barrulas, P.; Carvalho, M. Transcriptome Analysis of Wheat Roots Reveals a Differential Regulation of Stress Responses Related to Arbuscular Mycorrhizal Fungi and Soil Disturbance. Biology 2019, 8, 93. [Google Scholar] [CrossRef] [Green Version]
- Faria, J.M.S.; Pinto, A.P.; Teixeira, D.; Brito, I.; Carvalho, M. Diversity of Native Arbuscular Mycorrhiza Extraradical Mycelium Influences Antioxidant Enzyme Activity in Wheat Grown Under Mn Toxicity. Bull. Environ. Contam. Toxicol. 2021, 108, 451–456. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, H.; Yang, L.; Ye, B.; Wang, W. Dynamic changes of rhizosphere properties and antioxidant enzyme responses of wheat plants (Triticum aestivum L.) grown in mercury-contaminated soils. Chemosphere 2013, 93, 972–977. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Oxygen radicals in biological systems. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Aravind, P.; Prasad, M.N.V. Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L.: A free floating freshwater macrophyte. Plant Physiol. Biochem. 2003, 41, 391–397. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Teixeira, D.M.; Pinto, A.P.; Brito, I.; Barrulas, P.; Carvalho, M. The Protective Biochemical Properties of Arbuscular Mycorrhiza Extraradical Mycelium in Acidic Soils Are Maintained throughout the Mediterranean Summer Conditions. Agronomy 2021, 11, 748. [Google Scholar] [CrossRef]
- Pinto, E.; Aguiar, A.A.; Ferreira, I.M.P.L.V.O. Influence of soil chemistry and plant physiology in the phytoremediation of Cu, Mn, and Zn. CRC. Crit. Rev. Plant Sci. 2014, 33, 351–373. [Google Scholar] [CrossRef] [Green Version]
- Brito, I.; Goss, M.J.; Alho, L.; Brígido, C.; van Tuinen, D.; Félix, M.R.; Carvalho, M. Agronomic management of AMF functional diversity to overcome biotic and abiotic stresses—The role of plant sequence and intact extraradical mycelium. Fungal Ecol. 2019, 40, 72–81. [Google Scholar] [CrossRef]
- Goss, M.J.; Carvalho, M.; Brito, I. Functional Diversity of Mycorrhiza and Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2017; Volume 4, ISBN 9780128042441. [Google Scholar]
- Subrahmanyam, D.; Rathore, V.S. Influence of Manganese Toxicity on Photosynthesis in Ricebean (Vigna Umbellata) Seedlings. Photosynthetica 2000, 38, 449–453. [Google Scholar] [CrossRef]
- Hernández, L.E.; Sobrino-Plata, J.; Montero-Palmero, M.B.; Carrasco-Gil, S.; Flores-Cáceres, M.L.; Ortega-Villasante, C.; Escobar, C. Contribution of glutathione to the control of cellular redox homeostasis under toxic metal and metalloid stress. J. Exp. Bot. 2015, 66, 2901–2911. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, M.A.; Cardoso, E.J.B.N.; Hampp, R. Manganese toxicity and callose deposition in leaves are attenuated in mycorrhizal soybean. Plant Soil 2002, 246, 1–10. [Google Scholar] [CrossRef]
- Iwasaki, K.; Maier, P.; Fecht, M.; Horst, W.J. Effects of silicon supply on apoplastic manganese concentrations in leaves and their relation to manganese tolerance in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil 2002, 238, 281–288. [Google Scholar] [CrossRef]
- Rogalla, H.; Römheld, V. Role of leaf apoplast in silicon-mediated manganese tolerance of Cucumis sativus L. Plant Cell Environ. 2002, 25, 549–555. [Google Scholar] [CrossRef]
- Maksimović, J.D.; Mojović, M.; Maksimović, V.; Römheld, V.; Nikolic, M. Silicon ameliorates manganese toxicity in cucumber by decreasing hydroxyl radical accumulation in the leaf apoplast. J. Exp. Bot. 2012, 63, 2411–2420. [Google Scholar] [CrossRef] [Green Version]
- Blamey, F.P.C.; Mckenna, B.A.; Li, C.; Cheng, M.; Tang, C.; Jiang, H.; Daryl, L.; Paterson, D.J.; Kappen, P.; Wang, P.; et al. Manganese distribution and speciation help to explain the effects of silicate and phosphate on manganese toxicity in four crop species. New Phytol. 2017, 217, 1146–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Yi, K.; Chang, M.M.; Ling, G.Z.; Zhao, Z.K.; Li, X.F. Sequestration of Mn into the cell wall contributes to Mn tolerance in sugarcane (Saccharum officinarum L.). Plant Soil 2019, 436, 475–487. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, Z.; Xu, M.; Qian, Q.; Yu, J. Effect of excess manganese on the antioxidant system in Cucumis sativus L. under two light intensities. Environ. Exp. Bot. 2006, 58, 197–205. [Google Scholar] [CrossRef]
- Maksimović, J.D.; Bogdanović, J.; Maksimović, V.; Nikolic, M. Silicon modulates the metabolism and utilization of phenolic compounds in cucumber (Cucumis sativus L.) grown at excess manganese. J. Plant Nutr. Soil Sci. 2007, 170, 739–744. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Conceição, T.A.; Teixeira, D.M.; Brito, I.; Barrulas, P.; Pinto, A.P.; Vaz, M.; Carvalho, M. Arbuscular Mycorrhiza Extraradical Mycelium Promotes Si and Mn Subcellular Redistribution in Wheat Grown under Mn Toxicity. Int. J. Plant Biol. 2022, 13, 82-94. https://doi.org/10.3390/ijpb13020009
Faria JMS, Conceição TA, Teixeira DM, Brito I, Barrulas P, Pinto AP, Vaz M, Carvalho M. Arbuscular Mycorrhiza Extraradical Mycelium Promotes Si and Mn Subcellular Redistribution in Wheat Grown under Mn Toxicity. International Journal of Plant Biology. 2022; 13(2):82-94. https://doi.org/10.3390/ijpb13020009
Chicago/Turabian StyleFaria, Jorge M. S., Taiana A. Conceição, Dora Martins Teixeira, Isabel Brito, Pedro Barrulas, Ana Paula Pinto, Margarida Vaz, and Mário Carvalho. 2022. "Arbuscular Mycorrhiza Extraradical Mycelium Promotes Si and Mn Subcellular Redistribution in Wheat Grown under Mn Toxicity" International Journal of Plant Biology 13, no. 2: 82-94. https://doi.org/10.3390/ijpb13020009
APA StyleFaria, J. M. S., Conceição, T. A., Teixeira, D. M., Brito, I., Barrulas, P., Pinto, A. P., Vaz, M., & Carvalho, M. (2022). Arbuscular Mycorrhiza Extraradical Mycelium Promotes Si and Mn Subcellular Redistribution in Wheat Grown under Mn Toxicity. International Journal of Plant Biology, 13(2), 82-94. https://doi.org/10.3390/ijpb13020009








