Abstract
Soils and water resources of our ecosystems may contain Barium (Ba), a toxic metal naturally existent in the Earth’s crust and also can be derived from recycled wastes produced of several anthropogenic activities. As a result of this fact, the accumulation of Ba in agriculture soils would increase to reach the crops and eventually end up in the human food chain. The purpose of this work was to study tolerance and accumulation abilities in Limbarda crithmoides and Helianthus annuus treated with increasing concentrations of barium (from 0 to 500 µM) for 45 days. In order to evaluate the response of these species to Ba stress, the biomass production, the water status, and the accumulation of the secondary metabolites, macronutrients, total inorganic nitrogen (TIN), and Ba in shoots and roots, as well as chlorophyll levels, and metal tolerance index of the entire plant were assessed. Results showed an increase in plant biomass production and tolerance index in the two species with increasing Ba concentration. A significant increase in polyphenols and flavonoids levels was also shown with no negative effect on the macronutrients and TIN; however, the latter were found reduced in roots of L. crithmoides. Chlorophylls also were not affected. An average of 3000 µg·g−1 DW of Ba was accumulated in each organ of L. crithmoides while H. annuus accumulated up to 1350 µg·g−1 DW in the shoots. Our findings proved that L. crithmoides and H. annuus were susceptible to tolerate Ba-induced stress with high levels of Ba accumulation in the aboveground parts as well as in the roots during the 45 days of the experiments.
1. Introduction
Barium (Ba) is one of the alkaline trace metallic elements (TME) that constitutes roughly 0.05% of the Earth’s crust and is found present in the environment in relatively high levels [1,2]. Barite (barium sulphate) and witherite (barium carbonate) are the main common barium compounds that can be found in nature, specifically in sedimentary rocks, as underground ore deposits [3,4]. Due to the low water solubility of these two compounds, Ba is not predicted to be very mobile in soils, unless in acid soils [2,5,6]; this property may reduce the environmental risk in aerated soils [7]. However, the most harmful forms of water soluble barium compounds are barium acetate, barium hydroxide, barium sulphide, barium nitrate, and barium chloride, which are abundantly used in several industries [3].
Generally, Ba is used in wide production fields including glass, bricks, cement, ceramics, electronics, soaps, fluorescent lamps, insecticides, fertilisers and soil amendments, plastic stabilisers, railroad flares, paints, fireworks, explosives, fine chemicals, drilling fluids, lubricating oil additives, sugar refining, and paper coating [2,3,8]. Barium could be also discharged in wastewater from industrial and metallurgical processes, and the resulting wastes such as drilling fluids waste or mud are frequently disposed in farmlands [2,9]. The increased availability of Ba in various anthropogenic fields may induce an inappropriate accumulation of this metal in our ecosystems to reach water and agricultural soils [10]. It has been mentioned that the concentration of Ba in agricultural soils ranges from 10 to 5000 mg·kg−1, with an average abundance of about 500 mg·kg−1 [3].
As unessential element for all living organisms including humans, animals, and plants, Ba exposure can be toxic in high concentrations [11]. Human ingestion of Ba may induce various health issues including cardiac and renal failure, pulmonary edema, respiratory paralysis, and gastric and intestinal haemorrhages; Kravchenko et al. also stated that the consumption of BaCl2 causes vomiting, diarrhoea, liver and kidney failure, cardiac arrhythmia, anxiety, disorders of the nervous system, brain swelling, and even paralysis [12]. However, few studies have been performed to investigate the effect of barium on plant species and information concerning Ba phytotoxicity is also limited [10].
Marisamy et al. reported that photosynthetic pigments, including chlorophylls a and b and carotenoids, decreased in Amaranthus caudatus L. and Cyamopsis tetragonoloba L. with the increase of Ba concentrations in the growth medium; moreover, inhibitory effects of Ba on the growth of these two species were noted where root and shoot lengths, leaf area, and fresh and dry biomass productions were significantly reduced [13,14]. On the contrary, other plants grown in Ba-contaminated medium showed adaptation abilities to elevated Ba concentrations throughout the enhancement of plant biomass and the accumulation of high Ba amounts in the plant’s tissues, such as Typha domingensis [7], mustard, sunflower, and castor bean [15].
It has been mentioned that numerous halophytes are capable of tolerating metallic stress [16]. In this context, among halophilic species, Limbarda crithmoides L. (synonym Inula crithmoides L. Dumort.) is known for its extremely high tolerance to salinity [17] and was chosen in this study as the test halophyte to assess the behaviour of this plant against Ba-induced stress, when compared with Helianthus annuus (glycophyte). The present study was conducted to evaluate metal phytotoxicity and tolerance ability in these two plant species throughout the assessment of Ba effect on the growth, mineral nutrition, and chlorophyll synthesis and how antioxidants such as polyphenols and flavonoids were involved to defend against Ba stress. On the other hand, Ba contents were also determined, in the shoots and roots, to investigate barium accumulation potential in the present species and explore new Ba-hyperaccumulators species that could be used as an ecological alternative for the remediation of Ba-polluted soils.
2. Material and Methods
2.1. Plant Culture and Treatments Experimentations
Seeds of Limbarda crithmoides were collected from the north shore of the lagoon of Bizerte in Menzel Jemil, Bizerte, Tunisia (37°14′19″ N, 9°54′59″ E). Seeds of Helianthus annuus were obtained from a land farm of sunflower situated in the north of Tunisia (36°49′90″ N, 9°13′77″ E). Collected seeds were naturally dried before starting the culture.
Plants were grown in plastic pots containing a mixture of perlite and gravel substrate (2:1; v/v), in a semi-controlled greenhouse (natural photoperiod, temperature of 25 ± 5 °C, relative humidity ranging between 60% and 90%). After the germination, seedlings were regularly watered with Hewitt nutritive solution [18]. Subsequently, plants were divided into 5 groups of 10 plants and regularly treated with a volume of 100 mL/pot of nutritive solution supplemented with Ba at 0, 100, 200, 300, and 500 µM (until the water flowed out of the pot), 3 times a week for 45 days. Barium chloride (BaCl2, Sigma-Aldrich St. Louis, CA, USA) was used as a source of Ba.
Shoot and root fresh weights were separately determined immediately after the collection of the treated plants, which were subsequently dried in the oven to constant weight (70 ± 2 °C). After the drying process, dry weights were taken to determine the water content and metal tolerance index [19,20,21,22]. Tolerance index and water content were calculated according to the following equations, respectively:
2.2. Chlorophyll Fluorescence
Chlorophyll a fluorescence parameters were measured using a FluoroPen FP100-MAX (Photon systems Instruments, Dràsov, Czech Republic) and the measurements were performed in L. crithmoides and H. annuus old leaves after 45 days of Ba exposure [23]. The maximum primary yield of photochemistry of Photosystem II (Fv/Fo), the maximum quantum efficiency of Photosystem II (Fv/Fm), the photochemical efficiency of Photosystem II (Fv’/Fm’), and the Electron transport rate (ETR) were determined. Absorbed (ABS), trapped (TRo), dissipated (DIo), and transported (ETo) energy fluxes per reaction centre (RC) were also measured [24].
2.3. Chlorophyll Content Determination
Chlorophyll a and b extraction was preformed following Bankaji et al.’s protocol [25]. Briefly, approximately 250 mg of fresh leaves tissue were mixed in 10 mL of ethanol 80%. The prepared mixture was placed in a water bath at 80 °C for 40 min. The absorbance measurements of the extracts were read at 645 and 663 nm using a spectrophotometer (Genesys 10S UV-Visible, Thermo Scientific, Madison, WI, USA). Chlorophyll a and b concentrations were expressed in milligrams per gram of fresh weight (mg·g−1 FW) and calculated following Ayvaz et al. [26].
2.4. Minerals and Barium Accumulation
Minerals (K, Ca, and Mg) and Ba were quantified in the shoots and roots of the treated plants by atomic absorption spectrometry (Perkin Elmer PinAAcle900T, Waltham, MA, USA); after the digestion of 30 mg of dry samples (shoots or roots) in 3 mL of an acid mixture (containing HNO3:H2SO4:HClO4; 10:1:0.5; v/v/v) at 110 °C for 2 h, the extracts were diluted with 0.5% HNO3 (v/v) [21,22,27].
2.5. Total Inorganic Nitrogen
Total inorganic nitrogen (TIN) content was determined following Khjeldahl’s method [28] where 30 mg of the dry sample (shoots or roots) was gradually heated (from 150 to 350 °C for 2 h) in pure sulfuric acid (95%) to transform the organic nitrogen into its mineral form. The TIN content was determined in milligrams per gram of the dry weight of each organ.
2.6. Polyphenols and Flavonoids
Polyphenols and flavonoids contained in 30 mg of plant dry tissues (shoots or roots) were extracted in methanol 80% and determined following Velioglu et al. and Lamaison and Carnat’s methods [29,30], respectively. To determine polyphenols level, 150 µL of the extract was added to 500 µL of Folin–Ciocalteu reagent (10%, v/v) and 400 µL of Na2CO3 (7.5%, w/v); then, the mixture was incubated for 90 min in dark and the absorbance was read at 765 nm. A mixture consisting of 500 µL of the extract and 500 µL of AlCl3 (2%, w/v) was incubated for 30 min to determine flavonoids and the absorbance was read at 430 nm.
2.7. Statistical Analysis
Each analysis used 10 biological replicates and all the values were presented as means ± standard deviation (SD). Statistics were evaluated using ANOVA analysis and Tukey honest significant difference (HSD) tests by STATISTICA software. Significant differences among treatments were shown at p < 0.05 and p < 0.001. Associations analysis between the studied parameters was performed by correlation circle from principal component analysis (PCA) using STATISTICA 8.0 software.
3. Results and Discussion
3.1. Plant Growth
Although Ba is considered a non-essential metal for living organisms [8,12], research on the toxicity induced by this metal in plants is limited, where most of the studies reported its inhibitory effect on plant growth [31]. Among the physiological changes that could be induced by Ba stress is the inhibition of the photosynthetic process through the depletion of CO2 assimilation, resulting in a reduction in plant growth [32,33,34].
Contrarily to the findings stated in the previous studies, the results shown in Table 1 showed positive effects of Ba on the biomass production of the studied species. When compared with the control, Ba significantly increased shoots DW in L. crithmoides by increasing Ba concentration in the nutritive solution (p < 0.05). The roots showed also an elevation in DW with the increase of Ba levels; however, the increase was only significant at 500 µM Ba (p < 0.05; Table 1). Moreover, a similar trend was detected in the FW of the shoots and roots but the noted increase was only significant in the shoots of 500 µM Ba (p < 0.05; Table 1). Regarding plants morphology, L. crithmoides did not show any phytotoxicity signs after Ba exposure (Figure S1). In contrast, a slight increase was shown in the DW and FW of the shoots and roots of H. annuus in all Ba concentrations; however, the variation was not significant as compared with the untreated plants (Table 1). In addition, only plant height reduction was observed in all Ba-treated plants (Figure S2).

Table 1.
Assessment of the growth (DW: dry weight, FW: fresh weight, WC: water content) and the metal tolerance index (TI %) in the shoots and roots of Limbarda crithmoides and Helianthus annuus after 45 days of Ba exposure. Data are shown as means ± SD; values with lowercase denote significant differences at p < 0.05.
These findings were in agreement with Bouslimi et al.’s results [11], where the supplied Ba increased the fresh biomass production in Brassica juncea species shoots and roots. Other studies reported that shoots of Helianthus annuus, Brassica juncea, and Ricinus communis plants grown in a Rhodic Hapludox soil containing 150 and 300 mg·kg−1 of BaSO4 did not show any signs of plant intoxication induced by Ba stress [35]. Ribeiro et al. also mentioned the absence of Ba morphological toxicity signs in Oryza sativa, Eleocharis interstincta, and Cyperus papyrus species with non-significant variation in shoots and roots dry biomass in the two first species, while Cyperus papyrus plants treated with varying concentrations of Ba significantly increased the dry biomass of its shoots and roots [36]; it was reported that low metal concentrations can stimulate the increase of mitotic index, leading to the increase in plant growth [21].
In response to metallic stress, plants may develop adaptation characteristics through the increase of tolerance strategies to reduce or avoid the adverse effects of toxic metals. For example, aluminium induced the increase of chaperone proteins in the plant cells of soybean and citrus, resulting in the maintenance of cellular and protein homeostasis, and, therefore, this enhanced the tolerance of the plant to harmful environmental conditions [19,37,38]. This particularity can be evaluated by the determination of the metal tolerance index in plants [39,40]. Results showed (Table 1) that the TI of the entire plants of L. crithmoides and H. annuus treated with Ba were higher than 100% and it was found to increase with the increase of Ba concentration. This confirmed that both L. crithmoides and H. annuus species highly tolerated the different supplied concentrations of Ba in the growth medium. Additionally, our results were supported by Bouslimi et al., who observed that Brassica juncea was able to tolerate Ba stress [11]. Regarding the obtained results, Ba might be beneficial for plants; Srivastava et al. reported that Ba has been classified among the essential micronutrients, which remains associated with some metallo-enzymes; nonetheless, this metal is toxic at high concentrations [41].
The hydration state in stressed plants could be assessed by the estimation of water content [42]. It was reported that heavy metals such as Cd and Pb introduced the disturbance of water balance and transpiration rate in plants; indeed, a decrease in relative water content and transpiration was observed in Atriplex canscens [43]. In this study, Ba treatments did not induce any significant effect (p > 0.05; Table 1) on the water content of L. crithmoides and H. annuus when compared to the control (a negligible decrease was noted in WC in both shoots and roots under the effect of all Ba concentrations in L. crithmoides). It was also reported that the water status was not significantly affected by the application of Ba in Glycine max and Cucumis sativus plants [31,32]. This behaviour might be due to the accumulation of proline in plant cells, acting as a cytoplasmic osmotic solute, to preserve water and avoid the internal water deficit [44]. Thus, plants could develop an adaptive response to stress through the accumulation of this amino acid in order to increase the resistance to metals [44,45].
According to the results discussed above, it is possibly admitted that L. crithmoides and H. annuus, treated with different Ba concentrations, interestingly acquired tolerance to barium, which could be a result of the stimulation of some growth phytohormones such as auxin, abscisic acid, gibberellic acid, and cytokinin, etc. These phytohormones are known by their effective implication in metal toxicity mitigation and growth inhibition prevention in retaining plant growth plasticity during development [46]. A previous study reported that a metallophyte species (moss Scopelophila cataractae) maintained a good plant growth under high copper concentration and this was due to the accumulation of auxin that activated genes required for optimal growth and cell differentiation [47].
3.2. Chlorophyll Fluorescence
After 45 days of Ba treatments, the maximum primary yield of photosystem II (PSII) photochemistry (Fv/Fo), the maximum quantum efficiency of PSII (Fv/Fm), and the photochemical efficiency of PSII (Fv’/Fm’) in L. crithmoides and H. annuus-treated plants remained statistically the same as the control (p < 0.05; Table 2); the electron transport rate (ETR) of L. crithmoides significantly increased only under 500 µM Ba (p < 0.05). Contrarily, H. annuus plants showed no significant variation in all Ba concentrations (p > 0.05) when compared to the control. It is well known that the photosynthetic apparatus is sensitive to adverse environmental constraints [48]; thus, the assessment of the photosynthetic quenching parameters can provide estimation on plant leaf photosynthetic capacity to evaluate the adaptation potential of plants to abiotic stress [24]. Results showed that Ba exposure did not induce significant impacts on PSII photochemical performance including Fv/Fo, Fv/Fm, and Fv’/Fm’ in L. crithmoides and H. annuus (Table 2); these findings are in line with Reboredo et al. and Sghaier et al., who reported that metal stress induced by arsenic did not affect Fv/Fm and Fv’/Fm’ in Eucalyptus globulus [49] and Tamarix gallica [23] plants. Similar results were also reported in Elsholtzia argyi species treated with high Cd concentrations (Fv/Fo did not differ as well under the stress) [50].

Table 2.
Chlorophyll fluorescence parameters of Limbarda crithmoides and Helianthus annuus under the effect of different Ba concentrations after 45 days of the treatment. Data are shown as means ± SD; values with lower-case denote significant differences at p < 0.05. Fv/Fo: maximum primary yield of photochemistry of Photosystem II, Fv/Fm: maximum quantum efficiency of Photosystem II, Fv’/Fm’: photochemical efficiency of Photosystem II, ETR: electron transport rate, ABS/RC: absorption flux, TRo/RC: trapped energy flux, ETo/RC electron transport flux, DIo/RC: dissipation flux, RC: active reaction center.
Regarding energy fluxes, L. crithmoides showed a significant increase in the absorbed (ABS) and dissipated (DIo) energy fluxes in 500 µM Ba-treated plants only (p < 0.05), when compared with that of the control; however, no changes were marked in the trapped (TRo) and transported energy fluxes (ETo) in all treated plants (p > 0.05). H. annuus showed that the energy absorption and dissipation significantly increased in the highest Ba concentrations (300 and 500 µM) while the transported energy was significantly reduced at the same concentrations (p < 0.05) in comparison with the control. The trapped energy was not modified under Ba (p > 0.05; Table 2). The increase in ABS/RC under the effect of abiotic stress could be explained by the inactivation of some parts of PSII reaction centers (RCs) and that might be due to the inactivation of the oxygen-evolving complexes (OEC), or by the increase of antenna size [51,52,53]. Liu et al. also reported the significant increase in absorbed and dissipated energy fluxes in Melia azedarach treated with manganese [53]. Additionally, Pb stress induced an increase in energy absorption and dissipation within the PSII with a decline in electron transport [54]. It has been revealed that some plant species like halophytes (e.g., Tamarix gallica) are able to dissipate excessive energy in order to overcome the accumulation of excessive reducing energy that is known as the primary source of free radicals generation, and this could avoid the photo-destruction of the photosynthetic apparatus [23].
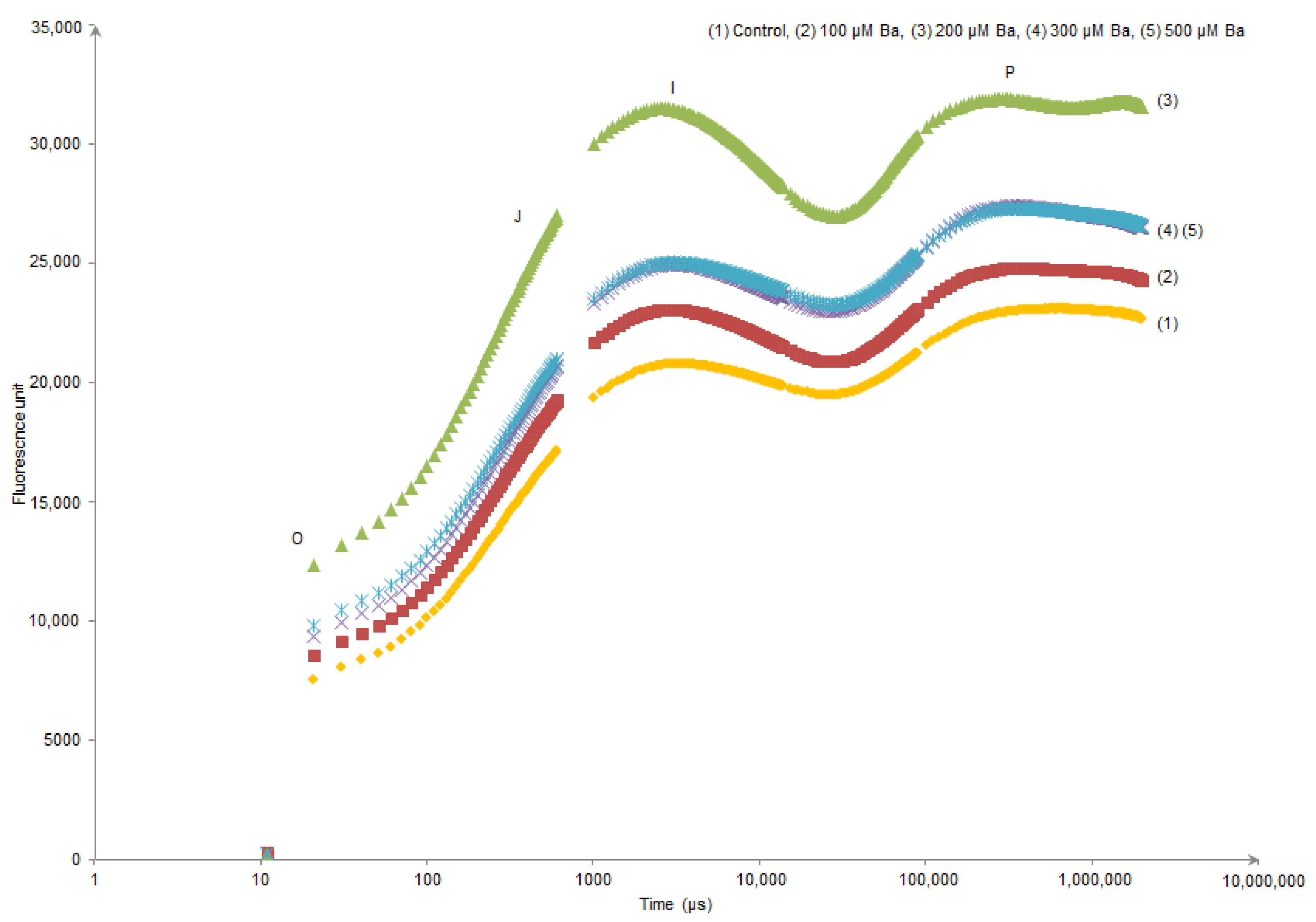
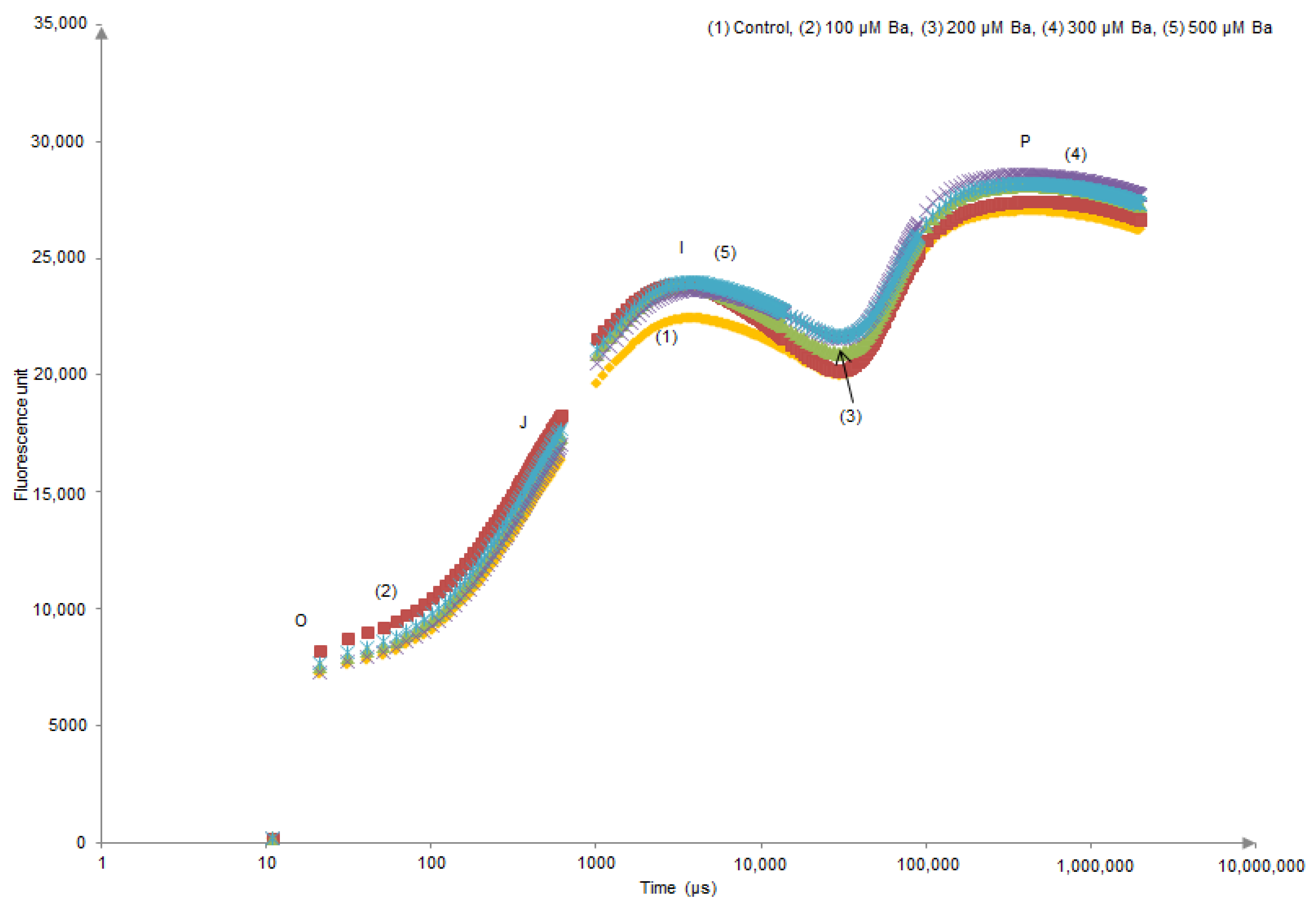
Kaustky plots derived from the OJIP transient analysis only showed an evident modification in (O–J) and (J–I) sides of the curve of L. crithmoides plants exposed to 200 µM Ba (Figure 1). In contrast, H. annuus did not show different behaviour in the Kautsky curves of all Ba treatments when compared to the control (Figure 2).
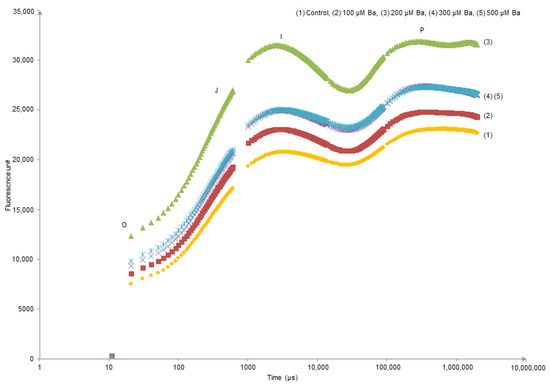
Figure 1.
Kautsky curves derived from the OJIP transient analysis in dark-adapted leaves of Limbarda crithmoides after 45 days of Ba exposure.
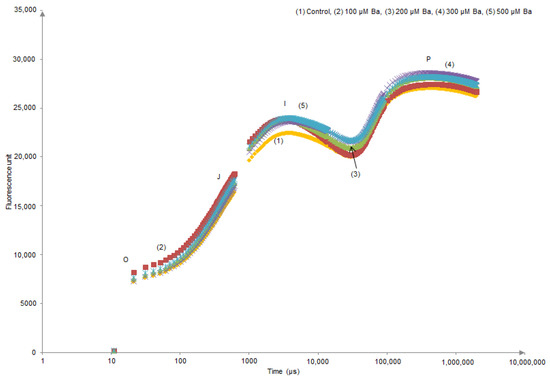
Figure 2.
Kautsky curves derived from the OJIP transient analysis in dark-adapted leaves of Helianthus annuus after 45 days of Ba exposure.
OJIP transient of dark-adapted leaves presents a polyphasic chlorophyll a fluorescence induction curve. In the results presented in Figure 1 and Figure 2, the OIJP curve was only higher in L. crithmoides of 200 µM Ba with no considerable changes observed in H. annuus. Indeed, the increase in chlorophyll fluorescence in the O–I part of the plot might be due to the net photochemical reduction of QA (first electron acceptor of PSII) to QA–, while the increase of J–I typically depends on a disturbance in the structure and function of OECs inducing the alteration of the rate of oxygen evolution [23,55]. Moreover, the rise in the I–P part is attributed to the reduction of electron transporters (e.g., ferredoxin, intermediary acceptors, NADP) of the Photosystem I acceptor side [56].
3.3. Chlorophylls Contents
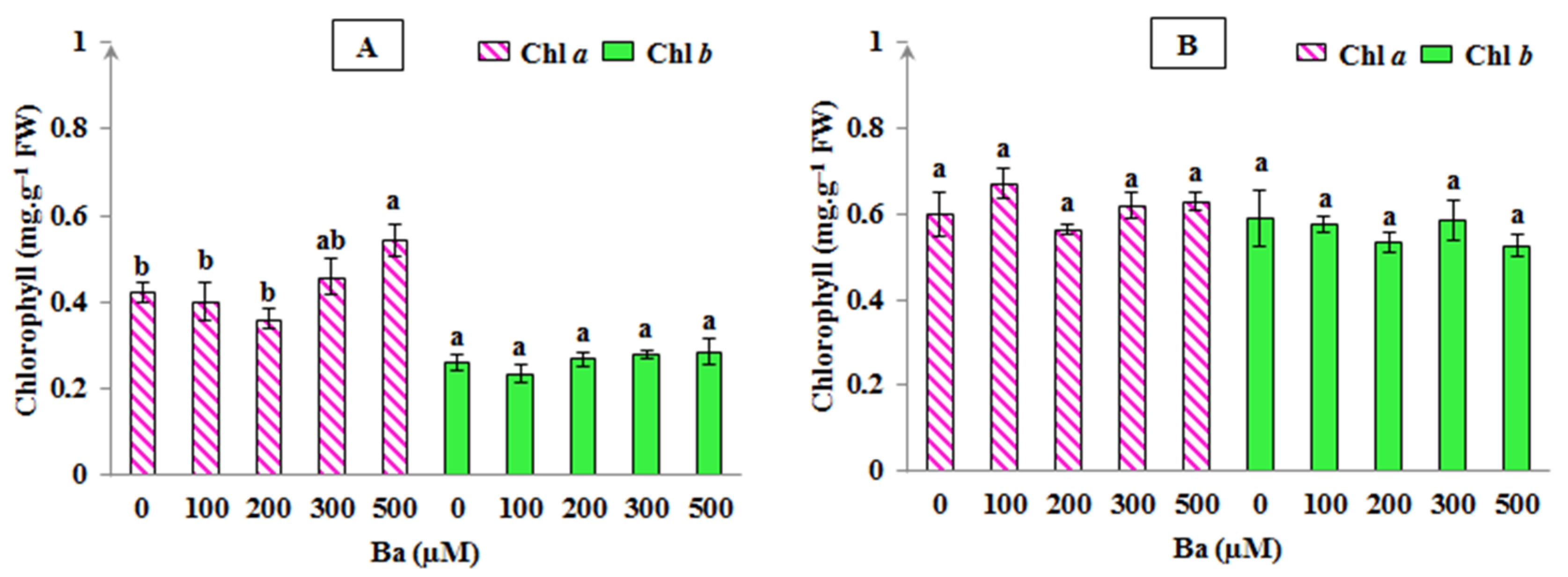
In addition, chlorophyll level can also reflect the sensitivity of plants to abiotic stresses where the decrease in these pigments presents a common symptom of metal toxicity in plants [25]. Despite the slight changes obtained in some chlorophyll fluorescence parameters under the effect of Ba, the results presented in Figure 3 showed that chlorophylls determined in plant leaves of L. crithmoides and H. annuus were not negatively affected by Ba-induced stress; Chl a content was found increased only in the highest Ba concentration (500 µM) in L. crithmoides, when compared with the control (p < 0.05). Furthermore, no variation in Chl b levels in all Ba concentrations was shown in the two species (p > 0.05). Our results are in line with several studies, for example, a study realised on Cyamopsis tetragonoloba plants, treated with high doses of BaCl2 (going from 2 to 10 mM Ba), showed that Chl a and Chl b contents were not affected in the lowest applied Ba concentration (2 mM), but a decrease in the level of these pigments was noted in the highest Ba ones [13]. This fact may clearly explain that the application of Ba might not affect photosynthesis in plants; however, at a very high Ba dose, the photosynthetic activity could be reduced. Lamb et al. also mentioned that Ba can be phytotoxic only when applied at very high doses [8]. In addition, Sosnowski and Drozłowska reported that the amount of chlorophyll was found to increase in marine algae treated with BaCl2, proving that these algae remained productive despite the toxic effects of Ba [43]. Overall, the maintenance of the photosynthetic efficiency and the biosynthesis of chlorophylls in L. crithmoides and H. annuus under varying Ba concentrations was in agreement with the increased biomass production in the present species throughout the experiment. Moreover, plant tolerance to Ba stress might be associated with a high ability of these two species to remove ROS through the activation of such protective mechanisms, e.g., increase in antioxidant enzymes activity and stimulation of the antioxidant active compounds synthesis [56].
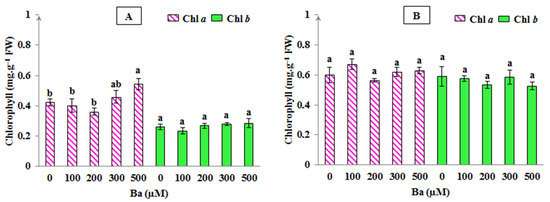
Figure 3.
Chlorophyll a and b levels in Limbarda crithmoides (A) and Helianthus annuus (B) leaves after 45 days of Ba exposure. Data are presented in mean values ± SD, n = 10. Bars marked with lower-case letters were significantly different at p < 0.05.
3.4. Polyphenols and Flavonoids Levels
The stimulation of the production of secondary metabolites such as polyphenols and flavonoids in plant cells is a response to a stress factor, which can be a metallic stress induced by barium [43]. The generation of reactive oxygen species (ROS) is the primary response of plants when exposed to toxic heavy metals (even to elevated concentrations of essential metals) [57,58]. The secondary metabolites, belonging to the non-enzymatic antioxidant system, are involved to scavenge free radicals and also to chelate metals to reduce their toxicity in the plant and eventually avoid the occurrence of plant damage [59,60]. Indeed, when exposed to metal stress, the antioxidant system is activated by the stimulation of the phenylpropanoid pathway using the phenylalanine to biosynthesise phenolic compounds (phenolic acids, flavonoids, rosmarinic acids, phytoalexins, and lignins) [61] and this is due to the increase of enzymes such as phenylalanine ammonia-lyase, polyphenol oxidase, shikimate dehydrogenase, and chalcone synthase involved in the biosynthetic pathway of phenolic compounds. Moreover, the chelation of metal ions with phenols can enable metal sequestration and reduce metal ions uptake to aerial parts of the plant [62]. Kisa et al. reported that phenolic compounds allow plants to adapt to different abiotic stress conditions in the environment, including metal toxicity [59].
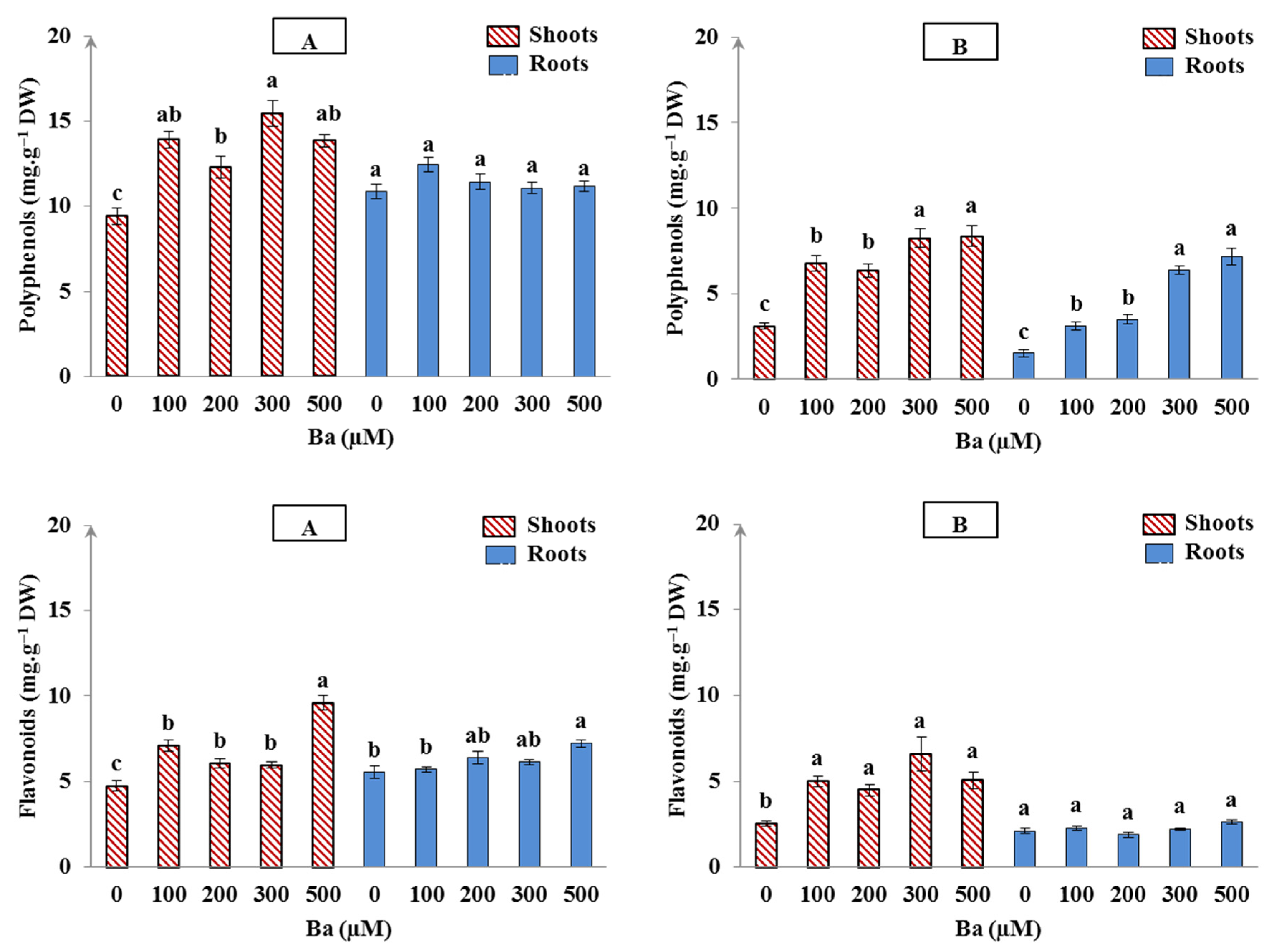
In our experiment, the addition of all Ba concentrations to the growth medium (from 100 to 500 µM) significantly promoted the increase of polyphenols in the shoots of L. crithmoides and H. annuus (p < 0.05), whereas an increase of more than 30% and 50% was detected, respectively, in comparison with the control (Figure 4A,B). The accumulation of polyphenols in the roots of L. crithmoides was not changed in the presence of Ba in the irrigation solutions when compared with the control (Figure 4A). In contrast, polyphenols in roots of H. annuus significantly increased with the increase of Ba (p < 0.05; Figure 4B). Moreover, the flavonoids biosynthesis was significantly stimulated in the shoots of all Ba-treated plants (p < 0.05); the concentrations of 100, 200, 300, and 500 µM Ba induced an increase of 1.5-, 0.85-, 1-, and 1.6-fold in L. crithmoides, and an increase of 2-, 1.8-, 2.6-, and 2-fold in H. annuus, respectively, in comparison with the untreated plants (Figure 4). However, a slight increase in flavonoids of the roots was observed by increasing Ba concentration but the enhancement was only significant at 500 µM Ba (p < 0.05). H. annuus did not show significant variation in flavonoids content in roots under Ba.
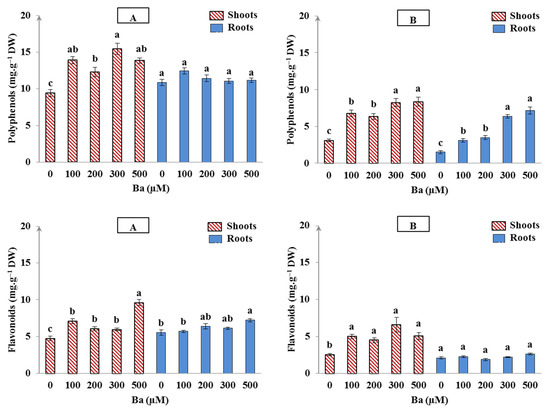
Figure 4.
Accumulation of polyphenols and flavonoids in shoots and roots of Limbarda crithmoides (A) and Helianthus annuus (B) plants after 45 days of Ba treatments. Data are presented in mean values ± SD, n = 10. Bars marked with different lower-case letters were significantly different at p < 0.05.
Our results are in accordance with Sosnowski and Drozłowska, who reported an increase in polyphenols in algae treated with BaCl2 [43]. Bouslimi et al. Also demonstrated that Ba stress exhibited polyphenols production in the shoots and roots of two Brassicaceae species, i.e., Brassica juncea and Cakile maritima, and no significant variation was reported in flavonoids level in roots of Brassica juncea [11].
3.5. Nutrients and Total Inorganic Nitrogen Contents
Table 3 showed different variations in the determined concentrations of K, Ca, Mg, and TIN in the shoots and roots of L. crithmoides and H. annuus under Ba stress. Regarding K levels, all Ba treatments significantly enhanced the content of this element in the upper parts of the two studied species and only in the roots of H. annuus in comparison with the control (p < 0.05). Conversely, the underground parts of L. crithmoides-treated plants accumulated less K than the untreated ones; this decrease was significant in all Ba treatments (p < 0.05). Moreover, Ba stress did not affect calcium levels in the shoots; however, as compared with the control, a significant reduction in Ca’ roots (up to 20%) from 100 to 500 µM Ba was marked (p < 0.05). In contrast, Ca contents in the shoots and roots of H. annuus were not affected by Ba when compared to the control (p > 0.05). Results also showed that Mg content was significantly increased only in the shoots of L. crithmoides in 500 µM Ba, and decreased in the roots of 100, 200, 300, and 500 µM Ba (1.4-, 1.4-, 1.2- and 1.5-fold decrease, respectively), as compared with the control (p < 0.05). Regarding H. annuus plants, a significant increase in Mg was noted only in shoots of 100 and 200 µM Ba (p < 0.05).

Table 3.
Nutrient elements uptake in the shoots and roots of Limbarda crithmoides and Helianthus annuus over 45 days of Ba exposure (K: potassium, Ca: calcium; Mg: magnesium, TIN: total inorganic nitrogen). Data are shown as means ± SD; values with lowercase denote significant differences at p < 0.05.
Regarding the obtained results, nutrients uptake was negatively affected only in the roots of Limbarda crithmoides in the presence of Ba. These results are in agreement with Suwa et al. and Monteiro et al., who stated the decrease of K in the roots of soybean and Tanzania Guineagrass plants treated with barium [32,43]. Suwa et al. also reported that the potassium ratio (epidermal cells K+/guard cells K+) in soybean decreased with increasing Ba concentrations [32]. This decline in potassium levels in these plants was explained by the fact that Ba is a competitive antagonist for K-channels, which can block the passive efflux of intracellular K [9], and by the inhibition of the opening of K channels in the membrane, K absorption would be reduced [32]. Similarly to the results of L. crithmoides, the concentrations of Ca and Mg were also reduced in roots of Tanzania Guineagrass when Ba rates increased, which may reflect an antagonistic relationship between these divalent cations [43], whereas Ba did not change Ca and Mg uptake levels in soybean species [32]. Regarding our evaluation, it was demonstrated that the reduction in K, Ca, and Mg contents in roots had no critical effects on the growth of L. crithmoides; this can be explained by the fact that Ba could play a crucial role in increasing plant growth and different physiological functions, or that the reduced nutrients was not a negative effect but rather a decrease in the luxury consumption. Plants have the ability to accumulate nutrients as an insurance strategy in order to survive such sudden environmental abiotic stress [63,64]. In addition to that, the increase in K content in the shoots of L. crithmoides and in both shoots and roots of H. annuus Ba-treated plants could be explained by the stimulation of Abscisic acid synthesis, the key hormone implicated in tuning responses to abiotic stresses [65,66]. The enhancement in Mg levels in shoots of L. crithmoides (500 µM Ba) and H. annuus (100 and 200 µM Ba) can be due to the involvement of this nutrient in metal toxicity alleviation via the maintenance of iron status, the increase in antioxidative activity, and the conservation of chlorophyll biosynthesis [21].
Moreover, when compared with the control, H. annuus showed a significant enhancement in TIN content in shoots of 100, 200, and 300 µM Ba and in roots of 100 and 200 µM Ba-treated plants (p < 0.05). A similar trend was observed in shoots of L. crithmoides in all applied Ba concentrations; however, TIN content decreased in roots of this species under Ba, but the decline was only significant at 100, 300, and 500 µM (p < 0.05). Marisamy et al. and Marisamy et al. reported that nitrate leaves contents in Cyamopsis tetragonoloba and Amanranthus caudatus treated with Ba were significantly increased to 78% and 119%, respectively, and the authors found that the increase in nitrate levels in these species was relative to the inhibition of nitrate reductase activity caused by Ba treatments [13,14]. Nitrogen was able to reduce or avoid metal toxicity, enhanced the photosynthetic activities through the increase of chlorophyll synthesis in plants, and increased the antioxidant enzymes activity [67]; therefore, this fact might also justify the increase of the studied species biomass. However, the decrease in the TIN in roots of L. crithmoides in this study can be a result of the increase of amino acids synthesis (from the nitrate sources) such as proline and peptides as GSH to tolerate and detoxify the metal through the elimination of hydroxyl radicals, the maintenance of osmoregulation, and the prevention of enzyme destruction [14,68,69].
3.6. Barium Content
Several plant species, growing on metal-contaminated soils, showed an adaptation to high concentrations of TME through the stabilisation, the translocation, and the extraction of metals such as Cd, Pb, Ni, Cu, Zn, Cr, and Ba, inducing as a result a sustained accumulation that mainly depends on biomass production and growth [31,36,70].
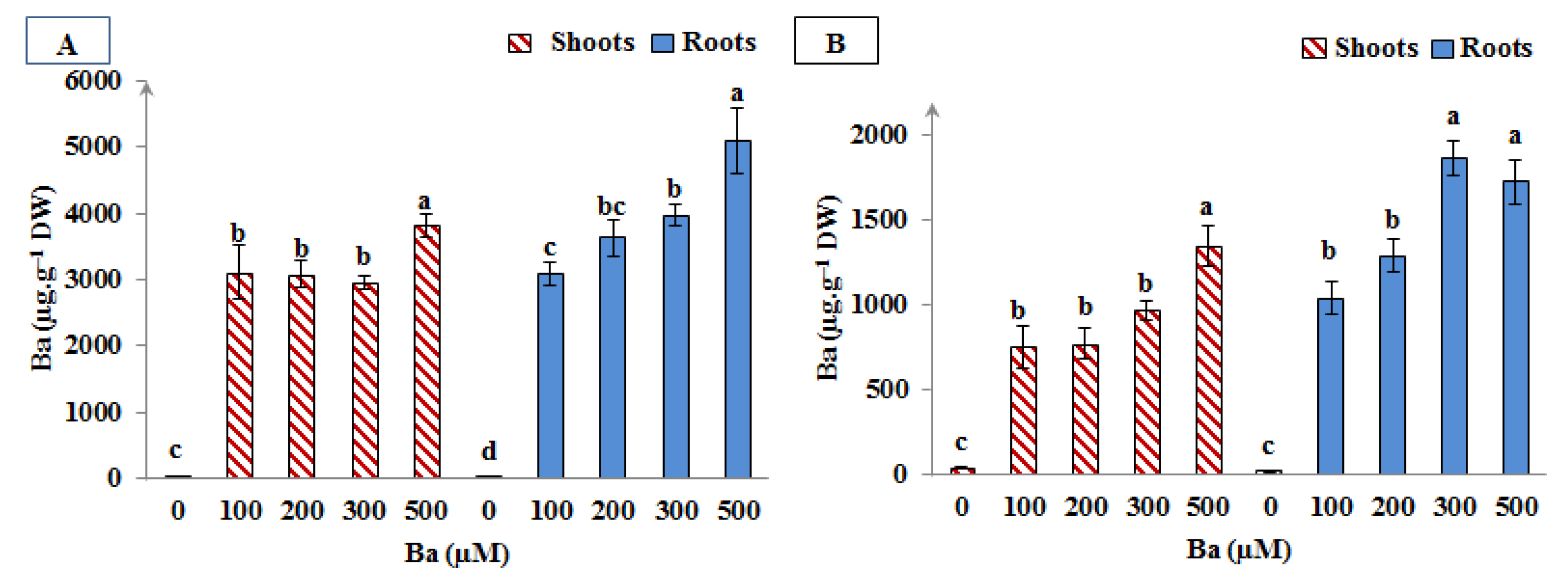
As can be seen in Figure 5, high levels of Ba were accumulated in L. crithmoides and H. annuus species after 45 days of Ba exposure. The accumulation of this metal was more notorious in L. crithmoides where Ba content was approximately distributed in equal proportions between the shoots and the roots of this plant in all Ba treatments. Adversely, H. annuus plants accumulated Ba in their roots more than in the shoots.
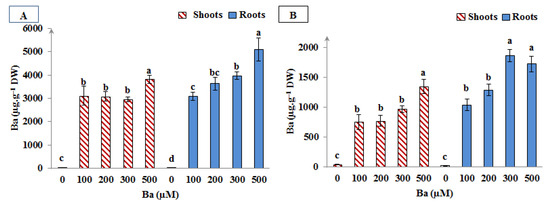
Figure 5.
Barium content in shoots and roots of Limbarda crithmoides (A) and Helianthus annuus (B) after 45 days of Ba treatment. Data are presented in mean values ± SD, n = 10. Bars marked with different lower-case letters were significantly different at p < 0.001.
Similarly, various species showed their capability to survive in barite-contaminated medium and also to accumulate Ba in their different organs such as Cucumis sativus species, which has accumulated an amount of 6 mg·g−1 DW of Ba in the roots and a similar quantity was accumulated in the shoots as well [31]. Ribeiro et al. also demonstrated high accumulating rates of Ba in the aerial parts or in roots or in both organs of Oryza sativa, Eleocharis acutangula, Eleocharis interstincta, Cyperus papyrus, and Typha domingensis after 120 days of BaCl2 treatment [43].
The translocation of metals to the shoot and the accumulation of these elements in non-toxic form are the most adopted resistance mechanisms by plants facing a metallic stress [21]. Kee et al. mentioned that metal hyperaccumulation mechanisms may rely on phytochelatins and metallothioneins, defined as metal binding-peptides, which might also be related to detoxification and vacuolar sequestrating of cationic metals [70].
Although several species have been identified as accumulators of Ba in previous studies, no specific definition exists to classify plant species as Ba hyperaccumulators [71]. However, taking into consideration similar toxic metals as Ba, the threshold values of metal concentrations retained in the upper parts of plants used as metal hyperaccumulators were fixed at 100 mg·kg−1 for Cd and 1000 mg·kg−1 for Pb, Al, Co, Ni, Cu, and As [72,73,74]. Thus, the fact that L. crithmoides and H. annuus were able to accumulate around 3000 and 1000 µg·g−1 DW of Ba, respectively, in their aerial parts makes it possible for them be identified as Ba-hyperaccumulator species.
3.7. Correlation Analysis
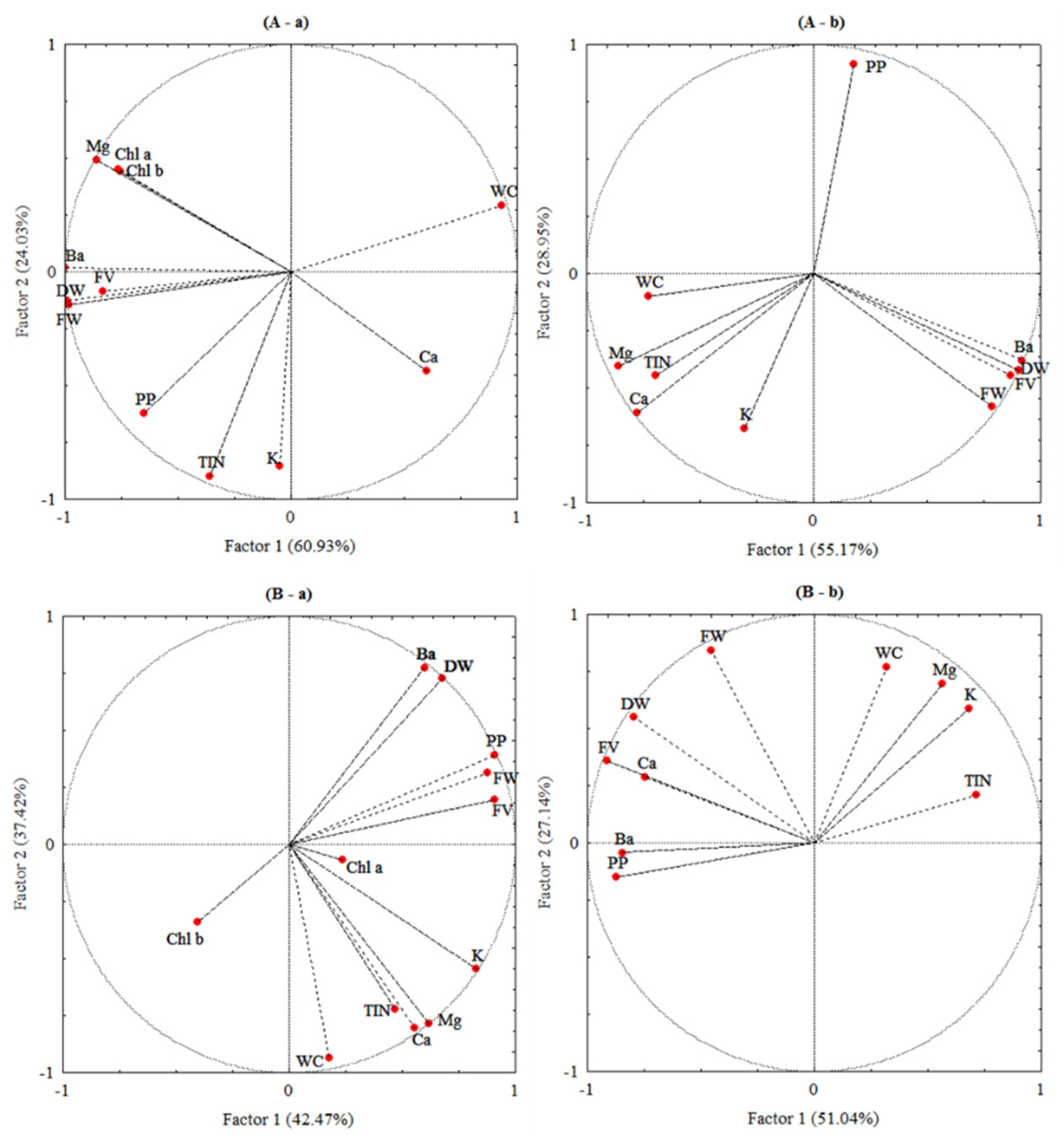
The correlations between the studied parameters were statistically analysed using principal component analysis (PCA) to reveal the effect of barium on the physiological and biochemical parameters in the present plant species. The statistical analysis showed that the multifactorial analysis of Ba influence indicated that axis were Factor 1 and Factor 2, where Factor 1 explained the variance of 60.93% and 55.17% in the shoots and roots of L. crithmoides (Figure 6A) and 42.41% and 51.04% in the same organs of H. annuus (Figure 6B), respectively, while Factor 2 represented only 24.03% and 28.95% of variance in shoots and roots of L. crithmoides, and H. annuus showed a variance of 37.42% and 27.14% in shoots and roots, respectively. The two components explained 84.96% and 84.12% in L. crithmoides and 79.89% and 78.18% in H. annuus of the total variance in the shoots and roots, respectively. These results were considered highly significant for this study. Shoots of L. crithmoides showed that Ba was positively correlated to DW, FW, Mg contents, flavonoids, polyphenols, and chlorophylls a and b, and negatively correlated to WC (Figure 6A(a)). A positive correlation between Ba and DW, FW, and flavonoids was marked in roots of the same plant (Figure 6A(b)). H. annuus showed that Ba was positively correlated to DW, FW, polyphenols, and flavonoids in shoots (Figure 6B(a)), and also positively correlated to Ca, polyphenols, and flavonoids in roots (Figure 6B(b)).
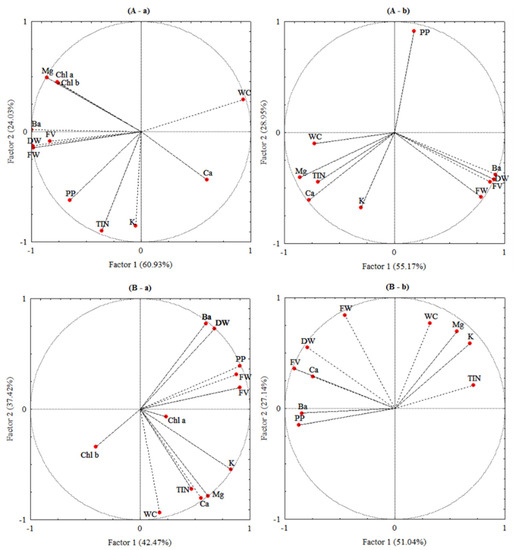
Figure 6.
Correlation circle from the principal component analysis (PCA) of barium concentration (Ba), dry biomass (DW), fresh biomass (FW), water content (WC), chlorophylls (Chl a and Chl b), polyphenols (PP), flavonoids (FV), and nutrient contents (potassium (K), calcium (Ca), magnesium (Mg), and total inorganic nitrogen (TIN)) data of the shoots (a) and roots (b) of Limbarda crithmoides (A) and Helianthus annuus (B).
4. Conclusions
Regarding the huge availability of Ba in various components of our ecosystems, the exploitation of new plant species that can survive, accumulate, and safely remove this metal from polluted area would be certainly a part of the solution to reduce all related Ba toxicity risks occurred in various living organisms up to humans. The present study showed the high tolerance susceptibility of L. crithmoides and H. annuus to barium-induced stress; indeed, Ba did not harmfully affect the physiological and biochemical processes of the plants, which maintained good mineral nutrition of the aerial parts as well as the photosynthetic activity assessed by chlorophylls levels in plant leaves. Despite the reduced amounts of K, Ca, and Mg determined in the roots of L. crithmoides, the plants did not show any deficiency of these nutrients followed by an increase in biomass production overall for the treated plants. The competitive antagonistic propriety of Ba for K, Ca, and Mg might explain the retention of Ba in the roots to replace these macronutrients without reducing biomass production. The increase in polyphenols and flavonoids biosynthesis in the two species indicated the efficiency of the antioxidant system against Ba stress. Results also showed that at 500 µM, the highest applied Ba concentration, the present plant species could accumulate a rate of 3800 and 1350 µg·g−1 DW of Ba in L. crithmoides and H. annuus aerial parts, respectively, during the 45 days of Ba exposure. Therefore, the accumulation potential of barium in aerial parts and roots of L. crithmoides and H. annuus associated with high plant biomass production (specifically L. crithmoides) allows these species to be exploited for phytoremediation schemes in barium-contaminated soils. Nonetheless, studying molecular responses could help to better understand the mechanisms involved to tolerate Ba constraint. In addition, experiencing higher Ba concentrations in the growth medium is recommended to assess the tolerance limit of Ba in L. crithmoides and H. annuus. According to the finding results, Ba could be a beneficial metal for some plant species when applied at low concentrations; however, the effect of barium on the physiological, biochemical, and molecular behaviours of other plant species grown in soils containing Ba needed to be further investigated.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb13020012/s1, Figure S1. Morphological aspect of Limbarda crithmoides after 45 days of Barium exposure; Figure S2. Morphological aspect of Helianthus annuus after 45 days of Barium exposure.
Author Contributions
Conceptualisation, N.S. and I.C.; methodology, N.D., H.B. and N.S.; formal analysis, N.D., H.B. and B.D.; writing—original draft preparation, N.D.; writing—review and editing, B.D. and I.C.; supervision, N.S. and I.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors have no funding received for this article.
Acknowledgments
The authors would like to thank Fundação para a Ciência e a Tecnologia (FCT) for funding MARE-Marine and Environmental Sciences Centre (UIDB/04292/2020 and UIDB/04292/2020), ARNET—Aquatic Research Infrastructure Network Associated Laboratory (LA/P/0069/2020) and the Project Rhizomis (PTDC/BIA-MIC/29736/2017). B. Duarte was supported by a FCT research contract (CEECIND/00511/2017).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kresse, R.; Baudis, U.; Jager, P.; Riechers, H.H.; Wagner, H.; Winkler, J.; Wolf, H.U. Barium and Barium Compounds. Encycl. Ind. Chem. 2007, 4, 621–640. [Google Scholar] [CrossRef]
- Oskarsson, A. Barium. In Handbook on the Toxicology of Metals, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume II. [Google Scholar] [CrossRef]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological Profile for Barium; Agency for Toxic Substances and Disease Registry; US Department of Health and Human Services: Atlanta, GA, USA, 2007.
- Abreu, C.A.; Cantoni, M.; Coscione, A.R.; Paz-Ferreiro, J. Organic Matter and Barium Absorption by Plant Species Grown in an Area Polluted with scrap metal residue. Appl. Environ. Soil Sci. 2012, 2012, 476821. [Google Scholar] [CrossRef]
- Madejon, P. Barium. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and their Bioavailability, Environmental Pollution; Alloway, B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 22, pp. 507–514. [Google Scholar] [CrossRef]
- Horner, T.J.; Pryer, H.V.; Nielsen, S.G.; Crockford, P.W.; Gauglitz, J.M.; Wing, B.A.; Ricketts, R.D. Pelagic barite precipitation at micromolar ambient sulfate. Nat. Commun. 2017, 8, 1342. [Google Scholar] [CrossRef] [PubMed]
- Viana, D.G.; Filho, F.B.E.; Pires, F.R.; Soares, M.B.; Ferreira, A.D.; Bonomo, R.; Martins, L.F. In situ barium phytoremediation in flooded soil using Typha domingensis under different planting densities. Ecotoxicol. Environ. Saf. 2021, 210, 111890. [Google Scholar] [CrossRef]
- Lamb, D.T.; Matanitobua, V.P.; Palanisami, T.; Megharaj, M.; Naidu, R. Bioavailability of barium to plants and invertebrates in soils contaminated by barite. Environ. Sci. Technol. 2013, 47, 4670–4676. [Google Scholar] [CrossRef]
- Verbruggen, E.M.J.; Smit, C.E.; Van-Vlaardingen, P.L.A. Environmental quality standards for barium in surface water. Proposal for an update according to the methodology of the Water Framework Directive. In RIVM Official Reports; National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2020; pp. 2–111. [Google Scholar] [CrossRef]
- Nogueira, T.A.R.; de Melo, W.J.; Fonseca, I.M.; Marques, M.O.; He, Z. Barium uptake by maize plants as affected by sewage sludge in a long-term field study. J. Hazard. Mater. 2010, 181, 1148–1157. [Google Scholar] [CrossRef]
- Bouslimi, H.; Ferreira, R.; Dridi, N.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Effects of Barium stress in Brassica juncea and Cakile maritima: The indicator role of some antioxidant enzymes and secondary metabolites. Phyton Int. J. Exp. Bot. 2021, 90, 145–158. [Google Scholar] [CrossRef]
- Kravchenko, J.; Darrah, T.H.; Miller, R.K.; Lyerly, H.K.; Vengosh, A. A review of the health impacts of barium from natural and anthropogenic exposure. Environ. Geochem. Health 2014, 36, 797–814. [Google Scholar] [CrossRef]
- Marisamy, K.; Sevugaperumal, R.; Ramasubramanian, V. Changes in growth, biochemical and enzymatic characteristics of Cyamopsis tetragonoloba (l.) Taub under metal stress due to barium. Int. J. Biol. Pharm. Res. 2015, 6, 935–938. [Google Scholar]
- Marisamy, K.; Duraipandian, M.; Sevugaperumal, R.; Ramasubramanian, V. Estimation of Barium Toxicity Mitigating Efficacy of Amaranthus caudatus L. Univers. J. Environ. Res. Technol. 2015, 5, 295–305. [Google Scholar]
- Coscione, A.R.; Berton, R.S. Barium extraction potential by mustard, sunflower and castor bean. Sci. Agric. 2009, 66, 59–63. [Google Scholar] [CrossRef][Green Version]
- Sleimi, N.; Bankaji, I.; Dallai, M.; Kefi, O. Accumulation des éléments traces et tolérance au stress métallique chez les halophytes colonisant les bordures de la lagune de Bizerte. Rev. D’Ecologie Terre Vie 2014, 69, 49–59. [Google Scholar]
- Ghabriche, R.; Ghnaya, T.; Mnasri, M.; Zaier, H.; Baioui, R.; Vromman, D.; Abdelly, C.; Lutts, S. Polyamine and tyramine involvement in NaCl-induced improvement of Cd resistance in the halophyte Inula chrithmoides L. J. Plant Physiol. 2017, 216, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, E.J. Sand and water culture methods used in the study of plant nutrition. J. Assoc. Off. Anal. Chem. 1966, 49, 888–889. [Google Scholar]
- Kouki, R.; Ayachi, R.; Ferreira, R.; Sleimi, N. Behavior of Cucumis sativus L. in presence of aluminum stress: Germination, plant growth, and antioxidant enzymes. Food Sci. Nutr. 2021, 9, 3280–3288. [Google Scholar] [CrossRef]
- Sleimi, N.; Abdelly, C. Salt-tolerance strategy of two halophytes species: Spartina alterniflora and Suaeda fruticosa. In Tasks for Vegetation Science; Cash Crop Halophytes; Lieth, H., Mochtchenko, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherland, 2003; Volume 38, pp. 79–85. [Google Scholar] [CrossRef]
- Dridi, N.; Ferreira, R.; Bouslimi, H.; Brito, P.; Martins-Dias, S.; Caçador, I.; Sleimi, N. Assessment of tolerance to lanthanum and cerium in Helianthus annuus plant: Effect on growth, mineral nutrition and secondary metabolism. Plants 2022, 11, 988. [Google Scholar] [CrossRef]
- Labidi, O.; Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M.; Sleimi, N. Assessing of growth, antioxidant enzymes, and phytohormone regulation in Cucurbita pepo under cadmium stress. Food Sci. Nutr. 2021, 9, 2021–2031. [Google Scholar] [CrossRef]
- Sghaier, D.B.; Duarte, B.; Bankaj, I.; Caçador, I.; Sleimi, N. Growth, chlorophyll fluorescence and mineral nutrition in the halophyte Tamarix gallica cultivated in combined stress conditions: Arsenic and NaCl. J. Photochem. Photobiol. B Biol. 2015, 149, 204–214. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Guo, P. Chlorophyll fluorescence: A useful tool in barley plant breeding programs. Photochem. Res. Prog. 2008, 29, 448–471. [Google Scholar]
- Bankaji, I.; Caçador, I.; Sleimi, N. Assessing of tolerance to metallic and saline stresses in the halophyte Suaeda fruticosa: The indicator role of antioxidative enzymes. Ecol. Indic. 2016, 64, 297–308. [Google Scholar] [CrossRef]
- Ayvaz, M.; Koyuncu, M.; Guven, A.; Fagerstedt, K.V. Does boron affect hormone levels of barley cultivars? Eur. Asian J. Biosci. 2012, 120, 113–120. [Google Scholar] [CrossRef]
- Sleimi, N.; Bankaji, I.; Kouki, R.; Dridi, N.; Duarte, B.; Caçador, I. Assessment of extraction methods of trace metallic elements in plants: Approval of a common method. Sustainability 2022, 14, 1428. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 11th ed.; Etats-Unis; Association of Official Analytical Chemists: Washington, DC, USA, 1970. [Google Scholar]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Carnat, A. Teneurs en acide rosmarinique, en dérivés hydroxycinnamiques totaux et activités antioxydantes chez les Apiacées, les Borraginacées et les Lamiacées médicinales. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Sleimi, N.; Kouki, R.; Hadj-Ammar, M.; Ferreira, R.; Pérez-Clemente, R. Barium effect on germination, plant growth, and antioxidant enzymes in Cucumis sativus L. plants. Food Sci. Nutr. 2021, 9, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Suwa, R.K.; Jayachandran, N.T.; Nguyen, A.; Boulenouar, K.; Fujita, K.; Saneoka, H. Barium toxicity effects in soybean plants. Arch. Environ. Contam. Toxicol. 2008, 55, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Gadgil, K.; Sharma, S. Comparative study of natural phytoextraction and induced phytoextraction of lead using mustard plant (Brassica juncea arawali). Int. J. Bioassay 2012, 2, 352–357. [Google Scholar]
- Caçador, I.; Duarte, B.; Marques, J.C.; Sleimi, N. Carbon mitigation: A salt marsh ecosystem service in times of change. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; pp. 83–110. [Google Scholar] [CrossRef]
- Monteiro, F.A.; Nogueirol, R.C.; Melo, L.C.A.; Artur, A.G.; da Rocha, F. Effect of barium on growth and macronutrient nutrition in Tanzania guinea grass grown in nutrient solution. Commun. Soil Sci. Plant Anal. 2011, 42, 1510–1521. [Google Scholar] [CrossRef]
- Ribeiro, P.R.C.; Viana, D.G.; Pires, F.R.; Filho, F.B.E.; Bonomo, R.; Filho, A.C.; Martins, L.F.; Cruz, L.B.S.; Nascimento, M.C.P. Selection of plants for phytoremediation of barium-polluted flooded soils. Chemosphere 2018, 206, 522–530. [Google Scholar] [CrossRef]
- Zhen, Y.; Qi, J.L.; Wang, S.S.; Su, J.; Xu, G.H.; Zhang, M.S.; Miao, L.V.; Peng, X.X.; Tian, D.; Yang, Y.H. Comparative proteome analysis of differentially expressed proteins induced by Al toxicity in soybean. Physiol. Plant. 2007, 131, 542–554. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.T.; Qi, Y.P.; Guo, P.; Lu, Y.B.; Chen, L.S. Aluminum toxicity-induced alterations of leaf proteome in two citrus species differing in aluminum tolerance. Int. J. Mol. Sci. 2016, 17, 1180. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2017, 255, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Bankaji, I.; Pérez-Clemente, R.M.; Caçador, I.; Sleimi, N. Accumulation potential of Atriplex halimus to zinc and lead combined with NaCl: Effects on physiological parameters and antioxidant enzymes activities. S. Afr. J. Bot. 2019, 123, 51–61. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, S.; Dwivedi, S.; Baghel, V.S.; Verma, S.; Tandon, P.K. Barium Phytoremediation Potential of Broad Bean, ViciafabaL., and Its Biochemical Responses. Bull. Environ. Contam. Toxicol. 1974, 74, 715–724. [Google Scholar] [CrossRef]
- Ikram, K.; Abdelhakim, R.Y.H.; Topcuoglu, B. Physiological and biochemical response of Atriplex canescens (pursh) nutt under metallic stress. Plant Arch. 2019, 19, 2747–2752. [Google Scholar]
- Sosnowski, W.; Drozłowska, E. Effect of barium chloride on growth and oxidative stress of saltwater algae. World Sci. News 2018, 111, 121–130. [Google Scholar]
- Bangajavalli, S.; Selvaraj, K.; Esakkiammal, B. Retrieval of barium affected Phaseolus mungo L. by Ulva lactuca. Int. J. Bot. Stud. 2021, 6, 59–63. [Google Scholar]
- Kumar, A.; Narasimha, M.P.V. Plant-lead interactions: Transport, toxicity, tolerance, and detoxification mechanisms. Ecotoxicol. Environ. Saf. 2018, 166, 401–418. [Google Scholar] [CrossRef]
- Bücker-Neto, L.; Paiva, L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef]
- Nomura, T.; Itouga, M.; Kojima, M.; Kato, Y.; Hasezawa, S. Copper mediates auxin signalling to control cell differentiation in the copper moss Scopelophila cataractae. J. Exp. Bot. 2015, 66, 1205–1213. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in barley. J. Photochem. Photobiol. B. 2012, 112, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reboredo, F.H.; Pelica, J.; Lidon, F.C.; Pessoa, M.F.; Silva, M.M.; Guerra, M.; Leitão, R.; Ramalho, J.C. The Tolerance of Eucalyptus globulus to Soil Contamination with Arsenic. Plants 2021, 10, 627. [Google Scholar] [CrossRef]
- Li, S.; Yang, W.; Yang, T.; Chen, Y.; Ni, W. Effects of Cadmium Stress on Leaf Chlorophyll Fluorescence and Photosynthesis of Elsholtzia argyi—A Cadmium Accumulating Plant. Int. J. Phytoremed. 2015, 17, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Van Heerden, P.D.R.; Swanepoel, J.W.; Kruger, G.H.J. Modulation of photosynthesis by drought in two desert scrub species exhibiting C3-mode CO2 assimilation. Environ. Exp. Bot. 2007, 61, 124–136. [Google Scholar] [CrossRef]
- Gomes, M.T.G.; da Luz, A.C.; dos Santos, M.R.; Batitucci, M.D.C.P.; Silva, D.M.; Falqueto, A.R. Drought tolerance of passion fruit plants assessed by the OJIP chlorophyll a fluorescence transient. Sci. Hortic. 2012, 142, 49–56. [Google Scholar] [CrossRef]
- Liu, M.S.; Huang, X.H.; Wang, R.J.; Xu, H.Y.; Zhu, F. Inhibition of photosynthesis in Melia azedarach and Ligustrum lucidum induced by manganese toxicity using OJIP chlorophyll a fluorescence transient. Photosynthetica 2021, 59, 148–159. [Google Scholar] [CrossRef]
- Lazar, D.; Jablonsky, J. On the approaches applied in formulation of a kinetic model of photosystem II: Different approaches lead to different simulations of the chlorophyll a fluorescence transients. J. Theor. Biol. 2009, 257, 260–269. [Google Scholar] [CrossRef]
- Panda, D.; Rao, D.N.; Sharma, S.G.; Strasser, R.J.; Sarkar, R.K. Submergence effects on rice genotypes during seedling stage: Probing of submergence driven changes of photosystem II by chlorophyll a fluorescence induction O-J-I-P transients. Photosynthetica 2006, 44, 69–75. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Chai, T.; Zhang, Y.; Tan, J.; Ma, S. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J. Plant Physiol. 2012, 169, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Kisa, D.; Elmastas, M.; Ozturk, L.; Kayir, O. Responses of the phenolic compounds of Zea mays under heavy metal stress. Appl. Biol. Chem. 2016, 59, 813–820. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Ali, M.B.; Singh, N.; Shohael, A.M.; Hahn, E.J.; Paek, K.Y. Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci. 2006, 171, 147–154. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, J.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Bergmann, E. Bergmann, H.W. Comparing diagrams of plant/leaf analysis presenting by rapid inspection the mineral nutrient element status of agricultural crop plants. Potash Rev. Sub. 1985, 5, 1–10. [Google Scholar]
- Kafkafi, U. The Functions of Plant K in Overcoming Environmental Stress Situations. In Proceedings of the 22nd Colloquium of IPI 1990, Soligorsk, USSR; IPI: Bern, Switzerland, 1990; pp. 81–93. [Google Scholar]
- Alazem, M.; Lin, N.S. Antiviral roles of Abscisic acid in plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef]
- Zhao, L.J.; Peralta-Videa, J.R.; Peng, B.; Bandyopadhyay, S.; Corral-Diaz, B.; Osuna-Avila, P.; Montes, M.O.; Keller, A.A.; Gardea-Torresdey, J.L. Alginate modifies the physiological impact of CeO2 nanoparticles in corn seedlings cultivated in soil. J. Environ. Sci. 2014, 26, 382–389. [Google Scholar] [CrossRef]
- Nawab, J.; Ghani, J.; Khan, S.; Khan, M.A.; Ali, A.; Rahman, Z.; Alam, M.; Hesham, A.; Lei, M. Nutrient Uptake and Plant Growth Under the Influence of Toxic Elements. In Sustainable Plant Nutrition under Contaminated Environments, Sustainable Plant Nutrition in a Changing World; Mahmood, Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 75–101. [Google Scholar] [CrossRef]
- Pourrut, B.; Shahid, M.; Douay, F.; Dumat, C.; Pinelli, E. Molecular mechanisms involved in lead uptake, toxicity and detoxification in higher plants. Heavy Met. Stress Plants 2013, 292, 275–281. [Google Scholar] [CrossRef]
- Rastgoo, L.; Alemzadeh, A.; Tale, A.M.; Tazangi, S.E.; Eslamzadeh, T. Effects of copper, nickel and zinc on biochemical parameters and metal accumulation in gouan Aeluropus littoralis. Plant Knowl. J. 2014, 3, 31–38. [Google Scholar]
- Kee, J.C.; Gonzales, M.J.; Ponce, O.; Ramírez, L.; León, V.; Torres, A.; Corpus, M.; Loayza-Muro, R. Accumulation of heavy metals in native Andean plants: Potential tools for soil phytoremediation in Ancash (Peru). Environ. Sci. Pollut. Res. 2018, 25, 33957–33966. [Google Scholar] [CrossRef]
- Kamachi, H.; Kitamura, N.; Sakatoku, A.; Tanaka, D.; Nakamura, S. Barium accumulation in the metalliferous fern Athyrium Yokoscense. Theor. Exp. Plant Physiol. 2015, 27, 99–107. [Google Scholar] [CrossRef]
- Jansen, S.; Broadley, M.R.; Robbrecht, E.; Smets, E. Aluminum hyperaccumulation in angiosperms: A review of Its phylogenetic significance. Bot. Rev. 2002, 68, 235–269. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q.; Wang, L.; Liu, W. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J. Hazard. Mater. 2009, 161, 808–814. [Google Scholar] [CrossRef] [PubMed]
- Baycu, G.; Tolunay, D.; Ozden, H.; Csatari, I.; Karadag, S.; Agba, T.; Rognes, S.E. An Abandoned Copper Mining Site in Cyprus and Assessment of Metal Concentrations in Plants and Soil. Int. J. Phytoremediat. 2015, 17, 622–631. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).