Can Milrinone Be a Therapeutic Alternative in Persistent Pulmonary Hypertension of the Newborn? A Case Series and Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Case Series
2.1.2. Narrative Literature Review
2.2. PPHN Definition
2.3. Echocardiography and Respiratory Support
3. Results
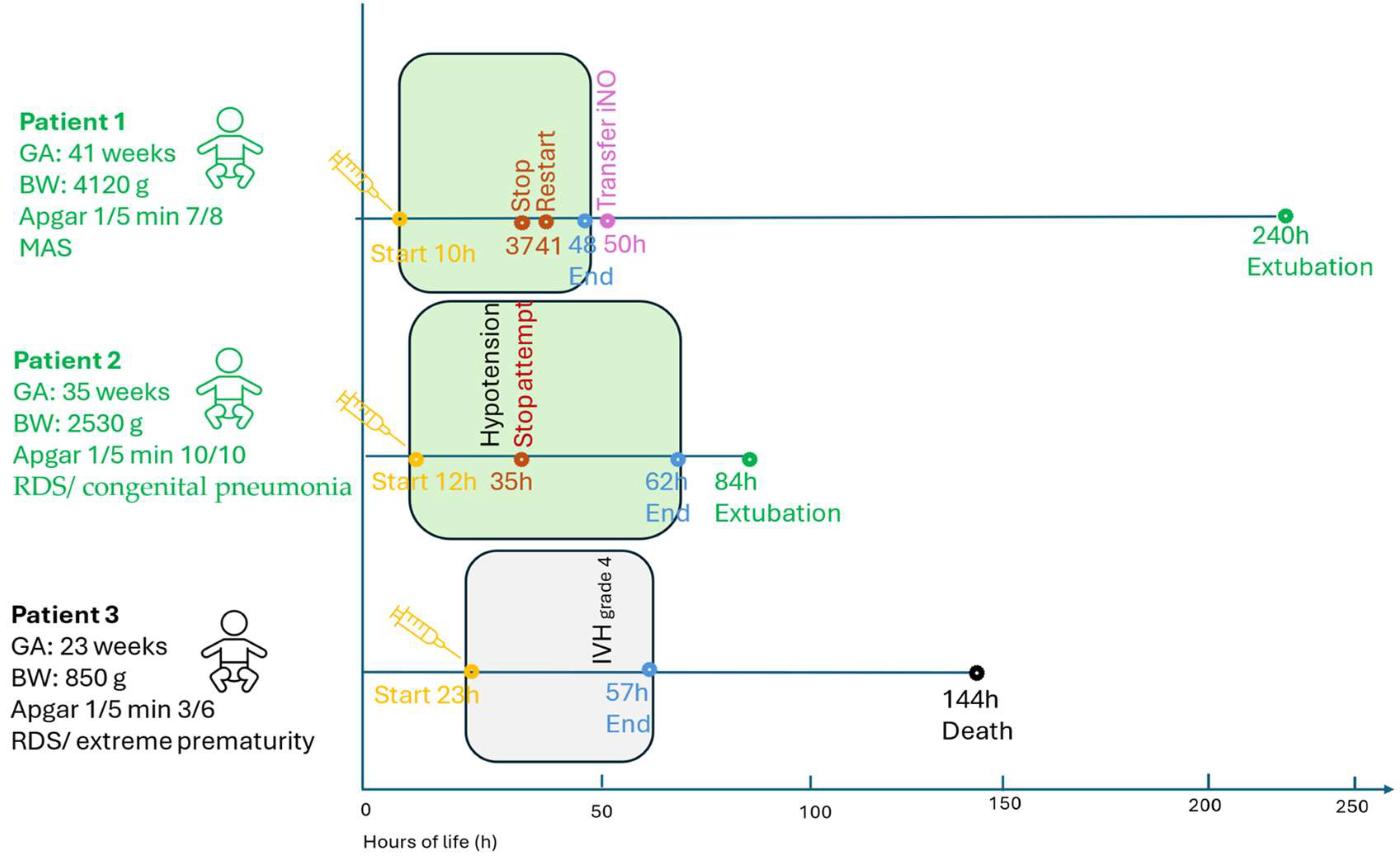
3.1. Summary of Patient Characteristics and Clinical Findings
3.1.1. Patient 1 (Term, 41 Weeks, 4120 g)
3.1.2. Patient 2 (Late-Preterm, 35 Weeks, 2530 g)
3.1.3. Patient 3 (Extremely Preterm, 23 Weeks, 820 g)
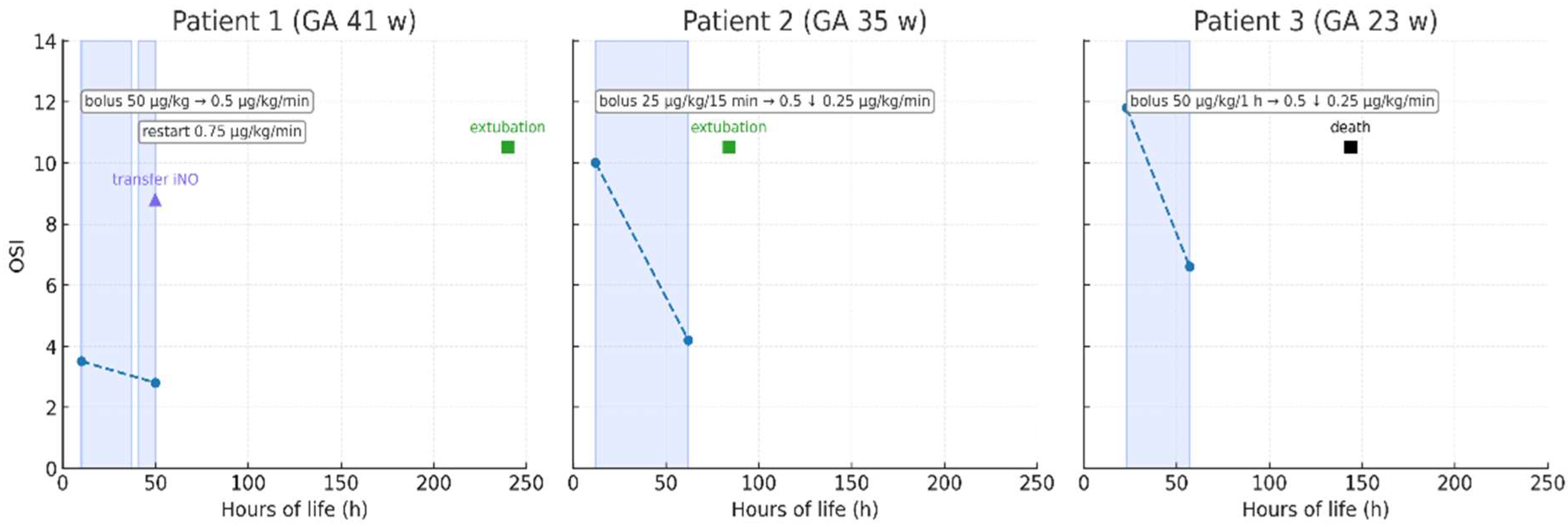
3.2. Milrinone Administration and Outcomes
3.3. Echocardiography and Respiratory Support
3.4. Summary of Findings
3.5. Literature Review on the Use of Milrinone in PPHN
3.5.1. Clinical Outcomes
3.5.2. Safety
3.5.3. Dosing
3.5.4. Applicability in Resource-Limited Settings
4. Discussion
4.1. Phenotype-Specific Responses and Mechanistic Insights
4.2. Safety Profile and Limitations in Preterm Neonates
4.2.1. Mechanisms and Clinical Context of Systemic Hypotension
4.2.2. Coagulopathy and Intraventricular Hemorrhage in Extreme Prematurity
4.3. Applicability in iNO-Limited Settings
4.4. Study Limitations and Future Directions
5. Conclusions
6. Clinical Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abman, S.H.; Hansmann, G.; Archer, S.L.; Ivy, D.D.; Adatia, I.; Chung, W.K.; Hanna, B.D.; Rosenzweig, E.B.; Raj, J.U.; Cornfield, D.; et al. Pediatric Pulmonary Hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015, 132, 2037–2099, Erratum in Circulation 2016, 133, e368. https://doi.org/10.1161/CIR.0000000000000363. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.K.; Seabrook, R.B.; Bonachea, E.M.; Chen, B.; Fathi, O.; Nankervis, C.A.; Osman, A.; Schlegel, A.B.; Magers, J.; Kulpa, T.; et al. Evidence-Based Guidelines for Acute Stabilization and Management of Neonates with Persistent Pulmonary Hypertension of the Newborn. Am. J. Perinatol. 2023, 40, 1495–1508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mukherjee, D.; Konduri, G.G. Pediatric Pulmonary Hypertension: Definitions, Mechanisms, Diagnosis, and Treatment. Compr. Physiol. 2021, 11, 2135–2190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hilgendorff, A.; Apitz, C.; Bonnet, D.; Hansmann, G. Pulmonary hypertension associated with acute or chronic lung diseases in the preterm and term neonate and infant. The European Paediatric Pulmonary Vascular Disease Network, endorsed by ISHLT and DGPK. Heart 2016, 102 (Suppl. S2), ii49–ii56. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Keszler, M. Persistent Pulmonary Hypertension of the Newborn. Neoreviews 2015, 16, e680–e692. [Google Scholar] [CrossRef] [PubMed]
- Kinsella, J.P.; Truog, W.E.; Walsh, W.F.; Goldberg, R.N.; Bancalari, E.; Mayock, D.E.; Redding, G.J.; DeLemos, R.A.; Sardesai, S.; McCurnin, D.C.; et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J. Pediatr. 1997, 131, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nelin, L.D.; Potenziano, J.L. Inhaled nitric oxide for neonates with persistent pulmonary hypertension of the newborn in the CINRGI study: Time to treatment response. BMC Pediatr. 2019, 19, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cookson, M.W.; Kinsella, J.P. Inhaled Nitric Oxide in Neonatal Pulmonary Hypertension. Clin. Perinatol. 2024, 51, 95–111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.Q.; Li, B.W.; Ma, Y.Q.; Zhang, H. Effect of NO inhalation on ECMO use rate and mortality in infants born at or near term with respiratory failure. Medicine 2019, 98, e17139. [Google Scholar] [CrossRef]
- Oliveira, C.A.; Troster, E.J.; Pereira, C.R. Inhaled nitric oxide in the management of persistent pulmonary hypertension of the newborn: A meta-analysis. Rev. Hosp. Clin. 2000, 55, 145–154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrington, K.J.; Finer, N.; Pennaforte, T.; Altit, G. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst. Rev. 2017, 1, CD000399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, G.B.; Wang, L.; Huang, S.Q.; Feng, B.Y.; Yao, M.L.; Fan, X.F.; Wang, M.J.; Zhu, L.; Zhang, J.; Zheng, Z.; et al. Clinical Analysis of Inhaled Nitric Oxide Therapy in Preterm Infants at Different Gestational Ages: A National Retrospective Multicenter Study. Am. J. Perinatol. 2025, 42, 732–741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fortas, F.; Di Nardo, M.; Yousef, N.; Humbert, M.; De Luca, D. Life-threatening PPHN refractory to nitric oxide: Proposal for a rational therapeutic algorithm. Eur. J. Pediatr. 2021, 180, 2379–2387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lakshminrusimha, S.; Mathew, B.; Leach, C.L. Pharmacologic strategies in neonatal pulmonary hypertension other than nitric oxide. Semin. Perinatol. 2016, 40, 160–173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- James, A.T.; Corcoran, J.D.; McNamara, P.J.; Franklin, O.; El-Khuffash, A.F. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiol. Young 2016, 26, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Australian Neonatal Medicines Formulary (ANMF) Guidelines. Milrinone Dosing in Neonates. 2021. Milrinone, Newborn Use Only. Available online: https://www.anmfonline.org/wp-content/uploads/2021/06/milrinone-18022021-3.0.pdf (accessed on 24 August 2025).
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D.S.; The CARE Group. The CARE guidelines: Consensus-based clinical case report guideline development. J. Clin. Epidemiol. 2014, 67, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Kreutzer, K.; McNamara, P.; Kirpalani, H. Milrinone for persistent pulmonary hypertension of the newborn. Cochrane Database Syst. Rev. 2010, CD007802. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Laique, F.; Muang-In, S.; Whyte, H.E. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J. Crit. Care 2006, 21, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Bassler, D.; Choong, K.; McNamara, P.; Kirpalani, H. Neonatal persistent pulmonary hypertension treated with milrinone: Four case reports. Biol. Neonate 2006, 89, 1–5. [Google Scholar] [CrossRef] [PubMed]
- McNamara, P.J.; Shivananda, S.P.; Sahni, M.; Freeman, D.; Taddio, A. Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatr. Crit. Care Med. 2013, 14, 74–84. [Google Scholar] [CrossRef]
- Choobdar, F.A.; Shahhosseini, P.; Vahedi, Z.; Khosravi, N.; Khalesi, N.; Ghassemzadeh, M. Comparison of the efficacy of inhaled versus infused milrinone in the management of persistent pulmonary hypertension of the newborn in resource-limited settings: A randomized clinical trial. Pediatr. Pulmonol. 2023, 58, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- El-Khuffash, A.; McNamara, P.J.; Breatnach, C.; Bussmann, N.; Smith, A.; Feeney, O.; Tully, E.; Griffin, J.; de Boode, W.P.; Cleary, B.; et al. The use of milrinone in neonates with persistent pulmonary hypertension of the newborn—A randomised controlled trial pilot study (MINT 1): Study protocol and review of literature. Matern. Health Neonatol. Perinatol. 2018, 4, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giaccone, A.; Zuppa, A.F.; Sood, B.; Cohen, M.S.; O’Byrne, M.L.; Moorthy, G.; Mathur, A.; Kirpalani, H. Pharmacokinetics and Pharmacodynamics in Neonates with Persistent Pulmonary Hypertension of the Newborn. Am. J. Perinatol. 2017, 34, 749–758. [Google Scholar] [CrossRef]
- Dillard, J.; Pavlek, L.R.; Korada, S.; Chen, B. Worsened short-term clinical outcomes in a cohort of patients with iNO-unresponsive PPHN: A case for improving iNO responsiveness. J. Perinatol. 2022, 42, 37–44. [Google Scholar] [CrossRef] [PubMed]
- El-Ghandour, M.; Hammad, B.; Ghanem, M.; Antonios, M.A.M. Efficacy of Milrinone Plus Sildenafil in the Treatment of Neonates with Persistent Pulmonary Hypertension in Resource-Limited Settings: Results of a Randomized, Double-Blind Trial. Paediatr. Drugs. 2020, 22, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; El-Farrash, R.A.; Taha, A.S.; Saleh, G.A. Milrinone Versus Sildenafil in Treatment of Neonatal Persistent Pulmonary Hypertension: A Randomized Control Trial. J. Cardiovasc. Pharmacol. 2022, 80, 746–752. [Google Scholar] [CrossRef]
- Qasim, A.; Jain, S.K. Milrinone Use in Persistent Pulmonary Hypertension of the Newborn. Neoreviews 2020, 21, e165–e178. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Agrawal, R. Persistent Pulmonary Hypertension of the Newborn: Recent Advances in the Management. Int. J. Clin. Pediatr. 2013, 2, 1–11. [Google Scholar] [CrossRef][Green Version]
- Darren, A.; Harris, C.; Greenough, A. Management of pulmonary hypertension in infants. Expert Opin. Orphan Drugs 2024, 12, 33–40. [Google Scholar] [CrossRef]
- Galis, R.; Mudura, D.; Trif, P.; Diggikar, S.; Prasath, A.; Ognean, M.L.; Mazela, J.; Lacatusu, A.; Ramanathan, R.; Kramer, B.W.; et al. Milrinone in persistent pulmonary hypertension of newborn: A scoping review. Pediatr. Res. 2024, 96, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, F.Y.; Krebs, V.L.J.; de Campos, C.V.; de Vincenzi Gaiolla, P.V.; de Carvalho, W.B. Reassessing the role of milrinone in the treatment of heart failure and pulmonary hypertension in neonates and children: A systematic review and meta-analysis. Eur. J. Pediatr. 2024, 183, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Pan, J.; Zhang, F.; Lin, Y.; Yuan, T. Comparison of Different Treatments of Persistent Pulmonary Hypertension of the Newborn: A Systematic Review and Network Meta-Analysis. Crit. Care Med. 2024, 52, e314–e322. [Google Scholar] [CrossRef] [PubMed]
- Ruoss, J.L.; Rios, D.R.; Levy, P.T. Updates on Management for Acute and Chronic Phenotypes of Neonatal Pulmonary Hypertension. Clin. Perinatol. 2020, 47, 593–615. [Google Scholar] [CrossRef] [PubMed]
| Patient 1 | Patient 2 | Patient 3 | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| TRV (m/s) | 5.1 | 2.6 | 2.7 | 2.5 | 3.0 | 2.25 |
| DA shunt | R→L | L→R | Bidirectional | L→R | R→L | Bidirectional |
| FO shunt | R→L | — | L→R | L→R | — | — |
| LV systolic function (qual.) | not reported | not reported | not reported | not reported | Depressed | Improved |
| TR (grade) | Mild | Trace | Mild | Trace | not reported | not reported |
| MR (grade) | not reported | not reported | not reported | not reported | Moderate | Mild |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewska, E.; Dera, N.; Minarowski, Ł.; Osiński, Ł.; Doboszynska, A.; Szajda, S.; Minarowska, A. Can Milrinone Be a Therapeutic Alternative in Persistent Pulmonary Hypertension of the Newborn? A Case Series and Narrative Review. Pediatr. Rep. 2025, 17, 116. https://doi.org/10.3390/pediatric17060116
Wasilewska E, Dera N, Minarowski Ł, Osiński Ł, Doboszynska A, Szajda S, Minarowska A. Can Milrinone Be a Therapeutic Alternative in Persistent Pulmonary Hypertension of the Newborn? A Case Series and Narrative Review. Pediatric Reports. 2025; 17(6):116. https://doi.org/10.3390/pediatric17060116
Chicago/Turabian StyleWasilewska, Eliza, Norbert Dera, Łukasz Minarowski, Łukasz Osiński, Anna Doboszynska, Sławomir Szajda, and Alina Minarowska. 2025. "Can Milrinone Be a Therapeutic Alternative in Persistent Pulmonary Hypertension of the Newborn? A Case Series and Narrative Review" Pediatric Reports 17, no. 6: 116. https://doi.org/10.3390/pediatric17060116
APA StyleWasilewska, E., Dera, N., Minarowski, Ł., Osiński, Ł., Doboszynska, A., Szajda, S., & Minarowska, A. (2025). Can Milrinone Be a Therapeutic Alternative in Persistent Pulmonary Hypertension of the Newborn? A Case Series and Narrative Review. Pediatric Reports, 17(6), 116. https://doi.org/10.3390/pediatric17060116







