Respiratory Syncytial Virus Infection in Children with Acute Lymphoblastic Leukemia (ALL): A Contemporary Emerging and Struggling Clinical Event
Abstract
1. Introduction
2. Case Presentations
2.1. Case 1 [Unique Patient Number (UPN) 1069066]
2.2. Case 2 (UPN 1077926)
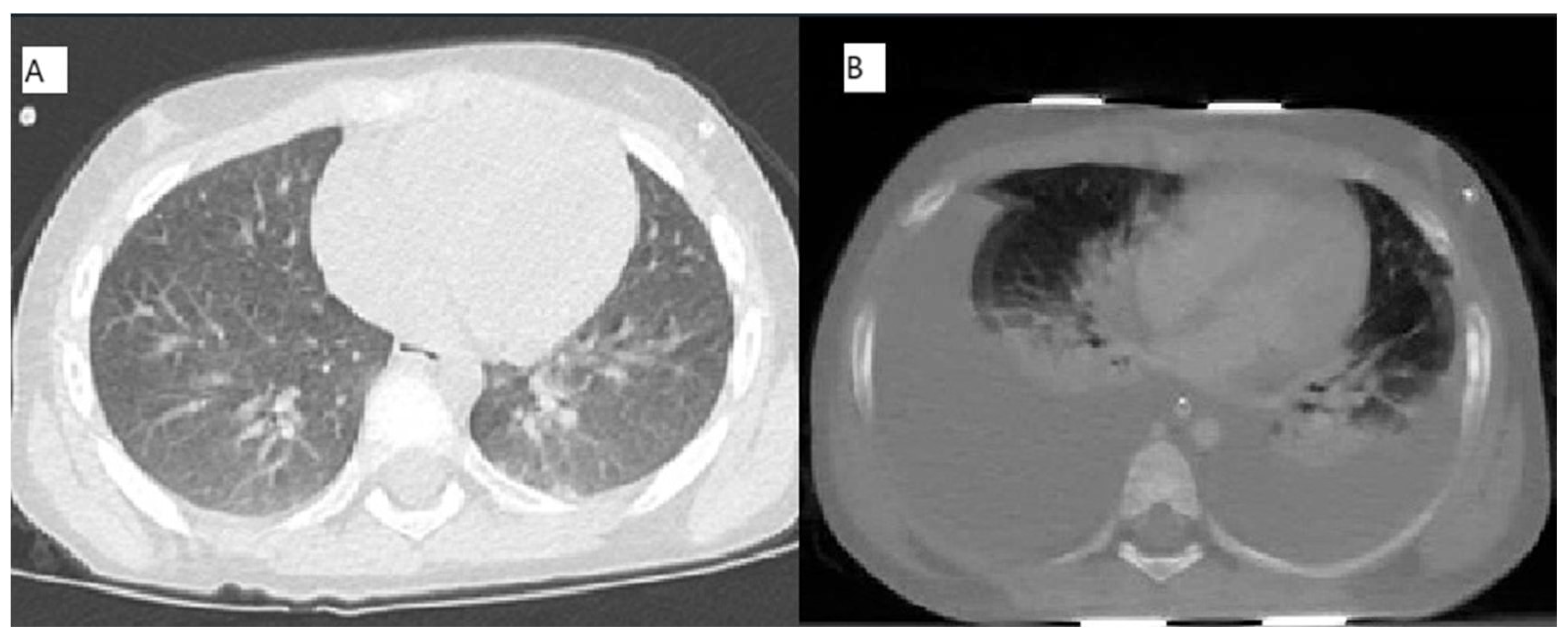
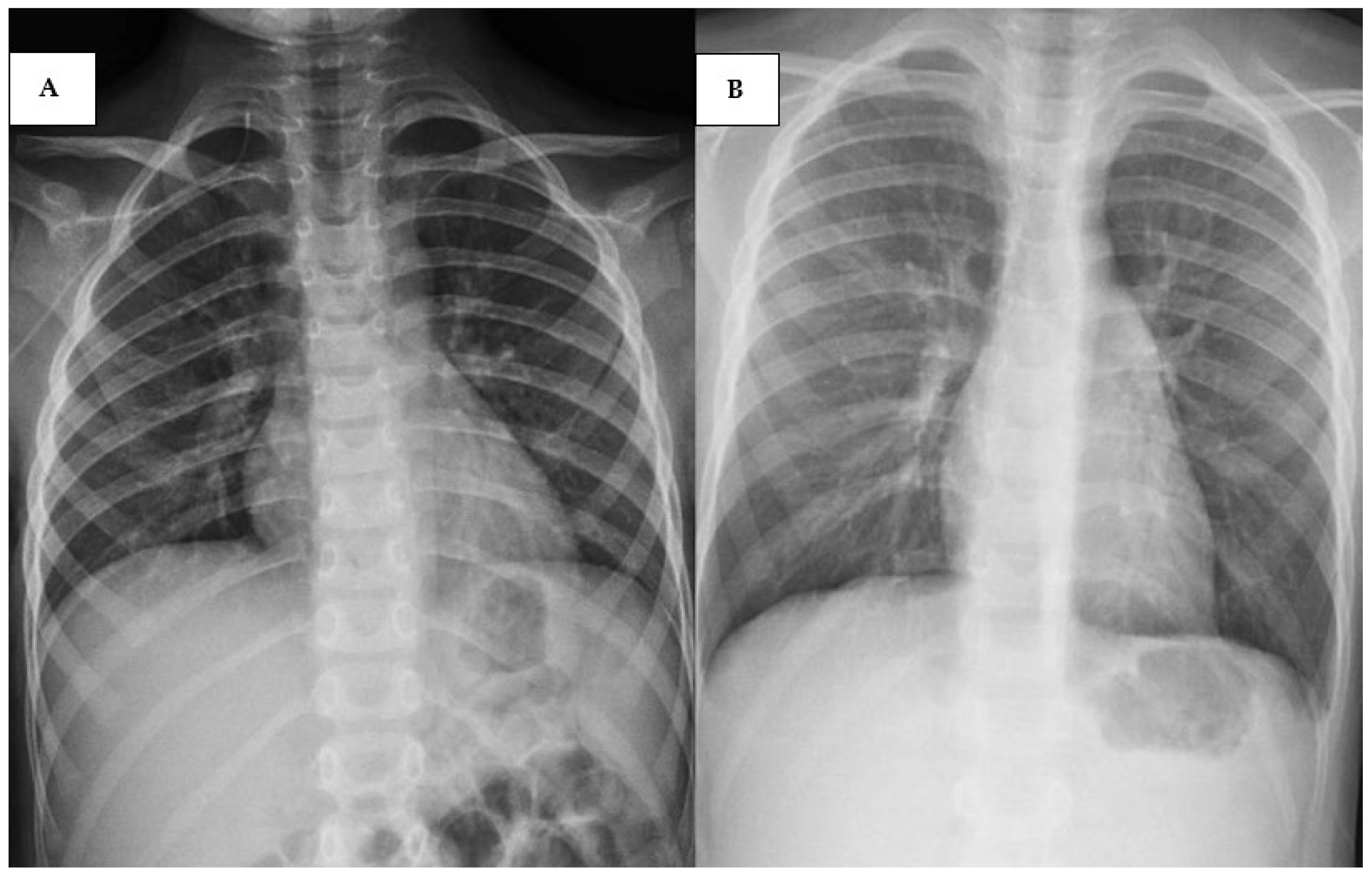
2.3. Cases 3 (UPN 1076952) and 4 (UPN 1077940)
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazur, N.I.; Caballero, M.T.; Nunes, M.C. Severe Respiratory Syncytial Virus Infection in Children: Burden, Management, and Emerging Therapies. Lancet 2024, 404, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory Syncytial Virus Infection in Adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef]
- Duan, Y.; Liu, Z.; Zang, N.; Cong, B.; Shi, Y.; Xu, L.; Jiang, M.; Wang, P.; Zou, J.; Zhang, H.; et al. Landscape of Respiratory Syncytial Virus. Chin. Med. J. 2024, 137, 2953–2978. [Google Scholar] [CrossRef]
- Colosia, A.; Costello, J.; McQuarrie, K.; Kato, K.; Bertzos, K. Systematic Literature Review of the Signs and Symptoms of Respiratory Syncytial Virus. Influenza Resp. Viruses 2023, 17, e13100. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Zhang, X.; Hua, W.; Xie, Z.-D.; Liu, H.-M.; Zhang, H.-L.; Chen, B.-Q.; Chen, Y.; Sun, X.; Xu, Y.; et al. Expert Consensus on the Diagnosis, Treatment, and Prevention of Respiratory Syncytial Virus Infections in Children. World J. Pediatr. 2024, 20, 11–25. [Google Scholar] [CrossRef]
- Dawson-Caswell, M.; Muncie, H.L. Respiratory Syncytial Virus Infection in Children. Am. Fam. Physician 2011, 83, 141–146. [Google Scholar]
- Li, X.; Willem, L.; Antillon, M.; Bilcke, J.; Jit, M.; Beutels, P. Health and Economic Burden of Respiratory Syncytial Virus (RSV) Disease and the Cost-Effectiveness of Potential Interventions against RSV among Children under 5 Years in 72 Gavi-Eligible Countries. BMC Med. 2020, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Benites, E.C.A.; Cabrini, D.P.; Silva, A.C.B.; Silva, J.C.; Catalan, D.T.; Berezin, E.N.; Cardoso, M.R.A.; Passos, S.D. Acute Respiratory Viral Infections in Pediatric Cancer Patients Undergoing Chemotherapy. J. Pediatr. 2014, 90, 370–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buus-Gehrig, C.; Bochennek, K.; Hennies, M.T.; Klingebiel, T.; Groll, A.H.; Lehrnbecher, T. Systemic Viral Infection in Children Receiving Chemotherapy for Acute Leukemia. Pediatr. Blood Cancer 2020, 67, e28673. [Google Scholar] [CrossRef]
- Hakim, H.; Dallas, R.; Zhou, Y.; Pei, D.; Cheng, C.; Flynn, P.M.; Pui, C.; Jeha, S. Acute Respiratory Infections in Children and Adolescents with Acute Lymphoblastic Leukemia. Cancer 2016, 122, 798–805. [Google Scholar] [CrossRef]
- Ross, H.S.; Dallas, R.H.; Ferrolino, J.A.; Johnson, M.B.; Allison, K.J.; Cross, S.J.; Hayden, R.T.; Mejias, A.; Hijano, D.R. Clinical Outcomes of Respiratory Syncytial Virus Infection Among Pediatric Immunocompromised Hosts. Pediatr. Blood Cancer 2025, 72, e31484. [Google Scholar] [CrossRef]
- Mogensen, T.H. Genetic Susceptibility to Viral Disease in Humans. Clin. Microbiol. Infect. 2022, 28, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-F.; Zhang, X.-Z.; Liu, C.-Y.; Li, W.; Li, W.-H.; Wang, Y.-Y.; Li, H.-Y.; Xiang, M.; Lu, R.; Yuan, T.-Y.; et al. A Novel Combined Nomogram for Predicting Severe Acute Lower Respiratory Tract Infection in Children Hospitalized for RSV Infection during the Post-COVID-19 Period. Front. Immunol. 2024, 15, 1437834. [Google Scholar] [CrossRef]
- Al-Maskari, N.; Mohsin, J.; Al-Maani, A.; Al-Macki, N.; Al-Ismaili, S. Atypical Presentations of Respiratory Syncytial Virus Infection: Case Series. Sultan Qaboos Univ. Med. J. 2016, 16, e86–e91. [Google Scholar] [CrossRef]
- Miura, H.; Hattori, F.; Uchida, H.; Hata, T.; Kudo, K.; Sato, M.; Yoshikawa, T. Case Report of Severe Myocarditis in an Immunocompromised Child with Respiratory Syncytial Virus Infection. BMC Pediatr. 2018, 18, 51. [Google Scholar] [CrossRef]
- Kristic Kirin, B.; Zrinski Topic, R.; Dodig, S. Hepatitis during Respiratory Syncytial Virus Infection—A Case Report. Biochem. Med. 2013, 23, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Bakalli, I. Liver Dysfunction in Severe Sepsis from Respiratory Syncytial Virus. J. Pediatr. Intensive Care 2018, 07, 110–114. [Google Scholar] [CrossRef]
- Hauer, J.; Fischer, U.; Borkhardt, A. Toward Prevention of Childhood ALL by Early-Life Immune Training. Blood 2021, 138, 1412–1428. [Google Scholar] [CrossRef]
- Piedimonte, G. New Insights into the Molecular Mechanisms of Respiratory Syncytial Virus (RSV) Disease. Pediatr. Respir. J. 2022, 1, 15. [Google Scholar] [CrossRef]
- Tejada, S.; Martinez-Reviejo, R.; Karakoc, H.N.; Peña-López, Y.; Manuel, O.; Rello, J. Ribavirin for Treatment of Subjects with Respiratory Syncytial Virus-Related Infection: A Systematic Review and Meta-Analysis. Adv. Ther. 2022, 39, 4037–4051. [Google Scholar] [CrossRef]
- Garegnani, L.; Styrmisdóttir, L.; Roson Rodriguez, P.; Escobar Liquitay, C.M.; Esteban, I.; Franco, J.V. Palivizumab for Preventing Severe Respiratory Syncytial Virus (RSV) Infection in Children. Cochrane Database Syst. Rev. 2021, 2021, CD013757. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lai, H.; Na, F.; Li, S.; Qiu, X.; Tian, J.; Zhang, Z.; Ge, L. Monoclonal Antibody for the Prevention of Respiratory Syncytial Virus in Infants and Children: A Systematic Review and Network Meta-Analysis. JAMA Netw Open 2023, 6, e230023. [Google Scholar] [CrossRef]
- Balbi, H. Nirsevimab: A Review. Pediatr. Allergy Immunol. Pulmonol. 2024, 37, 3–6. [Google Scholar] [CrossRef]
- Moline, H.L.; Tannis, A.; Toepfer, A.P.; Williams, J.V.; Boom, J.A.; Englund, J.A.; Halasa, N.B.; Staat, M.A.; Weinberg, G.A.; Selvarangan, R.; et al. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season—New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 209–214. [Google Scholar] [CrossRef]
- Muller, W.J.; Madhi, S.A.; Seoane Nuñez, B.; Baca Cots, M.; Bosheva, M.; Dagan, R.; Hammitt, L.L.; Llapur, C.J.; Novoa, J.M.; Saez Llorens, X.; et al. Nirsevimab for Prevention of RSV in Term and Late-Preterm Infants. N. Engl. J. Med. 2023, 388, 1533–1534. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Germano, C.; Avellis, V.; Tavella, E.; Dodaro, V.; Massaro, A.; Vitale, R.; Masturzo, B.; Manzoni, P. New Strategies for the Prevention of Respiratory Syncytial Virus (RSV). Early Hum. Dev. 2022, 174, 105666. [Google Scholar] [CrossRef]
- Dolgin, E. Antibody Therapies Set to Transform Respiratory Syncytial Virus Prevention for Babies. Nature 2023, 621, S55–S57. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xu, S.; Lu, Y.; Luo, Z.; Yan, Y.; Wang, C.; Ji, J. Development of mRNA Vaccines against Respiratory Syncytial Virus (RSV). Cytokine Growth Factor Rev. 2022, 68, 37–53. [Google Scholar] [CrossRef]

| d 0 | d + 8 ^ | d + 12 * | d + 15 | d + 18 ^ | d + 19 | d + 20 | d + 21 | d + 22 (8:00 a.m.) | d + 22 (4:00 p.m.) | d + 23 (8:00 a.m.) | d + 23 (4:00 p.m.) | d + 24 (Exitus) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (103/µL) | 12.9 | 2.2 | 1.3 | 1.4 | 0.540 | 0.620 | 0.420 | 0.570 | 0.570 | 0.440 | 0.540 | 0.900 | |
| Hb (g/dL) | 7 | 8.9 | 11.6 | 10.9 | 8.8 | 8.6 | 8.2 | 8.1 | 7.8 | 6.5 * | 9.2 | 7.9 | |
| PLT (103/µL) | 61 | 46 | 69 | 56 | 95 | 104 | 96 | 95 | 86 | 23 * | 24 | 52 | |
| CRP (mg/L) | 67 | 14 | 40 | 12 | 8.22 | 5.07 | 6.25 | 9.96 | 14.36 | 7.69 | |||
| PCT (mg/L) | 0.7 | 0.21 | 0.16 | 1.06 | 0.62 | 0.52 | |||||||
| AST (U/L) | 10 | 170 | 55 | 48 | 80 | 125 | 181 | 603 | 2643 | 3531 | 5884 | 5169 | |
| ALT (U/L) | 86 | 2857 | 4354 | 7735 | 6737 | ||||||||
| Bil. tot (mg/dl) | 0.27 | 0.43 | 0.38 | 0.59 | 0.67 | 0.55 | 1.39 | 2.26 | |||||
| PT (s) | 1.63 | 2.04 | 3.07 | 3.82 | not measurable | ||||||||
| PTT (s) | 44 | 52 | 57 | 69 | not measurable | ||||||||
| Fibrinogen (mg/dL) | 71 | 41 | 48 * | 67 * | 183 | ||||||||
| ATIII (%) | 59 * | 98 | 63 | 32 | |||||||||
| Pancreatic Amylase (U/L) | 15 | 19 | 27 | 26 | 27 | ||||||||
| LDH (U/L) | 374 | 160 | 248 | 7863 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrabito, M.; Cannata, E.; Lo Nigro, L. Respiratory Syncytial Virus Infection in Children with Acute Lymphoblastic Leukemia (ALL): A Contemporary Emerging and Struggling Clinical Event. Pediatr. Rep. 2025, 17, 95. https://doi.org/10.3390/pediatric17050095
Arrabito M, Cannata E, Lo Nigro L. Respiratory Syncytial Virus Infection in Children with Acute Lymphoblastic Leukemia (ALL): A Contemporary Emerging and Struggling Clinical Event. Pediatric Reports. 2025; 17(5):95. https://doi.org/10.3390/pediatric17050095
Chicago/Turabian StyleArrabito, Marta, Emanuela Cannata, and Luca Lo Nigro. 2025. "Respiratory Syncytial Virus Infection in Children with Acute Lymphoblastic Leukemia (ALL): A Contemporary Emerging and Struggling Clinical Event" Pediatric Reports 17, no. 5: 95. https://doi.org/10.3390/pediatric17050095
APA StyleArrabito, M., Cannata, E., & Lo Nigro, L. (2025). Respiratory Syncytial Virus Infection in Children with Acute Lymphoblastic Leukemia (ALL): A Contemporary Emerging and Struggling Clinical Event. Pediatric Reports, 17(5), 95. https://doi.org/10.3390/pediatric17050095







