A Decade of Transformation in the Management of Childhood Acute Lymphoblastic Leukemia: From Conventional Chemotherapy to Precision Medicine
Abstract
1. Introduction
2. The State of the Art in 2015: A Foundation for Progress
3. Transformative Advances in Diagnosis
3.1. Enhanced Minimal Residual Disease (MRD) Detection
- (1)
- (2)
- Mass cytometry: Allowing for the simultaneous analysis of multiple cellular markers, enhancing the characterization of residual leukemic cells [31].
- (3)
- Next Generation Sequencing MRD: The introduction of next-generation sequencing (NGS) represents the new frontier for MRD detection, enabling the identification of leukemic cells at levels as low as 10−6 (1 in 1,000,000 cells) [32,33]. This increased sensitivity allows for more precise risk stratification and treatment adjustments, improving patient outcomes. NGS-based MRD assays have demonstrated prognostic significance in both B-cell and T-cell ALL [34].
3.2. Advances in Genomic Diagnostics
4. Transformative Advances in Treatment
4.1. Hematopoietic Stem Cell Transplantation
4.2. Immunotherapy Breakthroughs
4.2.1. Blinatumomab
4.2.2. Inotuzumab Ozogamicin
4.3. Chimeric Antigen Receptor (CAR) T-Cell Therapy
- (1)
- (2)
- TRUCKs (T cells Redirected for Universal Cytokine Killing) represent fourth-generation CAR T cells that, upon antigen engagement, are programmed to secrete immunomodulatory cytokines such as IL-12, thereby augmenting T cell activity and reshaping the tumor microenvironment to overcome immunosuppression [74].
- (3)
- Off-the-shelf allogeneic CAR T-cells: Derived from healthy donors to improve accessibility and reduce manufacturing time [75].
4.4. Targeted Therapies
4.5. Philadelphia Chromosome-Positive ALL
4.6. Philadelphia-like ALL
4.7. Infant Acute Lymphoblastic Leukemia
4.8. Novel Targeted Agents Under Investigation
5. The Role of Artificial Intelligence
5.1. Artificial Intelligence in Diagnostics
5.2. Artificial Intelligence in Treatment
- (1)
- Automated morphological analysis: Convolutional neural networks have achieved >90% accuracy in classifying leukemic cells from peripheral blood smears [105].
- (2)
- Predictive modeling: Machine learning algorithms can predict treatment outcomes and identify patients at high risk of relapse based on clinical, genetic, and MRD data [106].
- (3)
- Drug response prediction: AI models trained on ex vivo drug testing data can predict patient-specific responses to chemotherapeutic agents, potentially guiding treatment selection [107].
- (4)
- Image analysis in flow cytometry: Deep learning algorithms can improve the accuracy and reproducibility of flow cytometry interpretation, reducing inter-observer variability [108].
6. Looking Ahead: Promises for the Next Five Years
6.1. Personalized Medicine
- 1.
- Pharmacogenomic-guided therapy: Adjusting drug dosages based on genetic variants affecting drug metabolism, such as TPMT and NUDT15 polymorphisms for thiopurines [111].
- 2.
- Risk-adapted therapy de-escalation: Identifying ultra-low-risk patients who may benefit from reduced treatment intensity, potentially decreasing long-term toxicities [112].
- 3.
- Integrated multi-omics approaches: Combining genomic, transcriptomic, proteomic, and metabolomic data to comprehensively characterize leukemia cells and identify therapeutic vulnerabilities [113].
6.2. Global Access and Equity
- 1.
- 2.
- Point-of-care diagnostics: Developing affordable and portable diagnostic tools for improved risk stratification [118].
- 3.
- 4.
- Generic drug production: Promoting the development of affordable generic versions of novel therapies [122].
- 5.
- 6.
- Global access initiatives: Implementation of programs designed to provide essential ALL medicines at no cost or very low cost—and, where feasible, through commitments by local health authorities. Examples include: the Max Foundation’s patient-assistance programs (e.g., GIPAP and Max Access Solutions), which have provided imatinib and other TKIs at no cost to eligible patients, including those with Ph+ disease [125]; the WHO–St. Jude Global Platform for Access to Childhood Cancer Medicines, which aims to ensure a continuous and reliable supply of quality-assured childhood cancer medicines to approximately 120,000 children in LMICs during 2022–2027 [126]; and the multi-stakeholder ACT for Children initiative, which has begun supplying pegylated L-asparaginase and other essential pediatric oncology medicines to hospitals in LMICs [127], with partners such as Resonance supporting implementation and data systems [128].
7. Challenges and Limitations
- Treatment toxicity: Acute and long-term side effects continue to impact quality of life for survivors, with neurocognitive impairment, cardiovascular disease, and secondary malignancies being major concerns [129].
- Therapy resistance: Approximately 10–15% of patients still experience relapse, often with chemo-resistant disease [130].
- Cost and accessibility: Novel therapies like CAR T-cell therapy remain prohibitively expensive, with costs exceeding $400,000 per treatment course, excluding hospitalization and management of complications [131].
- Biomarker standardization: Despite advances in MRD detection, there is still variability in methodologies, reporting, and interpretation across centers [132].
- Toxicity management: New immunotherapies are associated with unique toxicity profiles that require specialized expertise to manage effectively [133].
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hunger, S.P.; Mullighan, C.G. Acute lymphoblastic leukemia in children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 contributors. International incidence of childhood cancer, 2001–10: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Pui, C.-H.; Yang, J.J.; Hunger, S.P.; Pieters, R.; Schrappe, M.; Biondi, A.; Vora, A.; Baruchel, A.; Silverman, L.B.; Schmiegelow, K.; et al. Childhood acute lymphoblastic leukemia: Progress through collaboration. J. Clin. Oncol. 2015, 33, 2938–2948. [Google Scholar] [CrossRef]
- Teachey, D.T.; Pui, C.H. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 2019, 20, e142–e154. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, I.; Mullighan, C.G. Genetic basis of acute lymphoblastic leukemia. J. Clin. Oncol. 2017, 35, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Pei, D.; Campana, D.; Cheng, C.; Sandlund, J.T.; Bowman, W.P.; Hudson, M.M.; Ribeiro, R.C.; Raimondi, S.C.; Jeha, S.; et al. A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia 2021, 35, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Malard, F.; Mohty, M. Acute lymphoblastic leukaemia. Lancet 2020, 395, 1146–1162. [Google Scholar] [CrossRef]
- Conter, V.; Valsecchi, M.G.; Cario, G.; Zimmermann, M.; Attarbaschi, A.; Stary, J.; Niggli, F.; Dalla Pozza, L.; Elitzur, S.; Silvestri, D.; et al. Four Additional Doses of PEG-L-Asparaginase During the Consolidation Phase in the AIEOP-BFM ALL 2009 Protocol Do Not Improve Outcome and Increase Toxicity in High-Risk ALL: Results of a Randomized Study. J. Clin. Oncol. 2024, 42, 915–926. [Google Scholar] [CrossRef]
- Inaba, H.; Greaves, M.; Mullighan, C.G. Acute lymphoblastic leukaemia. Lancet 2013, 381, 1943–1955. [Google Scholar] [CrossRef]
- Roberts, K.G.; Mullighan, C.G. Genomics in acute lymphoblastic leukaemia: Insights and treatment implications. Nat. Rev. Clin. Oncol. 2020, 17, 415–430. [Google Scholar] [CrossRef]
- Möricke, A.; Zimmermann, M.; Valsecchi, M.G.; Stanulla, M.; Biondi, A.; Mann, G.; Locatelli, F.; Cazzaniga, G.; Niggli, F.; Aricò, M.; et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: Results of the randomized trial AIEOP-BFM ALL 2000. Blood 2016, 127, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Lu, X.; Devidas, M.; Camitta, B.M.; Gaynon, P.S.; Winick, N.J.; Reaman, G.H.; Carroll, W.L. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Schmiegelow, K.; Levinsen, M.F.; Attarbaschi, A.; Baruchel, A.; Devidas, M.; Escherich, G.; Gibson, B.; Heydrich, C.; Horibe, K.; Ishida, Y.; et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J. Clin. Oncol. 2013, 31, 2469–2476. [Google Scholar] [CrossRef]
- Schmiegelow, K.; Attarbaschi, A.; Barzilai, S.; Escherich, G.; Frandsen, T.L.; Halsey, C.; Hough, R.; Jeha, S.; Kato, M.; Liang, D.C.; et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: A Delphi consensus. Lancet Oncol. 2016, 17, e231–e239. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Roganovic, J.; Haupt, R.; Bárdi, E.; Hjorth, L.; Michel, G.; Pavasovic, V.; Scheinemann, K.; van der Pal, H.J.; Zadravec Zaletel, L.; Amariutei, A.E.; et al. Late Adverse Effects after Treatment for Childhood Acute Leukemia. Acta Med. Acad. 2024, 53, 59–80. [Google Scholar] [CrossRef]
- Mody, R.; Li, S.; Dover, D.C.; Sallan, S.; Leisenring, W.; Oeffinger, K.C.; Yasui, Y.; Robison, L.L.; Neglia, J.P. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. Blood 2008, 111, 5515–5523. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Hyun, G.; Ness, K.K.; Bhakta, N.; Pui, C.H.; Ehrhardt, M.J.; Krull, K.R.; Crom, D.B.; Chemaitilly, W.; Srivastava, D.K.; et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia: A retrospective analysis of the St Jude Lifetime Cohort Study. Lancet Haematol. 2019, 6, e306–e316. [Google Scholar] [CrossRef]
- Pieters, R.; De Lorenzo, P.; Ancliffe, P.; Aversa, L.A.; Brethon, B.; Biondi, A.; Campbell, M.; Escherich, G.; Ferster, A.; Gardner, R.A.; et al. Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the Interfant-06 protocol: Results from an international phase III randomized study. J. Clin. Oncol. 2019, 37, 2246–2256. [Google Scholar] [CrossRef]
- Burke, M.J.; Devidas, M.; Chen, Z.; Salzer, W.L.; Raetz, E.A.; Rabin, K.R.; Heerema, N.A.; Carroll, A.J.; Gastier-Foster, J.M.; Borowitz, M.J.; et al. Outcomes in adolescent and young adult patients (16 to 30 years) compared to younger patients treated for high-risk B-lymphoblastic leukemia: Report from Children’s Oncology Group Study AALL0232. Leukemia 2022, 36, 648–655. [Google Scholar] [CrossRef]
- Moorman, A.V. New and emerging prognostic and predictive genetic biomarkers in B-cell precursor acute lymphoblastic leukemia. Haematologica 2016, 101, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, D.; Pui, C.H. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. 2013, 14, e205–e217. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, M.; Schrauder, A.; Raff, T.; Pfeifer, H.; Dworzak, M.; Ottmann, O.G.; Asnafi, V.; Baruchel, A.; Bassan, R.; Benoit, Y.; et al. Standardized MRD quantification in European ALL trials: Proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18–20 September 2008. Leukemia 2010, 24, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Basso, G.; Veltroni, M.; Valsecchi, M.G.; Dworzak, M.N.; Ratei, R.; Silvestri, D.; Benetello, A.; Buldini, B.; Maglia, O.; Masera, G.; et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J. Clin. Oncol. 2009, 27, 5168–5174. [Google Scholar] [CrossRef]
- Borowitz, M.J.; Devidas, M.; Hunger, S.P.; Bowman, W.P.; Carroll, A.J.; Carroll, W.L.; Linda, S.; Martin, P.L.; Pullen, D.J.; Viswanatha, D.; et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: A Children’s Oncology Group study. Blood 2008, 111, 5477–5485. [Google Scholar] [CrossRef]
- Conter, V.; Bartram, C.R.; Valsecchi, M.G.; Schrauder, A.; Panzer-Grümayer, R.; Möricke, A.; Aricò, M.; Zimmermann, M.; Mann, G.; De Rossi, G.; et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 2010, 115, 3206–3214. [Google Scholar] [CrossRef]
- Schrappe, M.; Valsecchi, M.G.; Bartram, C.R.; Schrauder, A.; Panzer-Grümayer, R.; Möricke, A.; Parasole, R.; Zimmermann, M.; Dworzak, M.; Buldini, B.; et al. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: Results of the AIEOP-BFM-ALL 2000 study. Blood 2011, 118, 2077–2084. [Google Scholar] [CrossRef]
- Schrappe, M.; Bleckmann, K.; Zimmermann, M.; Biondi, A.; Möricke, A.; Locatelli, F.; Cario, G.; Rizzari, C.; Attarbaschi, A.; Valsecchi, M.G.; et al. Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: Results of an international randomized trial (AIEOP-BFM ALL 2000). J. Clin. Oncol. 2018, 36, 244–253. [Google Scholar] [CrossRef]
- Luib, L.; Kreyenberg, H.; Michaelis, S.; Handgretinger, R.; Mezger, M. Transferring measurable residual disease measurement in pediatric acute lymphoblastic leukemia from quantitative real-time PCR to digital droplet PCR. Pediatr. Transplant. 2023, 27, e14483. [Google Scholar] [CrossRef]
- Della Starza, I.; Chiaretti, S.; De Propris, M.S.; Elia, L.; Cavalli, M.; De Novi, L.A.; Soscia, R.; Messina, M.; Vitale, A.; Guarini, A.; et al. Digital-droplet PCR for precise quantification of minimal residual disease in acute lymphoblastic leukemia. Front. Mol. Biosci. 2021, 8, 730927. [Google Scholar]
- Bandyopadhyay, S.; Fisher, D.A.C.; Malkova, O.; Oh, S.T. Analysis of signaling networks at the single-cell level using mass cytometry: Focus on acute myeloid leukemia. Methods Mol. Biol. 2017, 1636, 327–347. [Google Scholar]
- Wood, B.; Wu, D.; Crossley, B.; Dai, Y.; Williamson, D.; Gawad, C.; Borowitz, M.J.; Devidas, M.; Maloney, K.W.; Larsen, E.; et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood 2018, 131, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, P.M.J.; de Bie, M.; van Zessen, D.; de Haas, V.; Stubbs, A.P.; van der Velden, V.H.J. Next-generation antigen receptor sequencing of paired diagnosis and relapse samples of B-cell acute lymphoblastic leukemia: Clonal evolution and implications for minimal residual disease target selection. Leuk. Res. 2019, 76, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Svaton, M.; Skotnicova, A.; Reznickova, L.; Rennerova, A.; Valova, T.; Kotrova, M.; van der Velden, V.H.J.; Brüggemann, M.; Darzentas, N.; Langerak, A.W.; et al. NGS better discriminates true MRD positivity for the risk stratification of childhood ALL treated on an MRD-based protocol. Blood 2023, 141, 529–533. [Google Scholar] [CrossRef]
- Ohki, K.; Kiyokawa, N.; Saito, Y.; Hirabayashi, S.; Nakabayashi, K.; Ichikawa, H.; Momozawa, Y.; Okamura, K.; Yoshimi, A.; Ogata-Kawata, H.; et al. Clinical and molecular characteristics of MEF2D fusion-positive B-cell precursor acute lymphoblastic leukemia in childhood, including a novel translocation resulting in MEF2D-HNRNPH1 gene fusion. Haematologica 2019, 104, 128–137. [Google Scholar] [CrossRef]
- Shinsuke, H.; Kentaro, O.; Kazuhiko, N.; Hitoshi, I.; Yukihide, M.; Kohji, O.; Akinori, Y.; Kazuki, T.; Yuya, S.; Ai, Y.; et al. ZNF384-related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype. Haematologica 2017, 102, 118–129. [Google Scholar]
- Marincevic-Zuniga, Y.; Dahlberg, J.; Nilsson, S.; Raine, A.; Nystedt, S.; Lindqvist, C.M.; Berglund, E.C.; Abrahamsson, J.; Cavelier, L.; Forestier, E.; et al. Transcriptome sequencing in pediatric acute lymphoblastic leukemia identifies fusion genes including MEF2D and ZNF384. J. Hematol. Oncol. 2017, 10, 123. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Li, Z.; Bai, L.; Wang, Q.; Li, J.; Jiang, M.; Xue, Q.; Cheng, N.; Zhang, W.; et al. Functional, structural, and molecular characterizations of the leukemogenic driver MEF2D-HNRNPUL1 fusion. Blood 2022, 140, 1390–1408. [Google Scholar] [CrossRef]
- Krali, O.; Marincevic-Zuniga, Y.; Arvidsson, G.; Enblad, A.P.; Lundmark, A.; Sayyab, S.; Zachariadis, V.; Heinäniemi, M.; Suhonen, J.; Oksa, L.; et al. Multimodal classification of molecular subtypes in pediatric B-cell precursor acute lymphoblastic leukemia. Nat. Prec. Oncol. 2023, 3, 4. [Google Scholar]
- Vicente-Garcés, C.; Fernández, G.; Esperanza-Cebollada, E.; Richarte-Franqués, M.; Crespo-Carrasco, A.; Montesdeoca, S.; Isola, I.; Sarrate, E.; Cuatrecasas, E.; Rives, S.; et al. RNA-sequencing: A reliable tool to unveil transcriptional landscape of paediatric B-other acute lymphoblastic leukaemia. Cancers 2025, 17, 456. [Google Scholar] [CrossRef]
- Péterffy, B.; Krizsán, S.; Egyed, B.; Bedics, G.; Benard-Slagter, A.; Palit, S.; Erdélyi, D.J.; Müller, J.; Nagy, T.; Hegyi, L.L.; et al. Molecular profiling reveals novel gene fusions and genetic markers for refined patient stratification in pediatric acute lymphoblastic leukemia. Mod. Pathol. 2025, 38, 100741. [Google Scholar] [CrossRef]
- Peters, C.; Dalle, J.H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J. Clin. Oncol. 2021, 39, 295–307. [Google Scholar] [CrossRef]
- Leonard, J.T.; Hayes-Lattin, B. Reduced-Intensity Conditioning Allogeneic Hematopoietic Transplantation for Children with ALL. Biol. Blood Marrow Transplant. 2018, 24, 207–214. [Google Scholar]
- Ruggeri, A.; Galimard, J.E.; Paina, O.; Fagioli, F.; Tbakhi, A.; Yesilipek, A.; Navarro, J.M.F.; Faraci, M.; Hamladji, R.M.; Skorobogatova, E.; et al. Outcomes of Unmanipulated Haploidentical Transplantation Using Post-Transplant Cyclophosphamide (PT-Cy) in Pediatric Patients with Acute Lymphoblastic Leukemia. Transpl. Cell Ther. 2021, 27, e1–e424. [Google Scholar] [CrossRef]
- Espinoza-Gutarra, M.; Mohty, R.; Jamy, O. Radiation-free conditioning in acute lymphoblastic leukemia: Is it time? Chin. Clin. Oncol. 2024, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wan, C.-L.; Xu, M.-Z.; Zhou, H.-X.; Liu, M.-J.; Gong, W.-J.; Kang, L.-Q.; Sun, A.-N.; Yu, L.; Wu, D.-P.; et al. Safety and efficacy of CD22 and CD19 CAR-T bridging auto-HSCT as consolidation therapy for AYA and adult B-ALL. Blood Cancer J. 2023, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, M.; Zhang, X.; Zhang, Z.; Zhong, T.; Yu, H.; Fu, Y.; Meng, H.; Feng, J.; Zou, X.; et al. CD19 chimeric antigen receptor-T cells as bridging therapy to allogeneic hematopoietic cell transplantation improves outcome in patients with refractory/relapsed B-cell acute lymphoblastic leukemia. Heliyon 2024, 10, e33937. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Locatelli, F.; Zugmaier, G.; Mergen, N.; Bader, P.; Jeha, S.; Schlegel, P.G.; Bourquin, J.P.; Handgretinger, R.; Brethon, B.; Rossig, C.; et al. Blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia: Results of the RIALTO trial, an expanded access study. Blood Cancer J. 2020, 10, 77. [Google Scholar] [CrossRef]
- Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Hogan, L.E.; Borowitz, M.J.; Raetz, E.A.; Zugmaier, G.; Sharon, E.; Bernhardt, M.B.; et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of B-cell acute lymphoblastic leukemia: A randomized clinical trial. JAMA 2021, 325, 833–842. [Google Scholar] [CrossRef]
- Locatelli, F.; Zugmaier, G.; Rizzari, C.; Morris, J.D.; Gruhn, B.; Klingebiel, T.; Parasole, R.; Linderkamp, C.; Flotho, C.; Petit, A.; et al. Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children with High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2021, 325, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Hogan, L.E.; Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Bhatla, T.; Borowitz, M.J.; Raetz, E.A.; Carroll, A.; Heerema, N.A.; et al. Children’s Oncology Group AALL1331: Phase III Trial of Blinatumomab in Children, Adolescents, and Young Adults with Low-Risk B-Cell ALL in First Relapse. J. Clin. Oncol. 2023, 41, 4118–4129. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rau, R.E.; Kairalla, J.A.; Rabin, K.R.; Wang, C.; Angiolillo, A.L.; Alexander, S.; Carroll, A.J.; Conway, S.; Gore, L.; et al. Blinatumomab in Standard-Risk B-Cell Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2025, 392, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef]
- Jabbour, E.; Sasaki, K.; Ravandi, F.; Huang, X.; Short, N.J.; Khouri, M.; Kebriaei, P.; Burger, J.; Khoury, J.; Jorgensen, J.; et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer 2018, 124, 4044–4055. [Google Scholar] [CrossRef]
- Bhojwani, D.; Sposto, R.; Shah, N.N.; Rodriguez, V.; Yuan, C.; Stetler-Stevenson, M.; O’Brien, M.M.; McNeer, J.L.; Quereshi, A.; Cabannes, A.; et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia 2019, 33, 884–892. [Google Scholar] [CrossRef]
- Kebriaei, P.; Cutler, C.; de Lima, M.; Giralt, S.; Lee, S.J.; Marks, D.; Merchant, A.; Stock, W.; van Besien, K.; Stelljes, M. Management of important adverse events associated with inotuzumab ozogamicin: Expert panel review. Bone Marrow Transplant. 2018, 53, 449–456. [Google Scholar] [CrossRef]
- Pennesi, E.; Brivio, E.; Ammerlaan, A.C.J.; Jiang, Y.; Van der Velden, V.H.J.; Beverloo, H.B.; Sleight, B.; Locatelli, F.; Brethon, B.; Rossig, C.; et al. Inotuzumab ozogamicin combined with chemotherapy in pediatric B-cell precursor CD22+ acute lymphoblastic leukemia: Results of the phase IB ITCC-059 trial. Haematologica 2024, 109, 3157–3166. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Gardner, R.A.; Finney, O.; Annesley, C.; Brakke, H.; Summers, C.; Leger, K.; Bleakley, M.; Brown, C.; Mgebroff, S.; Kelly-Spratt, K.S.; et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 2017, 129, 3322–3331. [Google Scholar] [CrossRef]
- O’LEary, M.C.; Lu, X.; Huang, Y.; Lin, X.; Mahmood, I.; Przepiorka, D.; Gavin, D.; Lee, S.; Liu, K.; George, B.; et al. FDA approval summary: Tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res. 2019, 25, 1142–1146. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Maude, S.L.; Rives, S.; Baruchel, A.; Boyer, M.W.; Bittencourt, H.; Bader, P.; Büchner, J.; Laetsch, T.W.; Stefanski, H.; et al. Updated analysis of the efficacy and safety of tisagenlecleucel in pediatric and young adult patients with relapsed/refractory acute lymphoblastic leukemia. Blood 2021, 138 (Suppl. 1), 1411. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transpl. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.-A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Buitrago, J.; Adkins, S.; Hawkins, M.; Iyamu, K.; van Oort, T. Adult Survivorship: Considerations following CAR T-cell therapy. Clin. J. Oncol. Nurs. 2019, 23, 2. [Google Scholar] [CrossRef]
- Shah, N.N.; Fry, T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019, 16, 372–385. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Fang, Y.; Xu, K.; Zhou, H.; Li, F.; Guan, X.; He, Y.; Feng, Y.; Lyu, L.; et al. Efficacy and Safety of Inaticabtagene Autoleucel in Children with Relapsed/Refractory (R/R) B-cell Acute Lymphoblastic Leukemia in China. Blood 2024, 144 (Suppl. 1), 7200. [Google Scholar] [CrossRef]
- Dulery, R.; Guiraud, V.; Choquet, S.; Thieblemont, C.; Bachy, E.; Barete, S.; Todesco, È.; Arnulf, B.; Boissel, N.; Baruchel, A.; et al. T cell malignancies after CAR T cell therapy in the DESCAR-T registry. Nat. Med. 2025, 31, 1130–1133. [Google Scholar] [CrossRef]
- Hsieh, E.M.; Myers, R.M.; Yates, B.; Annesley, C.; John, S.; Taraseviciute, A.; Steinberg, S.M.; Sheppard, J.; Chung, P.; Chen, L.; et al. Low rate of subsequent malignant neoplasms after CD19 CAR T-cell therapy. Blood Adv. 2022, 6, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Fousek, K.; Watanabe, J.; Joseph, S.K.; George, A.; An, X.; Byrd, T.T.; Morris, J.S.; Luong, A.; Martínez-Paniagua, M.A.; Sanber, K.; et al. CAR T-cells that target acute B-lineage leukemia irrespective of CD19 expression. Leukemia 2021, 35, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, K.; Du, H.; Xu, Y.; Shou, P.; Zhou, X.; Fucá, G.; Landoni, E.; Sun, C.; Chen, Y.; Savoldo, B.; et al. Dual Targeting CAR-T Cells with Optimal Costimulation and Metabolic Fitness enhance Antitumor Activity and Prevent Escape in Solid Tumors. Nat. Cancer 2021, 2, 904–918. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Rafiq, S.; Hackett, C.S.; Brentjens, R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020, 17, 147–167. [Google Scholar] [CrossRef]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Mirci-Danicar, O.C.; Lucchini, G.; Pinner, D.; Jain, N.; Kantarjian, H.; Boissel, N.; et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: Results of two phase 1 studies. Lancet 2020, 396, 1885–1894. [Google Scholar] [CrossRef]
- Gu, Z.; Churchman, M.L.; Roberts, K.G.; Moore, I.; Zhou, X.; Nakitandwe, J.; Hagiwara, K.; Pelletier, S.; Gingras, S.; Berns, H.; et al. PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat. Genet. 2019, 51, 296–307. [Google Scholar] [CrossRef]
- Brady, S.W.; Roberts, K.G.; Gu, Z.; Shi, L.; Pounds, S.; Pei, D.; Cheng, C.; Dai, Y.; Devidas, M.; Qu, C.; et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat. Genet. 2022, 54, 1376–1389. [Google Scholar] [CrossRef]
- Pölönen, P.; Di Giacomo, D.; Seffernick, A.E.; Elsayed, A.; Kimura, S.; Benini, F.; Montefiori, L.E.; Wood, B.L.; Xu, J.; Chen, C.; et al. The genomic basis of childhood T-lineage acute lymphoblastic leukaemia. Nature 2024, 632, 1082–1091. [Google Scholar] [CrossRef]
- Aricò, M.; Valsecchi, M.G.; Camitta, B.; Schrappe, M.; Chessells, J.; Baruchel, A.; Gaynon, P.; Silverman, L.; Janka-Schaub, G.; Kamps, W.; et al. Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N. Engl. J. Med. 2000, 342, 998–1006. [Google Scholar] [CrossRef]
- Schultz, K.R.; Carroll, A.; Heerema, N.A.; Bowman, W.P.; Aledo, A.; Slayton, W.B.; Sather, H.; Devidas, M.; Zheng, H.W.; Davies, S.M.; et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome–positive acute lymphoblastic leukemia: Children’s Oncology Group study AALL0031. Leukemia 2014, 28, 1467–1471. [Google Scholar] [CrossRef]
- Biondi, A.; Schrappe, M.; De Lorenzo, P.; Castor, A.; Lucchini, G.; Gandemer, V.; Pieters, R.; Stary, J.; Escherich, G.; Campbell, M.; et al. Imatinib after induction for treatment of children and adolescents with Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL): A randomised, open-label, intergroup study. Lancet Oncol. 2012, 13, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Gandemer, V.; De Lorenzo, P.; Cario, G.; Campbell, M.; Castor, A.; Pieters, R.; Baruchel, A.; Vora, A.; Leoni, V.; et al. Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): A prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol. 2018, 5, e641–e652. [Google Scholar] [CrossRef] [PubMed]
- Slayton, W.B.; Schultz, K.R.; Kairalla, J.A.; Devidas, M.; Mi, X.; Pulsipher, M.A.; Chang, B.H.; Mullighan, C.; Iacobucci, I.; Silverman, L.B.; et al. Dasatinib Plus Intensive Chemotherapy in Children, Adolescents, and Young Adults with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: Results of Children’s Oncology Group Trial AALL0622. J. Clin. Oncol. 2018, 36, 2306–2314. [Google Scholar] [CrossRef] [PubMed]
- Hunger, S.P.; Tran, T.H.; Saha, V.; Devidas, M.; Valsecchi, M.G.; Gastier-Foster, J.M.; Cazzaniga, G.; Reshmi, S.C.; Borowitz, M.J.; Moorman, A.V.; et al. Dasatinib with intensive chemotherapy in de novo paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (CA180-372/COG AALL1122): A single-arm, multicentre, phase 2 trial. Lancet Haematol. 2023, 10, e510–e520. [Google Scholar] [CrossRef]
- Shen, S.; Chen, X.; Cai, J.; Yu, J.; Gao, J.; Hu, S.; Zhai, X.; Liang, C.; Ju, X.; Jiang, H.; et al. Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 358–366. [Google Scholar] [CrossRef]
- Jabbour, E.; Kantarjian, H.M.; Aldoss, I.; Montesinos, P.; Leonard, J.T.; Gómez-Almaguer, D.; Baer, M.R.; Gambacorti-Passerini, C.; McCloskey, J.; Minami, Y.; et al. Ponatinib vs Imatinib in Frontline Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 2024, 331, 1814–1823. [Google Scholar] [CrossRef]
- Hijiya, N.; Zwaan, C.M.; Rizzari, C.; Foà, R.; Abbink, F.; Lancaster, D.; Landman-Parker, J.; Millot, F.; Moppett, J.; Nelken, B.; et al. Pharmacokinetics of Nilotinib in Pediatric Patients With Philadelphia Chromosome-Positive Chronic Myelogenous Leukemia or Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2020, 26, 812–820. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gu, Z.; Payne-Turner, D.; McCastlain, K.; Harvey, R.C.; Chen, I.-M.; Pei, D.; Iacobucci, I.; Valentine, M.; Pounds, S.B.; et al. High frequency and poor outcome of Philadelphia chromosome–like acute lymphoblastic leukemia in adults. J. Clin. Oncol. 2017, 35, 394–401. [Google Scholar] [CrossRef]
- Reshmi, S.C.; Harvey, R.C.; Roberts, K.G.; Stonerock, E.; Smith, A.; Jenkins, H.; Chen, I.-M.; Valentine, M.; Liu, Y.; Li, Y.; et al. Targetable kinase gene fusions in high-risk B-ALL: A study from the Children’s Oncology Group. Blood 2017, 129, 3352–3361. [Google Scholar] [CrossRef]
- Tasian, S.K.; Doral, M.Y.; Borowitz, M.J.; Wood, B.L.; Chen, I.-M.; Harvey, R.C.; Gastier-Foster, J.M.; Willman, C.L.; Hunger, S.P.; Mullighan, C.G.; et al. Aberrant STAT5 and PI3K/mTOR pathway activation in high-risk childhood acute lymphoblastic leukemia. Blood 2012, 120, 822–831. [Google Scholar] [CrossRef]
- Tran, T.H.; Tasian, S.K. How I treat Philadelphia chromosome-like acute lymphoblastic leukemia in children, adolescents, and young adults. Blood 2025, 145, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Tasian, S.K.; Hunter, D.S.; Chen, I.M.; Harvey, R.C.; Carroll, A.J.; Wagner, E.; Reshmi, S.C.; Borowitz, M.J.; Wood, B.L.; Daniel, J.; et al. A Phase 2 Study of Ruxolitinib with Chemotherapy in Children with Philadelphia Chromosome-like Acute Lymphoblastic Leukemia (AALL1521/INCB18424-269): Biologic Characteristics and Minimal Residual Disease Response of Patients with Non-CRLF2-Rearranged JAK Pathway Alterations. Blood 2022, 140 (Suppl. 1), 6117–6118. [Google Scholar] [CrossRef]
- Den Boer, M.L.; Cario, G.; Moorman, A.V.; Boer, J.M.; de Groot-Kruseman, H.A.; Fiocco, M.; Escherich, G.; Imamura, T.; Yeoh, A.; Sutton, R.; et al. Outcomes of paediatric patients with B-cell acute lymphocytic leukaemia with ABL-class fusion in the pre-tyrosine-kinase inhibitor era: A multicentre, retrospective, cohort study. Lancet Haematol. 2021, 8, e55–e66. [Google Scholar] [CrossRef] [PubMed]
- Górecki, M.; Kozioł, I.; Kopystecka, A.; Budzyńska, J.; Zawitkowska, J.; Lejman, M. Updates in KMT2A Gene Rearrangement in Pediatric Acute Lymphoblastic Leukemia. Biomedicines 2023, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Driessen, E.M.C.; de Lorenzo, P.; Campbell, M.; Felice, M.; Ferster, A.; Hann, I.; Vora, A.; Hovi, L.; Escherich, G.; Li, C.K.; et al. Outcome of relapsed infant acute lymphoblastic leukemia treated on the interfant-99 protocol. Leukemia 2017, 31, 2854. [Google Scholar] [CrossRef]
- Van der Sluis, I.M.; de Lorenzo, P.; Kotecha, R.S.; Attarbaschi, A.; Escherich, G.; Nysom, K.; Stary, J.; Ferster, A.; Brethon, B.; Locatelli, F.; et al. Blinatumomab Added to Chemotherapy in Infant Lymphoblastic Leukemia. N. Engl. J. Med. 2023, 388, 1572–1581. [Google Scholar] [CrossRef]
- Khaw, S.L.; Suryani, S.; Evans, K.; Richmond, J.; Robbins, A.; Kurmasheva, R.T.; Billups, C.A.; Erickson, S.W.; Guo, Y.; Houghton, P.J.; et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood 2016, 128, 1382–1395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Place, A.E.; Karol, S.E.; Forlenza, C.J.; Cooper, T.M.; Fraser, C.; Cario, G.; O’Brien, M.M.; Gerber, N.U.; Bourquin, J.P.; Reinhardt, D.; et al. Venetoclax Combined with Chemotherapy in Pediatric and Adolescent/Young Adult Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2025, 72, e31630. [Google Scholar] [CrossRef]
- Cooper, T.M.; Cassar, J.; Eckroth, E.; Malvar, J.; Sposto, R.; Gaynon, P.; Chang, B.H.; Gore, L.; August, K.; Pollard, J.A.; et al. A Phase I Study of Quizartinib Combined with Chemotherapy in Relapsed Childhood Leukemia: A Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Clin. Cancer Res. 2016, 22, 4014–4022. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. FDA Approves Revumenib for Relapsed or Refractory Acute Leukemia with a KMT2A Translocation (Revuforj). 15 November 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-revumenib-relapsed-or-refractory-acute-leukemia-kmt2a-translocation?utm_source=chatgpt.com (accessed on 5 September 2025).
- Stumpel, D.J.P.M.; Schneider, P.; van Roon, E.H.J.; Boer, J.M.; de Lorenzo, P.; Valsecchi, M.G.; de Menezes, R.X.; Pieters, R.; Stam, R.W. Specific promoter methylation identifies different subgroups of MLL-rearranged infant acute lymphoblastic leukemia, influences clinical outcome, and provides therapeutic options. Blood 2011, 117, 3247–3255. [Google Scholar] [CrossRef]
- Lygizou, E.M.; Reiter, M.; Maurer-Granofszky, M.; Dworzak, M.; Grosu, R. Automated Immunophenotyping Assessment for Diagnosing Childhood Acute Leukemia using Set-Transformers. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2024, Orlando, FL, USA, 15–19 July 2024; pp. 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Brown, L.M.; Ryland, G.L.; Lonsdale, A.; Kosasih, H.J.; Ludlow, L.E.; Majewski, I.J.; Blombery, P.; Ekert, P.G.; Davidson, N.M.; et al. ALLSorts: An RNA-Seq subtype classifier for B-cell acute lymphoblastic leukemia. Blood Adv. 2020, 6, 4093–4097. [Google Scholar] [CrossRef]
- Matek, C.; Schwarz, S.; Spiekermann, K.; Marr, C. Human-level recognition of blast cells in acute myeloid leukemia with convolutional neural networks. Nat. Mach. Intell. 2019, 1, 538–544. [Google Scholar] [CrossRef]
- Pan, L.; Liu, G.; Lin, F.; Zhong, S.; Xia, H.; Sun, X.; Liang, H. Machine learning applications for prediction of relapse in childhood acute lymphoblastic leukemia. Sci. Rep. 2017, 7, 7402. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andersen, A.N.; Brodersen, A.M.; Ayuda-Durán, P.; Piechaczyk, L.; Tadele, D.S.; Baken, L.; Fredriksen, J.; Stoksflod, M.; Lenartova, A.; Fløisand, Y.; et al. Clinical forecasting of acute myeloid leukemia using ex vivo drug-sensitivity profiling. Cell Rep. Methods 2023, 3, 100654. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aghaeepour, N.; Finak, G.; Dougall, D.; Hoos, H.; Mosmann, T.R.; Brinkman, R.; Gottardo, R.; FlowCAP Consortium; DREAM Consortium. Critical assessment of automated flow cytometry data analysis techniques. Nat. Methods 2013, 10, 228–238. [Google Scholar] [CrossRef]
- Relling, M.V.; Yang, J.J. Pharmacogenetics and personalized medicine in ALL. Nat. Rev. Clin. Oncol. 2015, 12, 344–357. [Google Scholar]
- Yang, J.J.; Cheng, C.; Devidas, M.; Cao, X.; Fan, Y.; Campana, D.; Yang, W.; Neale, G.; Cox, N.J.; Scheet, P.; et al. Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat. Genet. 2011, 43, 237–241. [Google Scholar] [CrossRef]
- Moriyama, T.; Nishii, R.; Perez-Andreu, V.; Yang, W.; Klussmann, F.A.; Zhao, X.; Lin, T.-N.; Hoshitsuki, K.; Nersting, J.; Kihira, K.; et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat. Genet. 2016, 48, 367–373. [Google Scholar] [CrossRef]
- Oh, B.L.; Hunger, S.P.; Yeoh, A.E.; Lee, S.H. Curing using the minimal—Strategies for treatment reduction in childhood acute lymphoblastic leukemia. EJC Paediatr. Oncol. 2025, 5, 100222. [Google Scholar] [CrossRef]
- Leo, I.R.; Aswad, L.; Stahl, M.; Kunold, E.; Post, F.; Erkers, T.; Struyf, N.; Mermelekas, G.; Joshi, R.N.; Gracia-Villacampa, E.; et al. Integrative multi-omics and drug response profiling of childhood acute lymphoblastic leukemia cell lines. Nat. Commun. 2022, 13, 1691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, S.; Howard, S.C.; Hunger, S.P.; Antillon, F.G.; Metzger, M.L.; Israels, T.; Harif, M.; Rodriguez-Galindo, C. Treating childhood cancer in low- and middle-income countries. Nat. Rev. Cancer 2015, 15, 219–228. [Google Scholar]
- Lam, C.G.; Howard, S.C.; Bouffet, E.; Pritchard-Jones, K. Science and health for all children with cancer. Science 2019, 363, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Israels, T.; Ribeiro, R.C.; Molyneux, E.M. Strategies to improve care for children with cancer in sub-Saharan Africa. Lancet Oncol. 2013, 14, e189–e195. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Rossi, E.; Brivio, E.; Carrillo, J.M.; Bonilla, M.; Vasquez, R.; Peña, A.; Fu, L.; Martinez, R.; Espinoza, C.M.; et al. Treatment of childhood acute lymphoblastic leukemia in central America: A lower-middle income countries experience. Pediatr. Blood Cancer 2014, 61, 803–809. [Google Scholar] [CrossRef]
- Witek, M.A.; Larkey, N.E.; Bartakova, A.; Hupert, M.L.; Mog, S.; Cronin, J.K.; Vun, J.; August, K.J.; Soper, S.A. Microfluidic Affinity Selection of B-Lineage Cells from Peripheral Blood for Minimal Residual Disease Monitoring in Pediatric B-Type Acute Lymphoblastic Leukemia Patients. Int. J. Mol. Sci. 2024, 25, 10619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Atun, R.; Bhakta, N.; Denburg, A.; Frazier, A.L.; Friedrich, P.; Gupta, S.; Lam, C.G.; Ward, Z.J.; Yeh, J.M.; Allemani, C.; et al. Sustainable care for children with cancer: A Lancet Oncology Commission. Lancet Oncol. 2020, 21, e185–e224. [Google Scholar] [CrossRef]
- Masera, G.; Baez, F.; Biondi, A.; Cavalli, F.; Conter, V.; Flores, A.; Fontana, G.; Fossati Bellani, F.; Lanfranco, P.; Malta, A.; et al. North-South twinning in paediatric haemato-oncology: The La Mascota programme, Nicaragua. Lancet 1998, 352, 1923–1926. [Google Scholar] [CrossRef]
- Rodriguez-Galindo, C.; Friedrich, P.; Alcasabas, P.; Antillon, F.; Banavali, S.; Castillo, L.; Israels, T.; Jeha, S.; Harif, M.; Sullivan, M.J.; et al. Toward the Cure of All Children with Cancer Through Collaborative Efforts: Pediatric Oncology as a Global Challenge. J. Clin. Oncol. 2015, 33, 3065–3073. [Google Scholar] [CrossRef]
- Briercheck, E.; Pyle, D.; Adams, C.; Atun, R.; Booth, C.; Dent, J.; Garcia-Gonzalez, P.; Ilbawi, A.; Jazieh, A.R.; Kerr, D.; et al. Unification of Efforts to Improve Global Access to Cancer Therapeutics: Report From the 2022/2023 Access to Essential Cancer Medicines Stakeholder Summit. JCO Glob. Oncol. 2024, 10, e2300256. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.M.; Barber, M.J.; Gotham, D. Estimated costs of production and potential prices for the WHO essential medicines list. BMJ Glob. Health 2018, 3, e000571. [Google Scholar] [CrossRef]
- Kantarjian, H.; Fojo, T.; Mathisen, M.; Zwelling, L. Cancer drugs in the United States: Justum pretium—The just price. J. Clin. Oncol. 2013, 31, 3600–3604. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, P.; Boultbee, P.; Epstein, D. Novel Humanitarian Aid Program: The Glivec International Patient Assistance Program—Lessons Learned from Providing Access to Breakthrough Targeted Oncology Treatment in Low- and Middle-Income Countries. J. Glob. Oncol. 2015, 1, 37–45. [Google Scholar] [CrossRef]
- World Health Organization; St. Jude Children’s Research Hospital; UNICEF; Pan American Health Organization (PAHO). Global Platform for Access to Childhood Cancer Medicines. Available online: https://www.who.int/teams/noncommunicable-diseases/ncds-management/cancer-programme/global-platform-for-access-to-childhood-cancer-medicines (accessed on 5 September 2025).
- ACT for Children. Access to Childhood Cancer Treatment in LMICs. Available online: https://act-4-children.org/ (accessed on 5 September 2025).
- International Society of Paediatric Oncology (SIOP). Tackling Inequities in Childhood Cancer Through Unique Global Collaboration. News Release; 12 February 2025. Available online: https://siop-online.org/news/tackling-inequities-in-childhood-cancer-through-unique-global-collaboration/ (accessed on 5 September 2025).
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Bhojwani, D.; Yang, J.J.; Pui, C.H. Biology of childhood acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015, 62, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Wolters Kluwer. Chimeric Antigen Receptor (CAR) T Cell Therapy: A Remarkable Breakthrough in Cancer Treatment. Expert Insights. 21 August 2023. Available online: https://www.wolterskluwer.com/en/expert-insights/car-t-cell-therapy-a-remarkable-breakthrough-in-cancer-treatment (accessed on 22 September 2025).
- Theunissen, P.; Mejstrikova, E.; Sedek, L.; van der Sluijs-Gelling, A.J.; Gaipa, G.; Bartels, M.; Sobral da Costa, E.; Kotrová, M.; Novakova, M.; Sonneveld, E.; et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017, 129, 347–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric antigen receptor T-cell therapy—Assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Kantarjian, H.; Pui, C.H.; Jabbour, E. Acute lymphocytic leukaemia. Lancet 2025. Epub ahead of print. [Google Scholar] [CrossRef]
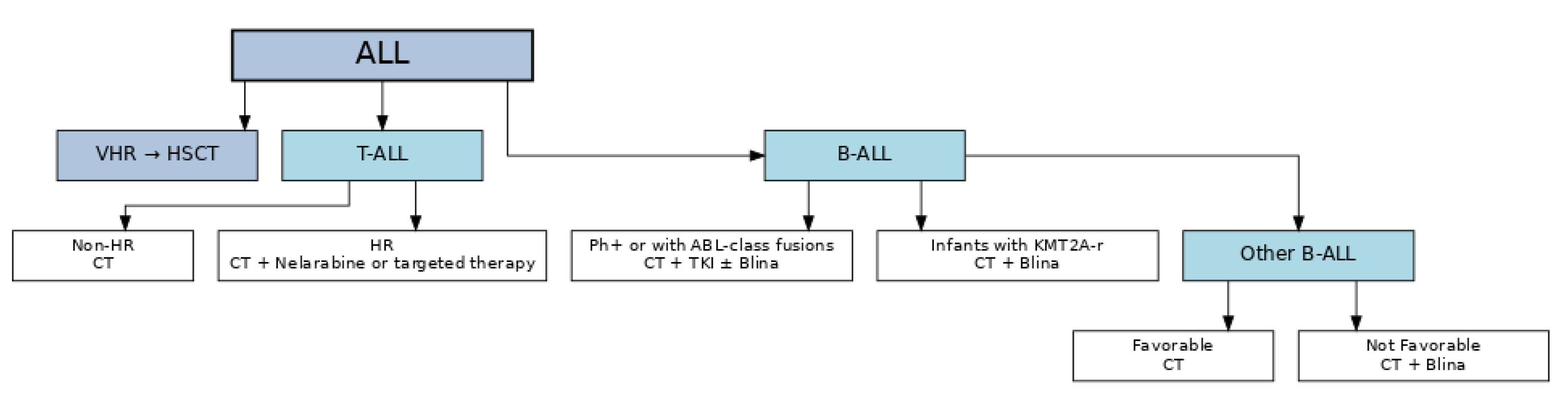
| Trial Name | Enrollment Period | Focus and Population | Key Findings |
|---|---|---|---|
| DFCI 95-01 | 1996–2000 | Intensive asparaginase-based treatment | 5-year EFS 81.6%, OS 89.6% |
| COG 1961 | 1996–2002 | Young adults (16–21 years) | Pediatric-style regimen improved 5-year EFS to ~71.5%; limited benefit from HSCT |
| Interfant-99 | 1999–2005 | Infants, especially MLL-rearranged | Poor prognosis in MLL+ cases |
| AIEOP-BFM ALL 2000 | 2000–2006 | MRD-guided risk stratification | MRD is strongest prognostic factor; 5-year EFS ~92% in MRD-negative at end of induction |
| COG AALL0031 | 2002–2006 | Imatinib for Ph+ disease | Imatinib plus chemotherapy achieved ~80% 3-year EFS; decreased need for HSCT |
| COG AALL0232 | 2004–2011 | High-risk B-lineage | MRD at end of induction is strongest predictor of outcome |
| SJCRH Total XV | 2004–2010 | Risk-directed therapy; no cranial radiation | 5-year EFS 85.6%, OS 93.5%; CNS relapse 2.7% without cranial irradiation |
| COG AALL1731 | 2015–2019 | Randomized Blinatumomab (2 non sequential courses in NCI standard-risk at average or higher risk of relapse | Blinatumomab significantly improved 3-year DFS (96% vs. 87.9%) |
| AIEOP-BFM ALL 2017 | 2017–2024 | Randomized additional Blinatumomab (one course) in MR patients; randomized 2 courses of Blinatumomab vs. chemo blocks in HR patients | favorable toxicity profile in HR patients; outcome data not yet published |
| Interfant-06 + blinatumomab | 2018–2021 | Infants with KMT2A-rearranged CD19+ B lineage disease; post-induction Blinatumomab | Phase 2 pilot (n = 30) vs. historical Interfant-06: 2-year DFS 81.6% vs. 49.4%; OS 93.3% vs. 65.8%; good MRD response; acceptable toxicity |
| Therapy | Target/Indication | Estimated Eligible Proportion |
|---|---|---|
| Imatinib/Dasatinib | Philadelphia chromosome–positive (Ph+) | 3–5% |
| Ruxolitinib | JAK-mutant/Ph-like | 10–15% |
| Blinatumomab | CD19+ B-lineage | 80–85% |
| Inotuzumab ozogamicin | CD22+ B-lineage | 60–70% |
| CAR T-cell therapy | CD19+ B-lineage; considered in first CR for refractory patients or those with persistent MRD | 1–2% of B-ALL |
| Application Area | AI Tool/Function |
|---|---|
| Diagnostics | AI-assisted immunophenotyping; automated morphological analysis |
| Risk Stratification | Predictive modeling integrating genetic features and minimal residual disease (MRD) |
| Treatment Planning | Drug response and therapy optimization models |
| Global Health Equity | Remote diagnostic support; AI-based classification for underserved regions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aricò, M.; Conter, V. A Decade of Transformation in the Management of Childhood Acute Lymphoblastic Leukemia: From Conventional Chemotherapy to Precision Medicine. Pediatr. Rep. 2025, 17, 108. https://doi.org/10.3390/pediatric17050108
Aricò M, Conter V. A Decade of Transformation in the Management of Childhood Acute Lymphoblastic Leukemia: From Conventional Chemotherapy to Precision Medicine. Pediatric Reports. 2025; 17(5):108. https://doi.org/10.3390/pediatric17050108
Chicago/Turabian StyleAricò, Maurizio, and Valentino Conter. 2025. "A Decade of Transformation in the Management of Childhood Acute Lymphoblastic Leukemia: From Conventional Chemotherapy to Precision Medicine" Pediatric Reports 17, no. 5: 108. https://doi.org/10.3390/pediatric17050108
APA StyleAricò, M., & Conter, V. (2025). A Decade of Transformation in the Management of Childhood Acute Lymphoblastic Leukemia: From Conventional Chemotherapy to Precision Medicine. Pediatric Reports, 17(5), 108. https://doi.org/10.3390/pediatric17050108







