Metabolic Syndrome Prevalence and Its Components in Adolescents from Western Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Size
2.3. Anthropometric Data, Blood Pressure, and Biochemical Parameters
2.4. Diagnosis of Metabolic Syndrome and Its Components
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics, Lifestyle, and Preexisting Cardiometabolic Conditions
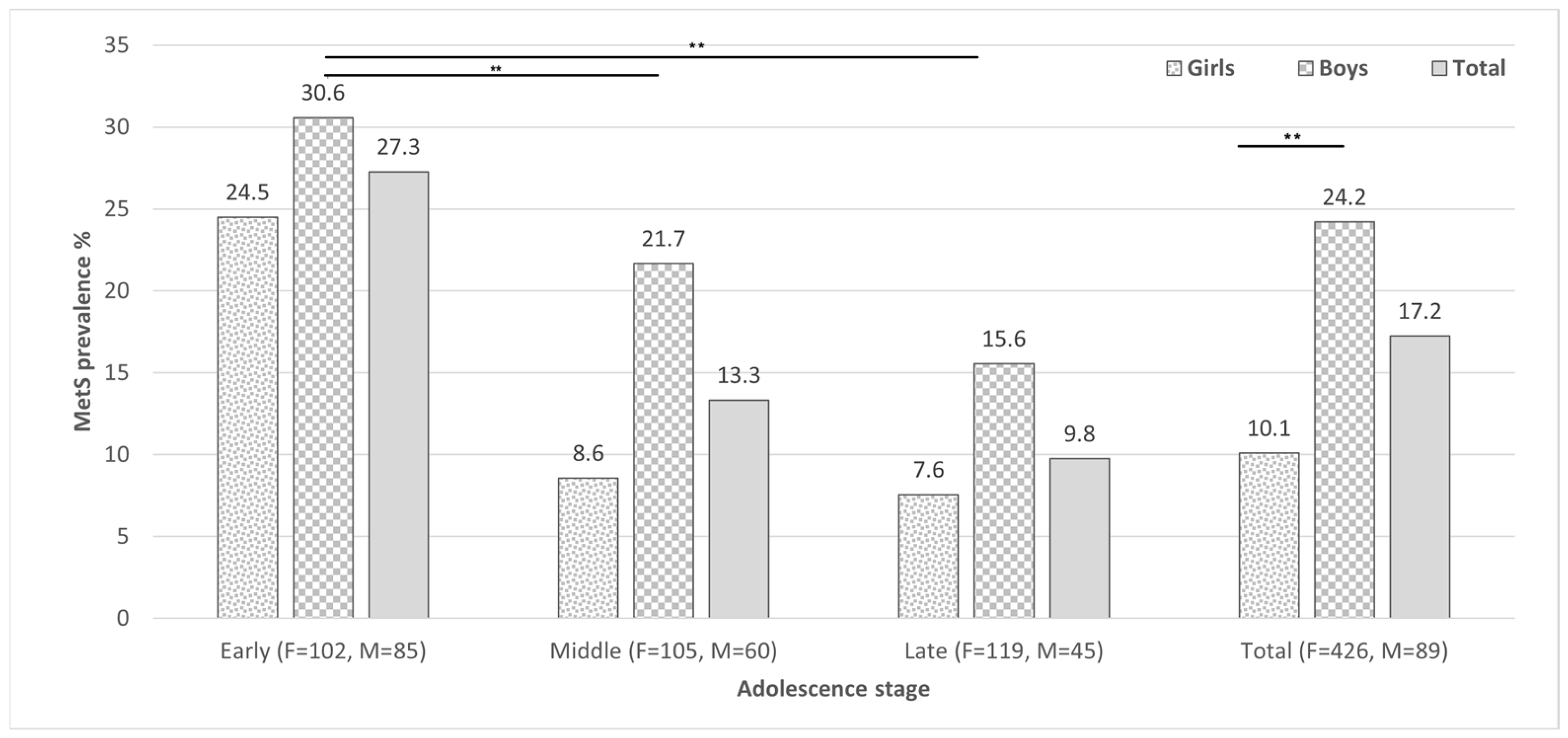
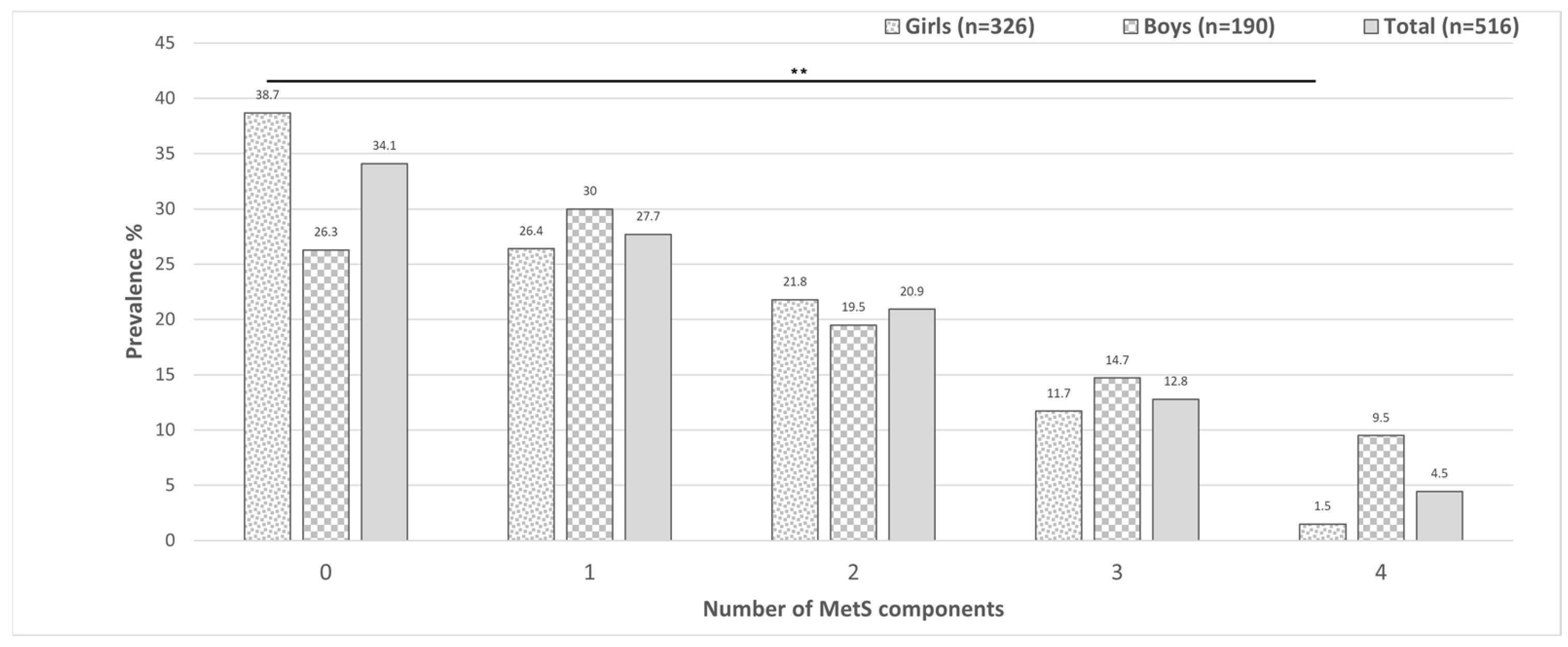
3.2. Metabolic Syndrome, Its Components and Related Parameters
3.3. Comparison of Quantitative Parameters and Risk Factors Associated with Metabolic Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MetS | Metabolic Syndrome |
| BMI | Body Index Mass |
| TyG index | Triglyceride–Glucose Index |
| IR | Insulin Resistance |
| WHtR | Waist-to-Height ratio |
| CVD | Cardiovascular diseases |
| HBP | High Blood Pressure |
| T2DM | Type 2 Diabetes Mellitus |
| HTG | Hypertriglyceridemia |
| Per | Percentile |
| HDL-C | High-density Lipoprotein |
| LDL-C | Low-density Lipoprotein |
| VLDL-C | Very-low-density Lipoprotein |
| GOT | Glutamic Oxalacetic Transaminase |
| GPT | Glutamic Pyruvic Transaminase |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| SD | Standard Deviation |
| HTN | Hypertension |
| PCOS | Polycystic Ovary Syndrome |
| CO | Central Obesity |
| CMRF | Cardiometabolic Risk Factors |
| ROS | Reactive Oxygen Species |
| UPR | Unfolded Protein Response |
| NAFLD | Non-alcoholic Fatty Liver Disease |
References
- OECD/The World Bank. Health at a Glance: Latin America and the Caribbean 2023; OECD Publishing: Paris, France, 2023; pp. 1–181. Available online: https://www.oecd.org/health/health-at-a-glance-latin-america-and-the-caribbean-2023-532b0e2d-en.htm (accessed on 15 February 2024).
- INEGI. Estadísticas de Defunciones Registradas (EDR). 2022. Available online: https://inegi.org.mx/contenidos/saladeprensa/boletines/2023/EDR/EDR2022.pdf (accessed on 15 February 2024).
- Organización Mundial de la Salud. Obesidad y Sobrepeso. 2017. Available online: http://www.who.int/mediacentre/factsheets/fs311/es/ (accessed on 15 February 2024).
- Shamah-Levy, T.; Gaona-Pineda, E.B.; Cuevas-Nasu, L.; Morales-Ruan, C.; Valenzuela-Bravo, D.G.; Méndez-Gómez Humaran, I.; Ávila-Arcos, M.A. Prevalencias de sobrepeso y obesidad en población escolar y adolescente de México. Ensanut Continua 2020–2022. Salud Publica Mex. 2023, 65, s218–s224. [Google Scholar] [CrossRef]
- Gaona-Pineda, E.B.; Rodríguez-Ramírez, S.; Medina-Zacarías, M.C.; Valenzuela-Bravo, D.G.; Martinez-Tapia, B.; Arango-Angarita, A. Consumidores de grupos de alimentos en población mexicana. Ensanut Continua 2020–2022. Salud Publica Mex. 2023, 65, s248–s258. [Google Scholar] [CrossRef]
- Medina, C.; Jáuregui, A.; Hernández, C.; González, C.; Olvera, A.G.; Blas, N.; Campos, I.; Barquera, S. Prevalencia de comportamientos del movimiento en población mexicana. Salud Publica Mex. 2023, 65, s259–s267. [Google Scholar] [CrossRef] [PubMed]
- Codazzi, V.; Frontino, G.; Galimberti, L.; Giustina, A.; Petrelli, A. Mechanisms and risk factors of metabolic syndrome in children and adolescents. Endocrine 2024, 84, 16–28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiarelli, F.; Mohn, A. Early diagnosis of metabolic syndrome in children. Lancet Child. Adolesc. Health 2017, 1, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.; Grundy, S.; Zimmet, P. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Lee, A.M.; Gurka, M.J.; DeBoer, M.D. A metabolic syndrome severity score to estimate risk in adolescents and adults: Current evidence and future potential. Expert. Rev. Cardiovasc. Ther. 2016, 14, 411–413. [Google Scholar] [CrossRef][Green Version]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- de Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef]
- World Health Organization. Global Accelerated Action for the Health of Adolescents (AA-HA!): Guidance to Support Country Implementation; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241512343 (accessed on 24 June 2025).
- PROYECTO de Norma Oficial Mexicana PROY-NOM-030-SSA2-2017, Para la Prevención, Detección, Diagnóstico, Tratamiento y Control de la Hipertensión Arterial Sistémica. Available online: https://www.isstech.gob.mx/portal/pdf/marcoJuridico/2024/NORMAS%20DE%20SALUD/PROY-NOM-030-SSA2-2017.pdf (accessed on 24 June 2025).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; RodrígUez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.L.; Weigensberg, M.J.; Huang, T.T.; Ball, G.; Shaibi, G.Q.; Goran, M.I. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J. Clin. Endocrinol. Metab. 2004, 89, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, C.; Nkeh-Chungag, B.N.; Fredriksen, P.M.; Goswami, N. The prevalence of pediatric metabolic syndrome-a critical look on the discrepancies between definitions and its clinical importance. Int. J. Obes. 2021, 45, 12–24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bitew, Z.W.; Alemu, A.; Ayele, E.G.; Tenaw, Z.; Alebel, A.; Worku, T. Metabolic syndrome among children and adolescents in low and middle income countries: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2020, 12, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child. Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Heiss, G.; Snyder, M.L.; Teng, Y.; Schneiderman, N.; Llabre, M.M.; Cowie, C.; Carnethon, M.; Kaplan, R.; Giachello, A.; Gallo, L.; et al. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: The Hispanic Community Health Study/Study of Latinos. Diabetes Care 2014, 37, 2391–2399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robinson, C.H.; Chanchlani, R. High Blood Pressure in Children and Adolescents: Current Perspectives and Strategies to Improve Future Kidney and Cardiovascular Health. Kidney Int. Rep. 2022, 7, 954–970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khoury, M.; Urbina, E.M. Hypertension in adolescents: Diagnosis, treatment, and implications. Lancet Child. Adolesc. Health 2021, 5, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Hisamatsu, T.; Kinuta, M. High blood pressure in childhood and adolescence. Hypertens. Res. 2024, 47, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.C.; Dunietz, G.L.; Matos-Moreno, A.; Solano, M.; Lazcano-Ponce, E.; Sánchez-Zamorano, L.M. Bedtimes and Blood Pressure: A Prospective Cohort Study of Mexican Adolescents. Am. J. Hypertens. 2020, 33, 269–277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, D.D.; Corkey, B.E.; Istfan, N.W.; Apovian, C.M. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction. J. Endocr. Soc. 2019, 3, 1727–1747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faruque, S.; Tong, J.; Lacmanovic, V.; Agbonghae, C.; Minaya, D.M.; Czaja, K. The Dose Makes the Poison: Sugar and Obesity in the United States—A Review. Pol. J. Food Nutr. Sci. 2019, 69, 219–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marketou, M.E.; Buechler, N.S.; Fragkiadakis, K.; Plevritaki, A.; Zervakis, S.; Maragkoudakis, S.; Tsiavos, A.; Simantirakis, E.; Kochiadakis, G. Visceral fat and cardiometabolic future in children and adolescents: A critical update. Pediatr. Res. 2023, 94, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Díaz-Orozco, L.E.; Santamaría-Arza, C.; Orozco-Morales, J.A.; Medina-Bravo, P.G. Metabolic-associated fatty liver disease in children and adolescents: Mexican experience. Lancet Gastroenterol. Hepatol. 2021, 6, 986. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Baek, K.S.; Han, S.; Kim, J.H.; Shin, Y.H. Serum alanine aminotransferase levels are closely associated with metabolic disturbances in apparently healthy young adolescents independent of obesity. Korean J. Pediatr. 2019, 62, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Congreso General de los Estados Unidos Mexicanos. DECRETO por el que se Reforman, Adicionan y Derogan Diversas Disposiciones de la Ley General de Educación, en Materia de Salud Alimentaria en las Escuelas. Diario Oficial de la Federación. 2023. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5711999&fecha=20/12/2023#gsc.tab=0 (accessed on 15 February 2024).
| Characteristic (Cut Point) | Total (n = 516) % (n) | Girls (n = 326) % (n) | Boys (n = 190) % (n) | p |
|---|---|---|---|---|
| Adolescence stage | ||||
| Pre-adolescence (10–11 years) | 36.2 (187) | 31.3 (102) | 44.7 (85) | 0.002 |
| Early adolescence (12–14 years) | 32 (165) | 32.2 (105) | 31.6 (60) | 0.8 |
| Late adolescence (15–17 years) | 31.8 (164) | 36.5 (119) | 23.7 (45) | 0.03 |
| Residence area | ||||
| Urban | 93.8 (484) | 94.5 (308) | 92.6 (176) | 0.7 |
| Rural | 6.2(32) | 5.5 (18) | 7.4 (14) | 0.7 |
| Lifestyle | ||||
| Alcohol consumption | 5.2 (27) | 6.4 (21) | 3.2 (6) | 0.1 |
| Tobacco consumption | 1.9 (10) | 2.3 (9) | 0.5(1) | 0.1 |
| Sedentarism | 31.8 (164) | 36.2 (118) | 24.2 (46) | 0.004 |
| Pathological history | ||||
| CVD Family history | 59.5 (307) | 61.7 (201) | 55.8 (106) | 0.2 |
| Previous diagnosis of T2DM, obesity, or CVD | 9.5 (49) | 11 (36) | 6.8 (13) | 0.1 |
| Treatment of T2DM, HBP, dyslipidemia | 0.1 (5) | 0.9 (3) | 1.1 (2) | 1 |
| Treatment of mood disorders | 1.2 (6) | 0.9 (3) | 1.6 (3) | 0.7 |
| Clinic characteristics | ||||
| MetS (NCEP-ATPIII) [17] | 9.3 (46) | 4.2 (13) | 13.3 (24) | 0.001 |
| MetS (Cruz et al., 2004) [18] | 7.5 (37) | 5.4 (17) | 16 (29) | 0.001 |
| Mets (de Ferranti et al., 2004) [12] | 17.8 (88) | 13.7 (43) | 24.9 (45) | 0.002 |
| High waist circumference (Per > 75) | 38.8 (200) | 33.7 (110) | 47.4 (90) | 0.002 |
| Hypertriglyceridemia (>100 mg/dL) | 29.7 (153) | 27.6 (90) | 33.2 (63) | 0.18 |
| Low HDL-C 1 | 22.5 (116) | 18.7 (61) | 28.9 (55) | 0.007 |
| High blood pressure (Per > 90) | 7.9 (41) | 4.9 (16) | 13.2 (25) | 0.001 |
| Altered fasting blood glucose (>110 mg/dL) | 0.8 (4) | 0.6 (2) | 1.1 (2) | 0.6 |
| Overweight + obesity (BMI Per > 85) | 43.6 (225) | 41.1 (134) | 47.9 (91) | 0.1 |
| Overweight (BMI Per > 85–95) | 17.6 (91) | 19.6 (64) | 14.2 (27) | 0.4 |
| Obesity (BMI Per > 95) | 26 (134) | 21.5 (70) | 33.7 (64) | 0.007 |
| High corporal fat (Per > 85) | 12.8 (66) | 16 (52) | 7.4 (14) | 0.005 |
| High trunk fat (Per > 85) | 9.8 (36) | 7.1 (23) | 6.8 (13) | 0.1 |
| Surrogate insulin resistance (TyG index > 4.68) | 14 (72) | 11.7 (38) | 17.9 (34) | 0.049 |
| Elevated LDL-C (>100 mg/dL) | 10.5 (54) | 10.7 (35) | 10 (19) | 0.7 |
| Elevated VLDL-C (>30 mg/dL) | 8.3 (43) | 6.4 (21) | 11.6 (22) | 0.04 |
| High total cholesterol (>165 mg/dL) | 26 (134) | 28.8 (94) | 21.1 (40) | 0.05 |
| Altered AST 2 | 31.4 (162) | 32.2 (105) | 30 (57) | 0.6 |
| Altered ALT 3 | 8.1 (42) | 5.8 (19) | 12.1 (23) | 0.01 |
| Clinical/Biochemical Characteristic | Age (Years) | Physical Exercise | Weight (kg) | BMI (kg/m2) | Corporal Fat (%) | Trunk Fat (%) | WC (cm) | HC (cm) | WHR | WHtR | SBP (mm/Hg) | DBP (mm/Hg) | HDL-C (mg/dL) | TG (mg/dL) | FBG (mg/dL) | TyG Index | LDL-C (mg/dL) | VLDL-C (mg/dL) | Chol (mg/dL) | AST (U/L) | ALT (U/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 1 | ||||||||||||||||||||
| Physical exercise | 0.18 ** | 1 | |||||||||||||||||||
| Weight (kg) | 0.29 ** | −0.01 | 1 | ||||||||||||||||||
| BMI (kg/m2) | 0.15 ** | −0.09 | 0.89 ** | 1 | |||||||||||||||||
| Corporal fat (%) | 0.01 | −0.211 * | 0.57 ** | 0.75 ** | 1 | ||||||||||||||||
| Trunk fat (%) | −0.01 | −0.25 ** | 0.55 ** | 0.69 ** | 0.92 ** | 1 | |||||||||||||||
| Waist circumference (cm) | 0.05 | −0.13 * | 0.87 ** | 0.86 ** | 0.62 ** | 0.60 ** | 1 | ||||||||||||||
| Hip circumference (cm) | 0.29 ** | −0.08 | 0.90 ** | 0.88 ** | 0.69 ** | 0.66 ** | 0.82 ** | 1 | |||||||||||||
| Waist–hip ratio | −0.26 ** | −0.11 * | 0.39 ** | 0.40 ** | 0.24 ** | 0.25 ** | 0.72 ** | 0.19 ** | 1 | ||||||||||||
| Height–waist ratio | −0.10 * | −0.19 ** | 0.70 ** | 0.85 ** | 0.69 ** | 0.65 ** | 0.93 ** | 0.72 ** | 0.73 ** | 1 | |||||||||||
| SBP (mm/Hg) | 0.01 | 0.02 | 0.44 ** | 0.33 ** | 0.10 * | 0.13 * | 0.41 ** | 0.35 ** | 0.27 ** | 0.30 ** | 1 | ||||||||||
| DBP (mm/Hg) | −0.02 | −0.11 * | 0.24 ** | 0.22 ** | 0.24 ** | 0.26 ** | 0.270 * | 0.24 ** | 0.17 ** | 0.24 ** | 0.54 ** | 1 | |||||||||
| HDL-C (mg/dL) | 0.34 ** | 0.22 ** | −0.19 ** | −0.19 ** | −0.15 ** | −0.19 ** | −0.31 ** | −0.18 ** | −0.32 ** | −0.31 ** | −0.21 ** | −0.16 ** | 1 | ||||||||
| Triglycerides (mg/dL) | −0.04 | −0.09 | 0.32 ** | 0.33 ** | 0.27 ** | 0.23 ** | 0.37 ** | 0.27 ** | 0.29 ** | 0.35 ** | 0.14 ** | 0.17 ** | −0.222 * | 1 | |||||||
| FBG (mg/dL) | −0.08 | 0.01 | −0.01 | −0.02 | −0.06 | −0.05 | 0.03 | −0.06 | 0.13 ** | 0.04 | 0.08 | 0.08 | 0.04 | 0.15 ** | 1 | ||||||
| TyG index | −0.09 * | −0.12 * | 0.29 ** | 0.32 ** | 0.28 ** | 0.25 ** | 0.36 ** | 0.25 ** | 0.31 ** | 0.37 ** | 0.16 ** | 0.18 ** | −0.22 ** | 0.90 ** | 0.33 ** | 1 | |||||
| LDL-C (mg/dL) | −0.17 ** | −0.14 * | 0.13 ** | 0.21 ** | 0.23 ** | 0.24 ** | 0.21 ** | 0.13 ** | 0.19 ** | 0.26 ** | 0.10 * | 0.17 ** | −0.22 ** | 0.19 ** | 0.09 * | 0.29 ** | 1 | ||||
| VLDL-C (mg/dL) | −0.05 | −0.09 | 0.32 ** | 0.33 ** | 0.27 ** | 0.23 ** | 0.37 ** | 0.27 ** | 0.29 ** | 0.35 ** | 0.14 ** | 0.17 ** | −0.22 ** | 1.0 ** | 0.15 ** | 0.90 ** | 0.19 ** | 1 | |||
| Total cholesterol (mg/dL) | 0.05 | −0.02 | 0.11 * | 0.17 ** | 0.19 ** | 0.17 ** | 0.11 * | 0.09 * | 0.07 | 0.14 ** | 0.01 | 0.11 * | 0.34 ** | 0.36 ** | 0.15 ** | 0.41 ** | 0.79 ** | 0.36 ** | 1 | ||
| AST (U/L) | 0.01 | 0.03 | 0.14 ** | 0.10 * | −0.01 | 0.01 | 0.14 ** | 0.08 | 0.13 ** | 0.11 * | 0.12 ** | 0.07 | 0.08 | 0.18 ** | 0.43 ** | 0.23 ** | 0.09 * | 0.18 ** | 0.19 ** | 1 | |
| ALT (U/L) | 0.15 ** | 0.05 | 0.22 ** | 0.18 ** | 0.04 | 0.05 | 0.18 ** | 0.17 ** | 0.10 * | 0.14 ** | 0.10 * | 0.04 | 0.13 ** | 0.14 ** | 0.31 ** | 0.19 ** | 0.12 ** | 0.14 ** | 0.23 ** | 0.71 ** | 1 |
| Indicators | OR (CI 99%) | p |
|---|---|---|
| Male | 3.22 (1.26–8.21) | 0.001 |
| WHtR > 0.5 | 7.22 (2.33–22.33) | <0.001 |
| Early adolescence stage | 1.98 (1.22–3.24) | <0.001 |
| Corporal fat percentage | 1.09 (1.02–1.17) | 0.001 |
| Subrogated IR | 4.54 (1.86–11.03) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Pacheco, D.; Rosales-Gómez, R.C.; García-Cobián, T.A.; Rubio-Chávez, L.A.; Gutiérrez-Rubio, A.A.; Rivera-Ramírez, J.H.; Gutiérrez-Rubio, S.A. Metabolic Syndrome Prevalence and Its Components in Adolescents from Western Mexico. Pediatr. Rep. 2025, 17, 83. https://doi.org/10.3390/pediatric17040083
Ortega-Pacheco D, Rosales-Gómez RC, García-Cobián TA, Rubio-Chávez LA, Gutiérrez-Rubio AA, Rivera-Ramírez JH, Gutiérrez-Rubio SA. Metabolic Syndrome Prevalence and Its Components in Adolescents from Western Mexico. Pediatric Reports. 2025; 17(4):83. https://doi.org/10.3390/pediatric17040083
Chicago/Turabian StyleOrtega-Pacheco, Diego, Roberto Carlos Rosales-Gómez, Teresa Arcelia García-Cobián, Lidia Ariadna Rubio-Chávez, Angélica Adriana Gutiérrez-Rubio, José Hugo Rivera-Ramírez, and Susan Andrea Gutiérrez-Rubio. 2025. "Metabolic Syndrome Prevalence and Its Components in Adolescents from Western Mexico" Pediatric Reports 17, no. 4: 83. https://doi.org/10.3390/pediatric17040083
APA StyleOrtega-Pacheco, D., Rosales-Gómez, R. C., García-Cobián, T. A., Rubio-Chávez, L. A., Gutiérrez-Rubio, A. A., Rivera-Ramírez, J. H., & Gutiérrez-Rubio, S. A. (2025). Metabolic Syndrome Prevalence and Its Components in Adolescents from Western Mexico. Pediatric Reports, 17(4), 83. https://doi.org/10.3390/pediatric17040083





