Antibiotic Effect on Clinical Response and Remission in Pediatric Inflammatory Bowel Disease
Abstract
1. Introduction
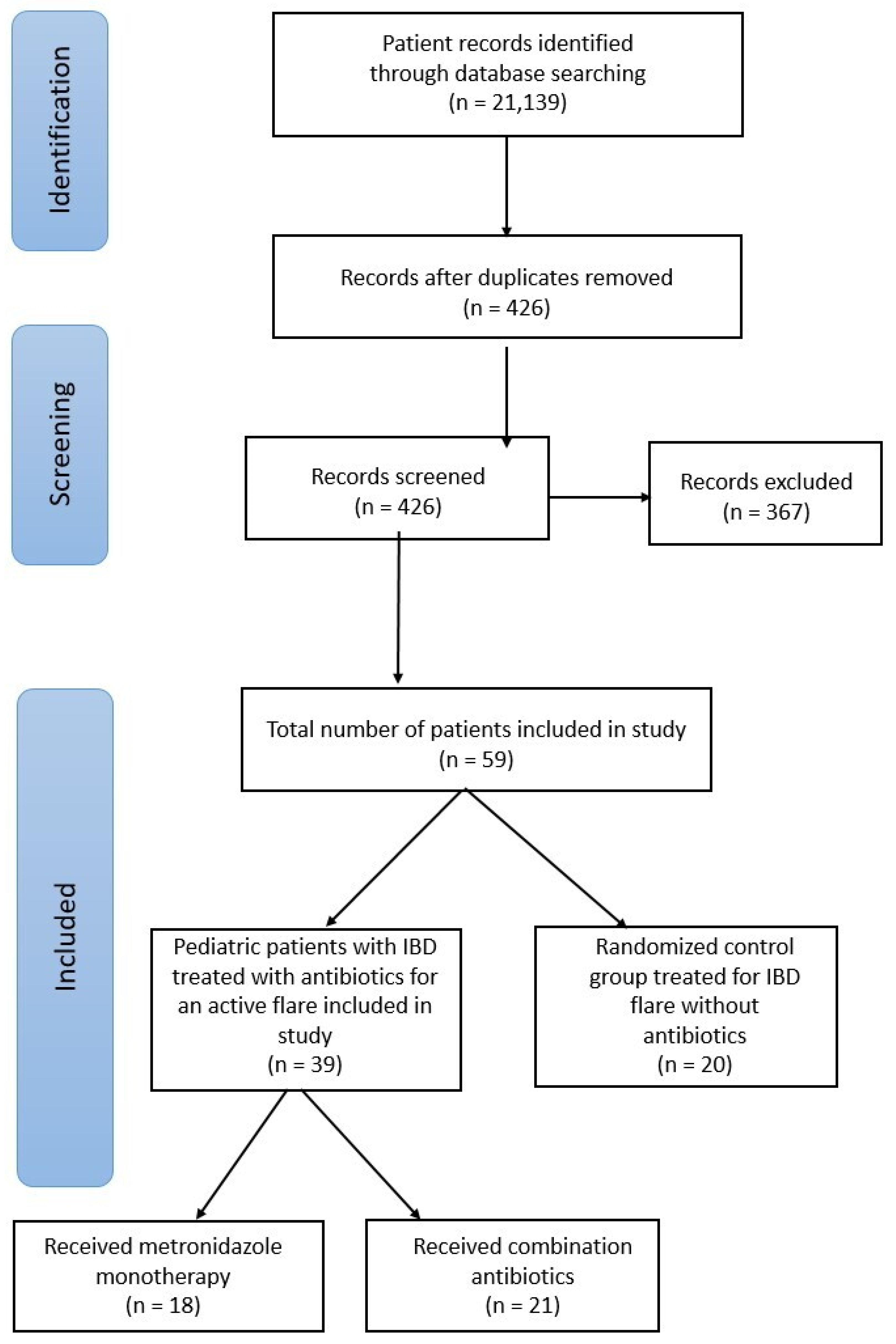
2. Materials and Methods
2.1. Patient Enrollment
2.2. Outcomes
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes
3.3. Changes in Clinical Scoring and Labs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| FDA | U.S. Food and Drug Administration |
| IBD | Inflammatory bowel disease |
| LOS | Length of stay |
| PCDAI | Pediatric Crohn’s Disease Activity Index |
| PUCAI | Pediatric Ulcerative Colitis Activity Index |
| SOC | Standard-of-care |
| UC | Ulcerative colitis |
References
- Elmaghrawy, K.; Fleming, P.; Fitzgerald, K.; Cooper, S.; Dominik, A.; Hussey, S.; Moran, G.P. The Oral Microbiome in Treatment-Naïve Pediatric IBD Patients Exhibits Dysbiosis Related to Disease Severity that Resolves Following Therapy. J. Crohns Colitis. 2022, 17, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Jha, D.K.; Mishra, S.; Dutta, U.; Sharma, V. Antibiotics for inflammatory bowel disease: Current status. Indian J. Gastroenterol. 2024, 43, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, N.A.; Lynch, S.V. Role of the microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 2012, 18, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Mechanisms of Disease: Pathogenesis of Crohn’s disease and ulcerative colitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 390–407. [Google Scholar] [CrossRef] [PubMed]
- Verburgt, C.M.; Heutink, W.P.; Kuilboer, L.I.; Dickmann, J.D.; van Etten-Jamaludin, F.S.; A Benninga, M.; de Jonge, W.J.; E Van Limbergen, J.; Tabbers, M.M. Antibiotics in pediatric inflammatory bowel diseases: A systematic review. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Kastl, A.; Hoffmann, N.; Rogers, R.; Grossman, A.B.; Mamula, P.; Kelsen, J.R.; Baldassano, R.N.; Albenberg, L. Efficacy of Combination Antibiotic Therapy for Refractory Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Gawronska, A.; Banasiuk, M.; Lachowicz, D.; Pituch, H.; Albrecht, P.; Banaszkiewicz, A. Metronidazole or Rifaximin for Treatment of Clostridium difficile in Pediatric Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ohkusa, T.; Terao, S.; Chiba, T.; Murakami, K.; Yanaka, A.; Uehara, T.; Ishii, Y.; Soma, M.; Tajiri, H. Adjunct antibiotic combination therapy for steroid-refractory or -dependent ulcerative colitis: An open-label multicentre study. Aliment. Pharmacol. Ther. 2014, 39, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Kato, K.; Terao, S.; Chiba, T.; Mabe, K.; Murakami, K.; Mizokami, Y.; Sugiyama, T.; Yanaka, A.; Takeuchi, Y.; et al. Newly Developed Antibiotic Combination Therapy for Ulcerative Colitis: A Double-Blind Placebo-Controlled Multicenter Trial. Am. J. Gastroenterol. 2010, 105, 1820–1829. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.; Kori, M.; Kierkus, J.; Boneh, R.S.; Sladek, M.; Escher, J.C.; Wine, E.; Yerushalmi, B.; Dias, J.A.; Shaoul, R.; et al. Azithromycin and metronidazole versus metronidazole-based therapy for the induction of remission in mild to moderate paediatric Crohn’s disease: A randomized controlled trial. Gut 2019, 68, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Su, J.W.; Ma, J.J.; Zhang, H.J. Use of antibiotics in patients with Crohn’s disease: A systematic review and meta-analysis. J. Dig. Dis. 2015, 16, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Sato, N. Antibacterial and antimycobacterial treatment for inflammatory bowel disease. J. Gastroenterol. Hepatol. 2005, 20, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W.; Selby, W.S.; Jewell, D.P. Controlled trial of intravenous metronidazole as an adjunct to corticosteroids in severe ulcerative colitis. Gut 1986, 27, 1210–1212. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Bishai, J.; Reshef, L.; Abitbol, G.; Focht, G.; Marcus, D.; Ledder, O.; Lev-Tzion, R.; Orlanski-Meyer, E.; Yerushalmi, B.; et al. Antibiotic Cocktail for Pediatric Acute Severe Colitis and the Microbiome: The PRASCO Randomized Controlled Trial. Inflamm. Bowel Dis. 2020, 26, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 2: Acute Severe Colitis—An Evidence-based Consensus Guideline From the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 292–310. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ruemmele, F.M.; Orlanski-Meyer, E.; Griffiths, A.M.; de Carpi, J.M.; Bronsky, J.; Veres, G.; Aloi, M.; Strisciuglio, C.; Braegger, C.P.; et al. Management of Paediatric Ulcerative Colitis, Part 1: Ambulatory Care—An Evidence-based Guideline From European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 257–291. [Google Scholar] [CrossRef] [PubMed]
- van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohn’s Coliti 2021, 15, 171–194. [Google Scholar] [CrossRef] [PubMed]
- FDA. What Is a Serious Adverse Event? [Internet]. U.S. Food and Drug Administration. 2019. Available online: https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event (accessed on 14 May 2025).
- Vasudevan, A.; Gibson, P.R.; van Langenberg, D.R. Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told? World J. Gastroenterol. 2017, 23, 6385–6402. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T. Induction of experimental ulcerative colitis by Fusobacterium varium isolated from colonic mucosa of patients with ulcerative colitis. Gut 2003, 52, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Denson, L.; Vlamakis, H.; Franzosa, E.A.; Thomas, S.; Gotman, N.M.; Rufo, P.; Baker, S.S.; Sauer, C.; Markowitz, J.; et al. Compositional and Temporal Changes in the Gut Microbiome of Pediatric Ulcerative Colitis Patients Are Linked to Disease Course. Cell Host Microbe 2018, 24, 600–610.e4. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Lutz, L.; Pereira, D.C.; Paiva, R.M.; Zavascki, A.P.; Barth, A.L. Macrolides decrease the minimal inhibitory concentration of anti-pseudomonal agents against Pseudomonas aeruginosa from cystic fibrosis patients in biofilm. BMC Microbiol. 2012, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Sprockett, D.; Fischer, N.; Boneh, R.S.; Turner, D.; Kierkus, J.; Sladek, M.; Escher, J.C.; Wine, E.; Yerushalmi, B.; Dias, J.A.; et al. Treatment-Specific Composition of the Gut Microbiota Is Associated with Disease Remission in a Pediatric Crohn’s Disease Cohort. Inflamm. Bowel Dis. 2019, 25, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Turner, D. Antibiotics in Refractory IBD: Not Without Risks but Are the Alternatives Better? Response to Gilmore et al. Inflamm. Bowel Dis. 2020, 26, e42. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristic | Metronidazole (n = 18) | Combination Antibiotics (n = 21) | Control Group (n = 20) |
|---|---|---|---|
| Age (years), mean ± SD 1 | 13 ± 3.9 | 14 ± 2.56 | 13 ± 3 |
| Sex (male), n (%) | 9 (50) | 10 (47) | 16 (80) |
| Weight (kg), median (min, max) | 109 (12, 120) | 52 (24, 75) | 41 (21, 112) |
| Race, n (%) | |||
| White | 15 (83) | 15 (71) | 18 (90) |
| Asian | 1 (6) | 1 (5) | 1 (5) |
| Not recorded | 2 (11) | 5 (24) | 1 (5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 9 (50) | 12 (57) | 10 (50) |
| Non-Hispanic | 9 (50) | 9 (43) | 10 (50) |
| Type of IBD, n (%) | |||
| Ulcerative Colitis | 9 (50) | 16 (76) | 10 (50) |
| Crohn’s Disease | 9 (50) | 5 (24) | 10 (50) |
| Maintenance Therapy, n (%) | |||
| Adalimumab | 3 (17) | 5 (24) | 4 (20) |
| Azathioprine | 1 (6) | 0 | 2 (10) |
| Budesonide | 0 | 1 (5) | 1 (5) |
| Hydrocortisone | 4 (22) | 2 (10) | 0 |
| Infliximab | 4 (22) | 7 (33) | 7 (35) |
| Mesalamine | 8 (44) | 7 (33) | 7 (35) |
| Methotrexate | 2 (11) | 1 (5) | 0 |
| Methylprednisolone | 4 (22) | 5 (24) | 4 (20) |
| Prednisone | 7 (39) | 12 (57) | 9 (45) |
| Sulfasalazine | 2 (11) | 1 (5) | 2 (10) |
| Ustekinumab | 1 (6) | 1 (5) | 3 (15) |
| Vedolizumab | 0 | 1 (5) | 1 (5) |
| 6-MMP | 1 (6) | 1 (5) | 0 |
| Primary Outcome | |||||
|---|---|---|---|---|---|
| Metronidazole (n = 18) | Control Group (n = 20) | Combination Antibiotics (n = 21) | p-Value | ||
| Time to clinical response, median (min, max) | 9 (2, 217) | 7.5 (1, 119) | 4 (1, 65) | 0.112 | |
| Secondary Outcome | |||||
| Time (days) | Metronidazole | Control Group | Combination Antibiotics | p-value | |
| Treatment failure, n (%) | No Treatment Failure | 16 (89) | 9 (45) | 16 (76) | 0.460 |
| 1 through 14 | 0 | 0 | 0 | ||
| 15 through 28 | 0 | 2 (10) | 0 | ||
| 29 through 100 | 1 (6) | 7 (35) | 2 (10) | ||
| >100 | 1 (6) | 2 (10) | 3 (14) | ||
| Clinical remission, n (%) | 1 through 14 | 5 (28) | 2 (10) | 5 (24) | 0.775 |
| 15 through 28 | 0 (0) | 0 | 1 (5) | ||
| 29 through 100 | 7 (39) | 6 (30) | 7 (33) | ||
| >100 | 4 (22) | 3 (15) | 2 (10) | ||
| Clinical response, n (%) | 1 through 14 | 11 (61) | 11 (55) | 18 (86) | 0.516 |
| 15 through 28 | 2 (11) | 3 (15) | 2 (10) | ||
| 29 through 100 | 3 (17) | 2 (10) | 1 (5) | ||
| >100 | 2 (11) | 2 (10) | 0 | ||
| Antibiotic Usage | Metronidazole (n = 18) | Combination Antibiotics (n= 21) |
|---|---|---|
| No bridge therapy, n (%) | 15 (83) | 13 (62) |
| Inpatient, n (%) | 8 (53) | 9 (69) |
| Outpatient, n (%) | 7 (46) | 4 (31) |
| Bridge therapy, n (%) | 3 (0.2) | 8 (38) |
| Inpatient, n (%) | 3 (100) | 7 (88) |
| Outpatient, n (%) | 0 | 1 (12) |
| Antibiotic regimens | ||
| Metronidazole, Amoxicillin, Doxycycline | - | 17 (81) |
| Metronidazole, Ciprofloxacin | - | 2 (9) |
| Metronidazole, Amoxicillin, | - | 1 (5) |
| Sulfamethoxazole–Trimethoprim | ||
| Ciprofloxacin monotherapy | - | 1 (5) |
| Antibiotic duration (days), median (min, max) | 11.5 (4, 94) | 14 (1, 124) |
| Patients receiving steroids on antibiotics, n | 4 | 15 |
| Days patient on steroids prior to antibiotic initiation, median (min, max) | 4 (0, 25) | 6 (0, 16) |
| Days from initiation of antibiotics to initiation of anti-TNF inhibitor | 17 (13, 19) | 3.5 (0, 17) |
| Lab | Timeframe 1 | Metronidazole | Control Group | Combination Antibiotics |
|---|---|---|---|---|
| Hemoglobin (g/dL) | Before Starting Treatment | 11 ± 2 | 11 ± 3 | 10 (5, 14) |
| After Completing Treatment | 11 ± 3 | 13 (7, 16) | 10 (8,12) | |
| Albumin (g/dL) | Before Starting Treatment | 4 ± 0.4 | 4 (1, 5) | 3 ± 1 |
| After Completing Treatment | 4 ± 1 | 4 ± 1 | 4 ± 1 | |
| C-reactive Protein (mg/dL) | Before Starting Treatment | 0.9 (0.3, 21.3) | 1.4 (0.3, 34.8) | 5 (0.3, 15) |
| After Completing Treatment | 1 ± 1 | 1.2 (0.1, 17) | 5 (0.3, 21) | |
| Erythrocyte Sedimentation Rate (mm/h) | Before Starting Treatment | 29 ± 21 | 37 ± 31 | 40 (2, 97) |
| After Completing Treatment | 17 ± 12 | 19 ± 18 | 40 (11, 87) | |
| Absolute Fecal Calprotectin (mcg/g) | Before Starting Treatment | 834 ± 499 | 1070 ± 1112 | 2149 ± 1011 |
| After Completing Treatment | 1066 ± 579 | 759 ± 1126 | 1442 ± 1045 | |
| PUCAI 2 Score | Before Starting Treatment | 45 ± 40 | 56 ± 6 | 55 (35, 85) |
| After Completing Treatment | 38 ± 33 | 15 ± 4 | 23 ± 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dye, C.; Sierra, C.M.; Bahjri, K.; Cohen, M.; Nagendra, G. Antibiotic Effect on Clinical Response and Remission in Pediatric Inflammatory Bowel Disease. Pediatr. Rep. 2025, 17, 77. https://doi.org/10.3390/pediatric17040077
Dye C, Sierra CM, Bahjri K, Cohen M, Nagendra G. Antibiotic Effect on Clinical Response and Remission in Pediatric Inflammatory Bowel Disease. Pediatric Reports. 2025; 17(4):77. https://doi.org/10.3390/pediatric17040077
Chicago/Turabian StyleDye, Caeley, Caroline M. Sierra, Khaled Bahjri, Mallory Cohen, and Gautam Nagendra. 2025. "Antibiotic Effect on Clinical Response and Remission in Pediatric Inflammatory Bowel Disease" Pediatric Reports 17, no. 4: 77. https://doi.org/10.3390/pediatric17040077
APA StyleDye, C., Sierra, C. M., Bahjri, K., Cohen, M., & Nagendra, G. (2025). Antibiotic Effect on Clinical Response and Remission in Pediatric Inflammatory Bowel Disease. Pediatric Reports, 17(4), 77. https://doi.org/10.3390/pediatric17040077






