Abstract
Neuroimaging has emerged as an innovative and essential tool for understanding the intricate relationship between brain development, emotions, and behavior. Investigating the neurobiological mechanisms underlying this interaction during the critical phase of brain maturation is crucial for promoting individual psychological well-being and mitigating the profound impact of mood disorders during childhood. This narrative scoping review synthesizes current pediatric neuroimaging evidence, filling a gap in the literature by integrating structural, functional, and emerging modalities, to provide clear translational pathways for clinical and behavioral observations. The contribution of major neuroimaging techniques, including fMRI, PET, DTI, and sMRI, is analyzed, emphasizing their ability to detect structural and functional alterations associated with mood disorders, enabling early diagnosis and personalized therapeutic strategies. Furthermore, the potential of these technologies to monitor the effects of psychotherapy is explored, demonstrating how such interventions can modulate neural circuits and enhance emotional processing. Despite significant advancements and growing interest, challenges remain, including the complexity of data interpretation, technological limitations, and ethical considerations related to the use of these interventions in pediatric populations. This review synthesizes the most recent scientific evidence, underscoring the potential of neuroimaging to improve diagnostic accuracy and therapeutic outcomes, while outlining future research directions aimed at enhancing interventions for children and adolescents with mood disorders.
1. Introduction
Pediatric mood disorders are complex and multifactorial conditions involving disruptions in emotional regulation, cognitive processes, and behavior. These dysfunctions are closely linked to the maturation of specific brain regions and networks, which undergo significant changes during childhood and adolescence.
Emotional development represents a critical aspect of this growth, shaping psychological well-being, social interactions, and individual temperament. Emotions not only drive social behavior but also influence cognitive processes and contribute to overall behavioral regulation. When this developmental process is disrupted, children may experience difficulties in managing emotions, increasing their risk of developing mood disorders such as depression and bipolar disorder [1]. According to the World Health Organization (WHO), approximately 10–20% of adolescents experience mental health disorders [2], with an estimated 6% of those aged 10–19-years affected by mood disorders [3]. Young individuals with mood disorders often face unfavorable long-term outcomes, and the safety of psychotropic medications in this demographic remains a significant concern [4].
In recent years, neuroimaging has emerged as a powerful tool for investigating the intricate interactions between brain development, emotions, and behavior. Advanced techniques, including functional magnetic resonance imaging (fMRI), positron emission tomography (PET), structural magnetic resonance imaging (sMRI), and diffusion tensor imaging (DTI), enable researchers to examine both the structural and functional dynamics of the brain in real time. These methodologies have been pivotal in identifying significant alterations in key brain regions, such as the prefrontal cortex, amygdala, and limbic system, which play a central role in emotional processing and regulation, particularly in children affected by mood disorders, offering valuable insights for early diagnosis and the optimization of therapeutic strategies [5]. These areas undergo profound changes during childhood and adolescence, reflecting the remarkable plasticity of the brain during these developmental stages. However, this same plasticity makes the brain particularly vulnerable to adverse influences, such as genetic predispositions or environmental stressors, which can compromise normal development and lead to emotional dysfunctions.
The application of neuroimaging extends beyond diagnosis. It offers a powerful means of tracking the effectiveness of treatments over time. By visualizing changes in brain structure and function, neuroimaging can provide objective evidence of how various therapies both psychological and pharmacological impact the neural circuits involved in mood regulation [6].
The aim of this review is to provide a comprehensive overview of scientific and clinical advancements in neuroimaging applied to pediatric mood disorders while simultaneously encouraging future research to improve diagnostic and therapeutic strategies.
By promoting targeted and personalized interventions, the goal is to enhance the long-term well-being of young patients. Importantly, this narrative scoping review aims to systematically map clinical, behavioral, and imaging findings across structural, functional, and emerging modalities in pediatric populations, explicitly overcoming the lack of of up-to-date literature on advanced neuroimaging applications for therapeutic monitoring and closed-loop interventions.
2. Emotions and Behavior: Brain Areas Involved
Emotions and human behavior are complex processes that involve several interconnected brain areas (Table 1). The main brain regions involved in the regulation of emotions and behaviors include the prefrontal cortex, the anterior cingulate cortex, the insula, the cerebellum, and the limbic system, as described in Figure 1 [7].

Table 1.
Connections between brain functions and emotional/behavioral disorders.
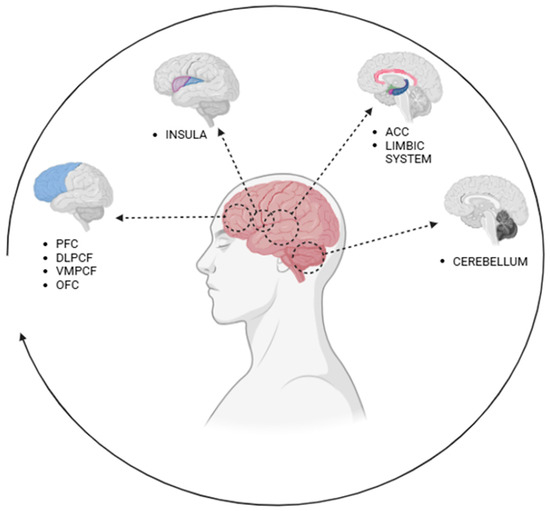
Figure 1.
Brain regions involved in the regulation of emotions and behaviors. Note: PFC: prefrontal cortex; DLPCF: dorsolateral PFC; VMPCF: ventromedial PFC; OFC: orbitofrontal cortex; ACC: anterior cingulate cortex.
The prefrontal cortex (PFC), located in the anterior part of the frontal lobes, is the center of emotional and behavioral regulation [8]. Its role in emotion regulation is evident in its control over emotional responses generated by the limbic system, such as the modulation of intense or inappropriate emotions, allowing for adaptive emotional control. It is essential for impulse control, inhibiting automatic or impulsive behaviors to enable thoughtful decision-making and socially appropriate behavior. The PFC is responsible for long-term planning and strategic decision-making, integrating emotional information to assess rewards and risks. It also plays a crucial role in stress regulation, modulating cortisol release and reducing the negative effects of chronic stress [9]. The PFC can be divided into subregions with specific functions. The dorsolateral prefrontal cortex (DLPFC) is involved in cognitive control, working memory, and planning, integrating emotional information with complex decision-making processes. The ventromedial prefrontal cortex (VMPFC) is crucial for emotional processing and value- and reward-based decision-making, with strong interactions with the amygdala, nucleus accumbens, and hippocampus [10]. The orbitofrontal cortex (OFC), connected to the dorsolateral prefrontal circuit, is involved in the manipulation and integration of sensory information from the external environment via the temporal cortex and the insula. In addition, it is implicated in mediating empathetic and socially appropriate responses by receiving information from the internal environment, with strong connections to the anterior cingulate cortex (ACC) [11]. In anxiety disorders, insufficient control over the amygdala leads to exaggerated fear responses [12]. Dysfunction of the OFC can lead to loss of control and compulsive behaviors [13,14]. In attention deficit hyperactivity disorder (ADHD), dysfunction of the DLPFC is associated with attention and self-control problems [15]. The cerebellum, traditionally associated with motor control, movement coordination, and balance, also plays a role in emotion processing and behavior regulation [16]. Recent studies have highlighted that this structure, through its connections with other brain areas, contributes to emotional identification and expression, as well as expression related to empathy [17]. Lesions in the posterior lobe result in cerebellar cognitive affective syndrome (CCAS), which includes deficits in executive function, visuospatial processing, language abilities, and affect regulation [18]. Anomalies in cerebello-limbic connections have been associated with maladaptive behaviors and social problems [19,20]. The insula is a region of the cerebral cortex located deep within the lateral sulcus, hidden between the temporal, parietal, and frontal lobes. It plays a fundamental role in integrating sensory, emotional, and cognitive signals. The insula monitors the body’s internal states, such as heartbeat, hunger, and body temperature, contributing to bodily awareness [21]; it regulates the subjective experience of emotions such as disgust, fear, empathy, and pleasure [22]; it plays a key role in the perception of physical and emotional pain and is involved in social suffering, such as rejection or loss [23,24]. The anterior insula is particularly active in empathy processes and in understanding others’ emotions, while the posterior insula is more related to sensory perception and visceral regulation [25,26,27]. This structure is also involved in addiction and compulsive behavior, contributing to the awareness of craving states such as the desire for drugs, food, or cigarettes [28,29,30]. It also plays an important role in decision-making and intuition, helping to connect bodily experience to choices. The limbic system is a set of brain structures involved in emotion processing, memory, and motivation and includes the amygdala, the hippocampus, the cingulate gyrus, the hypothalamus, and the nucleus accumbens [31].
The amygdala is an almond-shaped structure located in the limbic system, crucial for the perception and regulation of emotions [32]. The amygdala is responsible for processing emotionally salient stimuli and modulating behavioral responses based on the emotions perceived [33]. This crucial role manifests in several aspects. The amygdala detects emotional stimuli, quickly identifying signals of threat or emotional relevance in the environment, such as an angry person’s face or a dangerous situation. This automatic process prepares the organism to respond appropriately. Through its connection with other brain areas, such as the hypothalamus and brainstem, the amygdala coordinates behavioral and physiological responses, such as activating the “fight or flight” system during stressful or dangerous situations [34]. The amygdala interacts with the hippocampus to store memories associated with strong emotions, such as traumatic or significant events [35]. These emotional memories subsequently influence behavior, guiding the individual to avoid situations perceived as threatening or to repeat rewarding ones [36]. The amygdala works with the PFC to regulate the emotional response based on context. While the amygdala generates rapid emotional reactions, the PFC exerts top-down control, helping to modulate and adapt emotional behaviors to complex or socially appropriate situations [37]. Dysfunctions in amygdala activity can lead to altered emotional behaviors, such as impulsive aggression or chronic anxiety. Excessive activation can amplify inappropriate behavioral responses, while reduced activity can impair risk perception. Overall, the amygdala acts as a bridge between emotions and behaviors, transforming emotional perception into concrete responses. Its interaction with other brain structures ensures a balance between rapid reactions and contextual regulation, which is essential for adaptive behavior and survival. The ACC, located in the anterior portion of the gyrus, serves as a bridge between the limbic system, responsible for emotions, and the prefrontal cortex, involved in higher cognitive processes and behavior regulation [38]. The ACC is central to emotional regulation, monitoring and modulating emotional responses generated by the amygdala. Through connections with the limbic system, the ACC helps regulate the intensity of emotions such as fear, anxiety, and anger, playing a key role in reducing excessive emotional activation. A crucial aspect of the ACC is its contribution to error monitoring and conflict resolution [39]. When a behavior does not produce the expected outcome, the ACC is activated to detect discrepancies and direct attention to the error, promoting learning and adaptation. This mechanism is essential for behavioral control and maintaining motivation, especially in contexts of uncertainty or difficulty. The ACC is also involved in the management of both physical and emotional pain [40]. Its activation is associated with the processing of social pain, such as rejection or exclusion, as well as physical pain. This highlights its role in linking bodily experiences and emotional states, contributing to a coherent behavioral response. In social interactions, the ACC plays a role in regulating empathy and prosocial behavior, and helps us to understand others’ emotions, integrating social cues and emotional information to promote appropriate responses and support interpersonal connection [41]. In depression, reduced activity in the ACC can impair emotional regulation and the ability to cope with stressful events [42]. In schizophrenia, dysfunctions of the ACC are linked to issues with behavioral control and the inability to integrate emotional and cognitive information [43,44]. The hippocampus, also located in the temporal lobe, is crucial for the formation of long-term memory and learning [45]. It plays an important role in contextualizing emotions, associating specific events with emotional stimuli [46,47]. This function is essential for understanding and regulating appropriate behavioral responses based on past experiences, such as in anxiety disorders [48]. The hypothalamus plays a role in coordinating physiological responses to emotions: it regulates autonomic functions such as heart rate, blood pressure, and hormone release through the hypothalamic–pituitary–adrenal axis [49,50]. It is essential for maintaining homeostasis and modulating fundamental behaviors such as aggression, hunger, and mating [51].
The nucleus accumbens is involved in the perception of pleasure and the processing of rewards [52,53]. Its connections with the dopaminergic system make it crucial for behaviors related to the pursuit of gratification and for the regulation of addiction [54,55].
The limbic system integrates emotions, memory, and behaviors, allowing for adaptive responses to the demands of the environment. However, imbalances or dysfunctions in these structures can lead to emotional and behavioral difficulties, such as anxiety, depression, or uncontrolled aggression.
3. The Neuroplasticity of the Pediatric Brain
The neuroplasticity of the pediatric brain, or its ability to adapt and reorganize in response to experiences, environmental stimuli, and learning, is a fundamental aspect of child development [56,57]. Unlike the adult brain, which is more stable, the brain of a child has an incredible ability to reshape itself, making it highly adaptable but also vulnerable to external influences [58,59,60]. Brain plasticity in children is one of the most remarkable characteristics of the developing brain. During childhood, the brain has an exceptional ability to reorganize itself in response to environmental stimuli, strengthening or eliminating synaptic connections based on experience and in response to congenital or traumatic neurological conditions [61,62,63,64]. Siffredi et al. demonstrated that neuroplasticity in children with corpus callosum agenesis appears to allow functional connectivity comparable to that of a typically developing brain, increasing intra-hemispheric connectivity [65]. Childhood is characterized by high synaptic density and greater neural flexibility, enabling children to rapidly acquire new skills such as language, motor abilities, and cognitive functions, adapt to their environment, and modulate their emotional responses [66,67,68,69]. Emotional responses in children are profoundly influenced by synaptic plasticity, which shapes the neural circuits responsible for affective regulation [70,71]. During the early years of life, emotional experiences, both positive and negative, shape brain connectivity, influencing how a child will respond to stress, reward, and social interactions [72,73]. We differentiate between infancy (synaptogenesis peak), early childhood (pruning and language/emotion circuit refinement), and adolescence (pubertal maturational surge in limbic–PFC connectivity), each with distinct vulnerabilities and therapeutic windows [74].
Sensory, emotional, and cognitive experiences actively shape the structure and function of a child’s brain. Plasticity allows for a high degree of adaptability, supporting the development of effective emotional regulation strategies and the ability to recognize and interpret both one’s own emotions and those of others [75,76]. Positive experiences, such as emotional support and a sense of security, help build resilient neural circuits, foster emotional stability, and reduce susceptibility to mood disorders [77]. Conversely, exposure to chronic stress or emotional deprivation can lead to altered synaptic plasticity, increasing the risk of anxiety, depression, and difficulties in emotional regulation, as described in Figure 2 [78,79,80,81,82,83]. Self-dissatisfaction can influence brain neuroplasticity, which, in turn, affects self-perception [84]. Induced neuroinflammation, for example, from COVID-19, could alter microglial cell function, impairing brain plasticity and contributing to learning difficulties and neurodevelopmental disorders. This occurs through a reduction in brain-derived neurotrophic factor (BDNF), alteration an in communication between immune cells and microglia, increased inflammatory molecules, and the disruption of crucial signaling pathways for synaptic plasticity [85,86]. However, brain plasticity in childhood also offers recovery opportunities, enabling targeted therapeutic interventions to strengthen neural connections related to emotional well-being [87]. A key aspect of childhood plasticity is its compensatory ability [88,89]. If a brain region responsible for emotional regulation suffers a deficit, other regions may step in to support these functions, making effective adaptation possible. This property is essential for early interventions aimed at children with emotional difficulties, allowing the modulation of responses through educational and therapeutic strategies based on neuroplasticity. After the early years of life, neuroplasticity begins to slow down [90]. This property is essential for early interventions aimed at children with emotional difficulties, allowing emotional responses to be modulated through educational and therapeutic strategies based on neuroplasticity.
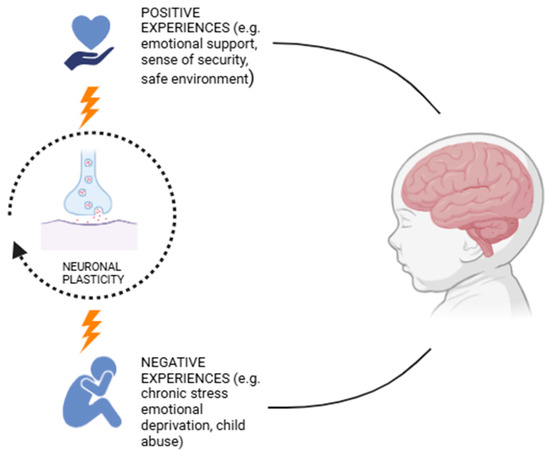
Figure 2.
Effect of emotional experiences on neuroplasticity.
4. Role of Neuroimaging in the Study of Mood Disorders
Neuroimaging has become an indispensable tool to investigate the neurobiological mechanisms underlying mood disorders, especially given that these disorders are a leading cause of disability worldwide. By providing a non-invasive, real-time window into brain structure and function, brain imaging techniques have helped us to understand how brain networks are involved in emotion regulation, cognitive control, and mood stability development and function in both adults and children, including adolescents [91,92]. These imaging techniques not only aid in the detection of early biomarkers, but also allow clinicians to monitor disease progression, to assess the efficacy of treatments, and to develop more personalized therapeutic interventions. The imaging techniques available in clinical settings include the following: magnetic resonance (MRI), which assessed anatomy (structural MRI) and brain activity (functional MRI); positron emission tomography (PET), which evaluates brain metabolism and neurotransmitter activity; and diffusion tensor imaging (DTI), which examines the brain’s white matter connections (Table 2).

Table 2.
Brain imaging techniques.
Structural, or volumetric, neuroimaging has been used to investigate the correlation between pathophysiology and measurable changes in neuroanatomy, identifying several anatomical abnormalities in brain regions that are thought to affect regulation and emotional expression.
MRI generates images through the differential magnetic properties of hydrogen in different tissues.
Structural MRI (sMRI), which provides high-resolution anatomical imaging, has been instrumental in identifying structural abnormalities associated with mood and behavioral disorders. Studies have reported reductions in gray matter volume in key emotion-regulating regions such as the prefrontal cortex, hippocampus, and amygdala, with these deficits being more pronounced in individuals with early-onset depression and recurrent mood disorders [93,94].
Functional MRI (fMRI) detects brain activity through blood-oxygen-level-dependent (BOLD) signals, making it possible to map functional networks involved in emotional regulation. A different component of hydrogen atoms is measured which reflects the ferromagnetic nature of deoxygenated vs. oxygenated blood. Thus, an area with more oxygenated blood will appear more intense compared to when there is less oxygenated blood.
Research has consistently shown hyperactivity in the amygdala and reduced connectivity between the prefrontal cortex and limbic system in children with depression, highlighting mechanisms that contribute to emotional dysregulation and heightened sensitivity to negative stimuli [95].
PET imaging, through the use of radioactive neurotransmitter derivatives, produces a three-dimensional image of the brain, allowing the study of metabolic activity and neurotransmitter function, thereby clarifying the neurochemical imbalances in mood disorders. Findings from PET studies, for example, have linked pediatric depression to serotonin and dopamine dysregulation, as well as reduced glucose metabolism in the DLPFC, OFC, PFC, and CCA—patterns that correlate with symptoms such as low motivation, cognitive dysfunction, and emotional blunting [96,97].
DTI, an advanced MRI-based technique, is crucial for mapping white matter pathways and evaluating brain connectivity. By examining disruptions in major tracts such as the corpus callosum, uncinate fasciculus, and cingulum bundle, DTI has provided a valuable insight into BD, where abnormal connectivity is thought to contribute to mood instability, impulsivity, and cognitive deficits [98,99,100,101]. Together, these neuroimaging techniques offer a comprehensive view on the neural basis of emotional development in children and adolescents, leading the way for earlier diagnosis, targeted treatments, and a deeper understanding of pediatric mood disorders.
5. Psychotherapy and Neuroimaging
In recent decades, functional neuroimaging techniques have revolutionized our understanding of the neural mechanisms underlying psychiatric disorders. More recently, their use has expanded to psychotherapy, allowing not only for the confirmation of brain changes induced by psychotherapeutic interventions but also for the development of new approaches based on neural biomarkers. The integration of neuroimaging into clinical practice is transforming psychotherapy from a model based solely on symptoms and behaviors to a biologically informed approach, in which modifications in brain networks can be used to monitor treatment response and personalize therapeutic interventions.
A growing body of research has demonstrated that psychotherapy does not merely modify behavior and cognition but also induces measurable neurobiological changes. Among the most extensively studied approaches, cognitive behavioral therapy (CBT) has been shown to have a significant impact on brain connectivity. In particular, it has been observed that in patients with anxiety disorders, CBT strengthens prefrontal control over subcortical structures involved in fear regulation, such as the amygdala and ACC, thereby contributing to better modulation of emotional responses [102]. This effect extends to mood disorders as well, where activation of the subgenual ACC has been found to predict treatment efficacy: elevated metabolic activity in this region has been associated with poorer therapy response, highlighting the need for more targeted therapeutic approaches [103].
The use of neuroimaging to monitor the effects of psychotherapy is also expanding to the pediatric population, particularly in the study of mood and anxiety disorders. One study examined the association between reward circuit function and psychotherapy response in young individuals with anxiety disorders, revealing that higher pre-treatment activation in the medial prefrontal cortex and nucleus accumbens was correlated with better therapy outcomes. These findings suggest that neuroimaging could serve as a tool to identify young patients who are more likely to benefit from psychotherapeutic interventions [104].
In the context of major depressive disorder (MDD), CBT has been shown to induce significant modifications in brain connectivity. A study on adolescents with remitted MDD found that rumination-focused cognitive behavioral therapy (RF-CBT) not only improved clinical symptoms but also induced changes in neural circuits involved in rumination regulation, such as the left precuneus and angular gyrus. Additionally, the low stability of brain activations in treated patients suggests that therapy does not merely reinforce existing circuits but promotes the dynamic reorganization of neural networks involved in repetitive thinking [105]. Other studies have demonstrated that CBT can enhance connectivity between the subgenual ACC, the amygdala, and frontal regions, suggesting that the treatment does not simply normalize brain activity but contributes to the functional readjustment of neural networks in MDD patients [106].
Another key aspect concerns the interaction between sleep, emotional regulation, and neurobiology. Insomnia is a known risk factor for depression, and neuroimaging studies have shown that targeted interventions can significantly mitigate this risk. In particular, CBT for insomnia (CBT-I) combined with circadian rhythm support (CRS) has been found to enhance amygdala response and improve reactivity in the left insula, both crucial regions for emotional regulation [107].
Alongside CBT, mindfulness and meditation-based interventions are emerging as complementary tools for emotional regulation, with distinct neurobiological effects. Notably, mindfulness-based cognitive therapy for children (MBCT-C) has shown improvements in brain connectivity and emotional regulation in youth with a familial risk of bipolar disorder, suggesting a positive impact on resilience [108]. The efficacy of mindfulness has also been confirmed by the Mindfulteen study, which demonstrated that mindfulness can reduce stress reactivity and anxiety in adolescents by modulating connections between the PFC and the limbic system [109].
The benefits of meditation have been further validated by studies examining brain connectivity in adolescents with major depression. Body–mind relaxation meditation (BMRM) has been shown to normalize alterations in thalamocortical connectivity, with positive effects on attention and self-referential processes [110]. Additionally, mindfulness has been found to increase gray matter volume in the right hippocampus, a crucial region for memory and stress regulation, with significant benefits in reducing depressive symptoms and perceived stress in individuals with a history of childhood maltreatment [111].
Finally, a pilot study showed that a mindfulness-based intervention (MBI) increased connectivity between the PCC and the DLPFC, regions involved in emotional regulation. This change was associated with reduced suppression of negative emotions and increased awareness, suggesting that these practices could serve as a therapeutic target for mood stabilization in at-risk youth [112].
Overall, these findings confirm that neuroimaging not only allows for the evaluation of psychotherapy’s direct effects on brain circuits, but is also emerging as a key tool for optimizing therapeutic interventions. The identification of neurofunctional biomarkers could enable the personalization of treatment selection and the prediction of the likelihood of success for a given psychotherapeutic approach. While CBT remains an effective strategy for reshaping brain connectivity in patients with anxiety and depression, mindfulness and meditation practices are emerging as complementary strategies for improving emotional regulation and enhancing resilience. The integration of neuroscience and psychotherapy could therefore represent a promising avenue for improving the efficacy and precision of therapeutic interventions.
6. Role of Neuroimaging and Emotion Regulation in Adolescence: A Window into the Developing Brain
Modern neuroimaging techniques, including fMRI, magnetic resonance spectroscopy (MRS), and PET, provide an advanced means of investigating structural and functional abnormalities in the brain. These methods are particularly valuable in studying younger populations, where the brain is still undergoing critical developmental processes. By enabling researchers to visualize neural activity, connectivity, and biochemical composition, these imaging modalities serve as a powerful tool for examining how different brain regions interact, activate, and, in some cases, exhibit dysfunctions linked to psychiatric disorders. This approach not only deepens our understanding of neurodevelopmental and psychiatric conditions but also supports the development of more targeted therapeutic interventions.
Adolescence is a critical period of emotional vulnerability due to the ongoing maturation of emotion regulation mechanisms and the development of self-identity [113]. Emotion regulation encompasses the process through which individuals modulate their emotional responses, determining which emotions they experience and when they occur. The immaturity of neural networks responsible for emotion regulation may contribute to the onset and exacerbation of mood disorders, as adolescents often struggle to sustain positive emotions while exhibiting an exaggerated response to negative stimuli. Impairments in these regulatory mechanisms, along with the reliance on maladaptive regulatory strategies, are linked to various psychiatric disorders and play a significant role in their persistence [114].
This heightened emotional sensitivity increases the risk of early-onset mood disorders, which are often associated with greater symptom severity [115] and a poorer long-term prognosis [116]. Moreover, adolescent depression is strongly linked to an elevated risk of suicide, emphasizing the need for early and targeted interventions. The combination of impaired emotion regulation and a tendency toward self-focused rumination further exacerbates vulnerability to depression and increases the risk of suicide attempts [117].
Recent advancements in brain imaging techniques have greatly enhanced the identification of neural markers linked to mood disorders in children and adolescents. Early detection of these conditions is essential for ensuring timely interventions and improving long-term outcomes. Advanced imaging methods allow for the identification of brain biomarkers that may indicate a predisposition to mood disorders even before the onset of overt clinical symptoms, providing valuable insights into early pathological changes and potential targets for preventive strategies.
A consistently observed finding is the reduced functional connectivity between the amygdala and the PFC, a pattern associated with impaired emotion regulation and heightened vulnerability to depression in young individuals. Neurodevelopmentally, adolescence is characterized by increased activity in limbic structures, particularly the amygdala, while the medial prefrontal cortex (mPFC), essential for regulating emotions, remains underdeveloped [118]. Consequently, adolescents experience emotions with greater intensity due to reduced top-down regulatory control by the mPFC [119].
Given these neurodevelopmental dynamics, understanding the neural basis of emotion regulation in adolescents is crucial for developing targeted interventions that mitigate the risk of mood disorders.
In this context, emotion regulation has become a primary focus in real-time functional magnetic resonance imaging neurofeedback (rt-fMRI-NF) research, a promising tool for enhancing emotion regulation capacities [120]. This method shows potential in achieving long-term symptom improvement in psychiatric conditions such as post-traumatic stress disorder (PTSD) [121], borderline personality disorder (BPD) [122], and attention deficit hyperactivity disorder (ADHD) [123]. Empirical studies indicate that healthy individuals can actively modulate their brain activity in response to rt-fMRI-NF across multiple regions implicated in emotion regulation, such as the amygdala, anterior insula, and ACC. Furthermore, brain activity regulation via rt-fMRI-NF has been demonstrated to be feasible in modulating both prefrontal–limbic connectivity and specific, individually targeted brain regions [120]. These findings highlight the potential of rt-fMRI-NF as a novel intervention for enhancing emotion regulation, particularly in vulnerable populations such as adolescents. Early identification of at-risk individuals is essential to implementing preventive and therapeutic strategies that may mitigate long-term consequences. In this context, neuroimaging has emerged as a powerful tool for detecting structural and functional brain alterations linked to mood disorders before clinically evident symptoms appear. It has also facilitated the identification of biomarkers associated with pediatric bipolar disorder (PBD), enhancing early diagnosis and treatment precision. Numerous neuroimaging studies have documented neuroanatomical abnormalities in PBD, though findings remain inconsistent, likely due to the intrinsic complexity and heterogeneity of the disorder itself [124]. The study by Otten and Meeter [125] found that bipolar disorder (BD) is associated with a reduction in hippocampal volume, particularly in early-onset cases. These findings suggest that hippocampal abnormalities may contribute to BD pathophysiology from its earliest stages, offering important insights for early intervention and targeted therapies. Another study by Kiani et al. [126] has identified notable white matter alterations in PBD, particularly a reduction in fractional anisotropy (FA) within the ACC, anterior corona radiata, and the genu of the corpus callosum, suggesting a link between these structural changes and clinical symptoms. Additionally, the uncinate fasciculus demonstrated atypical developmental patterns, emphasizing its potential role in the disorder. In terms of structural connectivity, graph analysis revealed extensive disruptions, particularly in the OFC, frontal gyrus, and basal ganglia. A key finding was the weakened connectivity between the prefrontal and limbic regions, which may underlie the emotional instability frequently observed in PBD. These structural and connectivity alterations could serve as potential neurobiological biomarkers, aiding in early diagnosis, risk assessment, and the development of targeted therapeutic interventions.
For children and adolescents with mood disorders that do not respond to conventional treatments, advanced brain imaging techniques are proving to be invaluable for refining therapeutic strategies and improving clinical outcomes. By offering a deeper understanding of the neural circuits involved in these conditions, it enables more precise and personalized interventions.
One significant application is in targeting dysfunctional neural pathways. By identifying the specific brain regions associated with symptoms, these imaging techniques help guide neuromodulatory treatments such as repetitive transcranial magnetic stimulation (rTMS), which may restore activity in affected areas and offer relief for adolescents with treatment-resistant depression [127].
Another major advantage is optimizing pharmacological interventions [128]. By identifying biomarkers predictive of treatment response, neuroimaging assists clinicians in selecting the most suitable medication, reducing the time spent on ineffective therapies and lowering the risk of adverse effects. This precision medicine approach moves psychiatric treatment toward a model where interventions are tailored to individual neurobiological profiles.
Moreover, neuroimaging is advancing the development of innovative therapies. Research has supported the use of ketamine for severe depression in young patients, and brain imaging may help identify individuals who are most likely to benefit from this treatment [129]. Unlike traditional antidepressants, which can take weeks or months to exert their effects, ketamine has been shown to induce rapid improvements in mood and suicidality, with some patients experiencing significant changes within just a few hours after administration, sometimes as early as four hours post-treatment [130]. By integrating neuroimaging into clinical practice, healthcare providers can design more effective and individualized treatment plans, offering new possibilities for children and adolescents struggling with mood disorders that do not respond to standard care.
Brain imaging has become an essential tool in the follow-up of pediatric patients with mood disorders, offering valuable insights into the course of the illness and treatment response. By detecting subtle changes in brain activity before symptoms reappear, it allows for the early identification of relapse risk, enabling timely interventions to prevent full recurrence [131]. This proactive approach can improve long-term outcomes and reduce the burden of chronic mood instability. Moreover, advanced imaging techniques provide an objective assessment of treatment effectiveness, helping clinicians evaluate how the brain responds to different pharmacological and psychotherapeutic interventions. Beyond monitoring treatment response, brain imaging also enhances our understanding of the neurobiological factors that contribute to disease persistence [132]. Identifying these underlying mechanisms can lead to the development of personalized prevention strategies, minimizing the risk of long-term impairment.
While conventional MRI and PET approaches have laid the foundation for pediatric neuroimaging, several advanced techniques promise to deepen our understanding of brain–behavior relationships and enable earlier, more precise clinical interventions.
In addition to fMRI, PET, DTI, and sMRI, emerging techniques such as magnetoencephalography (MEG), magnetic resonance spectroscopy (MRS), high-density EEG with source localization, and hybrid MR-PET systems are gaining traction in pediatric research. MEG offers millisecond temporal resolution of circuit dynamics; MRS quantifies neurochemical changes (e.g., GABA, glutamate); EEG source localization can non-invasively track functional connectivity in free-behaving children; and MR-PET combines molecular and structural imaging for unparalleled insight into neurotransmitter metabolism. By integrating these emerging modalities, each with its unique strengths in temporal resolution, biochemical specificity, or hybrid imaging potential, researchers can construct a richer, multimodal portrait of pediatric brain development. Even the brief inclusion of representative plates or example studies for MEG, MRS, EEG source maps, and MR-PET underscores the field’s trajectory toward truly comprehensive, translational neuroimaging.
Representative plates are high-quality images, often arranged as multi-panel figures, that serve as visual exemplars of the key methods, findings, or analytical steps used to link brain structure/function with clinical or behavioral measures. They may facilitate understanding the imaging modality (e.g., what an fMRI activation map looks like in children performing an emotion-processing task), clarifying the definition of regions or pathways (e.g., overlays of an anatomical atlas on structural images), and appreciating the correlation between imaging metrics and clinical/behavioral scores (e.g., scatterplots of fractional anisotropy vs. attention performance). In other words, representative plates can be considered visual examples of how neuroimaging data are acquired, processed, and statistically linked to meaningful clinical and behavioral endpoints in pediatric populations. Figure 3 presents an example of a task-based pediatric fMRI activation map.
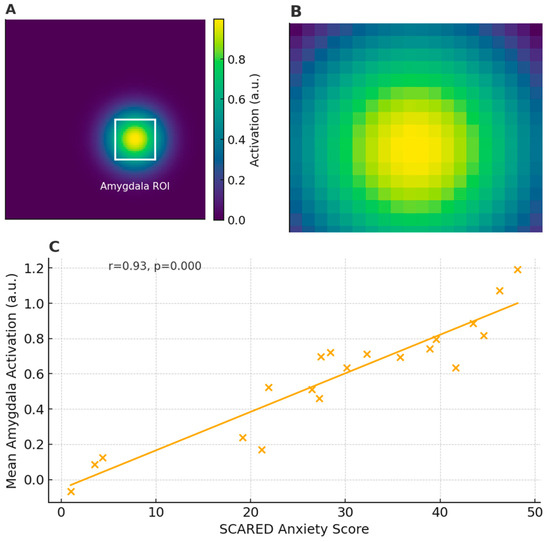
Figure 3.
Example of task-based pediatric fMRI activation map: (A) Pediatric fMRI activation map overlaid on pediatric template; white box indicates amygdala ROI (Region of Interest). (B) Zoomed-in view of amygdala ROI. (C) Scatterplot of mean amygdala activation vs. SCARED (Screen for Child Anxiety-Related Emotional Disorders) anxiety scores.
In this figure, the amygdala ROI (Region of Interest) refers to a rectangular zone placed over the amygdala, which plays a key role in processing emotions such as fear and anxiety. By averaging the BOLD (blood-oxygen-level-dependent) signal within this ROI, researchers obtain a single value (e.g., mean activation intensity) that reflects how strongly the amygdala responds during the task across each subject. This approach reduces noise from other brain regions and allows a direct comparison between subjects or conditions. The Screen for Child Anxiety-Related Emotional Disorders (SCARED) is a standardized, parent- and/or self-report questionnaire used to assess symptoms of anxiety in children and adolescents. It consists of 41 items across five subscales (panic/somatic, generalized anxiety, separation anxiety, social phobia, and school phobia), each rated on a three-point scale (0 = “Not True or Hardly Ever True”, 1 = “Somewhat True or Sometimes True”, 2 = “Very True or Often True”). The total scores range from 0 to 82, with higher scores indicating greater anxiety symptomatology. In the scatterplot (Panel C), each child’s total SCARED score is plotted against their mean amygdala activation, allowing us to see how neural reactivity relates to clinically assessed anxiety levels. A strong positive correlation (r = 0.93, p < 0.001) suggests that children reporting more anxiety symptoms also exhibit higher amygdala responses during the emotional-processing task.
7. Limitations in Neuroimaging Techniques
Despite the significant advancements in neuroimaging, its application in studying the relationship between brain activity, emotions, and behavior remains complex. While these techniques provide valuable insights into which brain regions are activated during emotional processing, the connection between neural activity and subjective emotional experience is not always straightforward. Emotions emerge from intricate interactions between multiple brain networks, and their expression can vary depending on context, individual differences, and environmental influences.
Interpreting neuroimaging data is further complicated by the fact that changes in brain activity in specific regions may not be exclusive to a single psychiatric condition or cognitive process. For instance, alterations observed in areas such as the prefrontal cortex, amygdala, or hippocampus may be associated with multiple disorders, including anxiety, depression, or bipolar disorder. This overlapping neural signature makes it difficult to determine whether observed changes are disorder-specific or reflect broader variations in brain function [133].
Another major challenge is differentiating between normal neurodevelopment and pathological alterations. The pediatric brain is highly plastic and continuously evolving, with structural and functional modifications occurring naturally during growth. Some brain changes identified in neuroimaging studies may reflect typical developmental processes rather than early markers of psychiatric disorders. As a result, establishing clear-cut criteria to distinguish between normative and pathological brain alterations remains a significant limitation in pediatric research [134].
Moreover, sample size limitations and population heterogeneity present challenges for the robustness and generalizability of findings. Many neuroimaging studies involve small or clinically diverse samples, leading to variability in results and difficulties in replicating findings across different populations. Additionally, psychiatric conditions are often heterogeneous, with symptoms manifesting differently among individuals, further complicating efforts to establish universal neural biomarkers [135].
Beyond biological factors, socio-cultural and environmental influences play a critical role in shaping brain development and mental health. However, these variables are often underrepresented or inadequately controlled in neuroimaging studies. Differences in socioeconomic status, educational background, early-life stress, and cultural upbringing can significantly impact both brain function and mental health outcomes, yet their effects are frequently overlooked. This selection bias limits the applicability of findings to broader populations and may introduce confounding factors that affect the interpretation of results.
Additionally, technological and methodological constraints pose further obstacles, particularly when studying young children. Techniques such as fMRI require participants to remain still for extended periods, which can be particularly challenging for young children or those with mood disorders and emotional dysregulation. Motion artifacts, compliance difficulties, and variations in attentional capacity can reduce the reliability of neuroimaging data in pediatric populations, making it difficult to draw definitive conclusions [136].
While neuroimaging remains an invaluable tool for advancing our understanding of the neural mechanisms underlying emotion and behavior, these methodological and conceptual challenges highlight the need for multidisciplinary approaches, improved study designs, and larger, more representative samples. Addressing these limitations is essential for ensuring that neuroimaging findings translate into meaningful clinical applications, particularly in the early identification and treatment of psychiatric disorders in children and adolescents.
8. Conclusions and Future Directions
Neuroimaging has become an essential tool for exploring the intricate relationship between brain development, emotional regulation, and mood disorders in children and adolescents. Research has consistently demonstrated structural and functional changes in key neural circuits, including the prefrontal cortex, amygdala, and limbic system, shedding light on the neurobiological underpinnings of emotional dysregulation. These discoveries have paved the way for early diagnosis and more tailored therapeutic approaches, enhancing treatment strategies for young patients with mood disorders.
However, several challenges remain. The interpretation of neuroimaging data is complicated by individual differences in brain maturation, the overlapping characteristics of various psychiatric conditions, and technical limitations such as motion artifacts in pediatric studies. To overcome these hurdles, future research should prioritize the integration of multiple neuroimaging techniques, leverage Artificial Intelligence for more refined data analysis, and conduct large-scale longitudinal studies to better capture the evolving nature of brain development.
Moving forward, the field offers exciting possibilities. The combination of advanced neuroimaging with Artificial Intelligence (AI)-driven analytics may enhance diagnostic accuracy and help identify early biomarkers for mood disorders. Additionally, innovative treatment models, such as real-time neurofeedback and personalized interventions, hold promise for improving clinical outcomes. Building on identified gaps, we propose (1) longitudinal multimodal imaging studies to chart normative and pathological trajectories; (2) AI-driven analytics for individualized biomarker discovery; (3) the integration of real-time neurofeedback into clinical protocols; (4) standardized pipelines for combining nutrition/gut–brain axis assessments with brain imaging; and (5) pediatric-specific ethical frameworks and trial designs to accelerate closed-loop interventions.
Conducting prospective, repeated-measures studies that combine structural MRI, diffusion tensor imaging (DTI), functional MRI (task-based and resting-state), and even PET or MR spectroscopy allows us to chart both normative brain development (e.g., synaptic pruning trajectories from infancy through adolescence) and the divergent paths seen in children who go on to develop mood or anxiety disorders. For example, leveraging cohorts like the NIH MRI Study of Normal Brain Development alongside specialized clinical samples can help disentangle age-related changes in prefrontal-amygdala connectivity from disease-related alterations [137].
Machine learning frameworks, ranging from random forests and support vector machines to deep learning convolutional neural networks, can ingest high-dimensional imaging and behavioral data to uncover latent phenotypes or “brain signatures” predictive of treatment response. For instance, recent studies have shown that gradient-boosted decision trees applied to DTI metrics can identify children at highest risk for persistent anxiety with >85% accuracy [138]. Embedding these algorithms within clinical workflows could one day enable “precision neuropsychiatry,” where each child’s unique connectivity profile informs a targeted intervention plan.
Real-time fMRI neurofeedback (rt-fMRI-NF) and EEG-based neurofeedback platforms allow children to see, in seconds, how their own brain activity (e.g., amygdala or prefrontal cortex activation) changes as they engage in emotion regulation strategies. Pilot trials in adolescents with major depressive disorder demonstrate that training to down-regulate amygdala hyperactivity via rt-fMRI-NF can produce sustained mood improvements at follow-up [139]. Embedding these protocols into existing cognitive behavioral therapy sessions may enhance skill acquisition and long-term resilience.
Robust evidence links early-life nutrition and microbiota-derived neuroactive metabolites to myelination, neurotransmitter synthesis, and emotional regulation. Deficiencies in iron or docosahexaenoic acid (DHA) impair white matter integrity and synaptic plasticity, increasing the risk for mood and attention disorders. The bidirectional gut–brain axis modulates neuroinflammation and HPA-axis activity, suggesting that combined dietary and microbiota-targeted interventions might serve as effective adjunctive therapies in pediatric mood disorders. Nutrition and the gut microbiome critically shape neurodevelopment: iron and omega-3 fatty acid deficiencies impair myelination, while microbial metabolites like short-chain fatty acids modulate neuroinflammation. We advocate for harmonized protocols that collect dietary records, blood biomarkers (e.g., ferritin, DHA levels), stool microbiome sequencing, and multimodal imaging in the same participants. Such pipelines, exemplified by the emerging “NutriBrain” project, will permit direct correlations between nutrient status, microbial diversity, and imaging metrics like fractional anisotropy or functional connectivity [140,141,142].
Children require special protections in neuroimaging research, such as minimizing sedation, limiting radiation exposure (for PET), and securing both parental consent and age-appropriate assent. It is necessary to recommend developing standardized ethical guidelines that address data privacy (especially as AI analytics become more powerful), the minimal-risk threshold for repeated scans, and culturally sensitive consent processes. Furthermore, adaptive trial designs (e.g., Bayesian or N-of-1 trials) can tailor intervention parameters, such as the neurofeedback target region or dietary supplement dose, in real time based on each child’s interim imaging and behavioral data, thereby closing the loop between measurement and treatment.
By advancing research along these five vectors, rooted in rigorous, pediatric-tailored methodology and clear translational pathways, we can move beyond descriptive neuroimaging toward interventions that dynamically adapt to each child’s developmental trajectory and clinical needs. Finally, by embracing technological advancements and fostering interdisciplinary collaboration, future research can refine our understanding of pediatric mood disorders and develop more effective, individualized treatment approaches.
Author Contributions
Conceptualization, G.M. and M.M. (Marianna Mazza).; methodology, G.M. and M.M. (Marianna Mazza); resources, E.G., G.T., G.S., and R.P.; data curation, C.B., C.C., F.B., F.M.L., M.A., M.B.A., M.M. (Miriam Milintenda), and O.M.; writing—original draft preparation, G.M., G.T., and M.M. (Marianna Mazza); writing—review and editing, G.M. and M.M. (Marianna Mazza); supervision, G.M., E.G., G.S., R.P., G.T., and M.M. (Marianna Mazza). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AI | Artificial Intelligence |
| WHO | World Health Organization |
| MRI | magnetic resonance |
| sMRI | structural magnetic resonance imaging |
| fMRI | functional magnetic resonance imaging |
| PET | positron emission tomography |
| DTI | diffusion tensor imaging |
| PFC | prefrontal cortex |
| DLPFC | dorsolateral prefrontal cortex |
| VMPFC | ventromedial prefrontal cortex |
| OFC | orbitofrontal cortex |
| ACC | anterior cingulate cortex |
| ADHD | attention deficit hyperactivity disorder |
| CCAS | cerebellar cognitive affective syndrome |
| BDNF | brain-derived neurotrophic factor |
| BOLD | blood-oxygen-level-dependent |
| CBT | cognitive behavioral therapy |
| RF-CBT | rumination-focused cognitive behavioral therapy |
| MDD | major depressive disorder |
| CBT-I | CBT for insomnia |
| CRS | circadian rhythm support |
| MBCT-C | mindfulness-based cognitive therapy for children |
| BMRM | body–mind relaxation meditation |
| MBI | mindfulness-based intervention |
| MRS | magnetic resonance spectroscopy |
| mPFC | medial prefrontal cortex |
| rt-fMRI-NF | real-time functional magnetic resonance imaging neurofeedback |
| PTSD | post-traumatic stress disorder |
| BPD | borderline personality disorder |
| PBD | pediatric bipolar disorder |
| BD | bipolar disorder |
| FA | fractional anisotropy |
| rTMS | repetitive transcranial magnetic stimulation |
References
- Wu, Y.; Zhong, Y.; Zhang, G.; Wang, C.; Zhang, N.; Chen, Q. Distinct functional patterns in child and adolescent bipolar and unipolar depression during emotional processing. Cereb. Cortex 2024, 34, bhad461. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Kurian, S.; Kumar, R.; Arnold, L.E.; Simkin, D.R. Mood disorders in youth: Complementary and integrative medicine. Child Adolesc. Psychiatr. Clin. N. Am. 2023, 32, 367–394. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation (IHME). Global Burden of Disease Study 2024 Results; IHME: Seattle, WA, USA, 2024. [Google Scholar]
- Shahidullah, J.D.; Roberts, H.; Parkhurst, J.; Ballard, R.; Mautone, J.A.; Carlson, J.S. State of the evidence for use of psychotropic medications in school-age youth. Children 2023, 10, 1454. [Google Scholar] [CrossRef] [PubMed]
- Bore, M.C.; Liu, X.; Huang, X.; Kendrick, K.M.; Zhou, B.; Zhang, J.; Klugah-Brown, B.; Becker, B. Common and separable neural alterations in adult and adolescent depression—Evidence from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 2024, 164, 105835. [Google Scholar] [CrossRef]
- La Buissonniere-Ariza, V.; Fitzgerald, K.; Meoded, A.; Williams, L.L.; Liu, G.; Goodman, W.K.; Storch, E.A. Neural correlates of cognitive behavioral therapy response in youth with negative valence disorders: A systematic review of the literature. J. Affect. Disord. 2021, 282, 1288–1307. [Google Scholar] [CrossRef]
- Malezieux, M.; Klein, A.S.; Gogolla, N. Neural circuits for emotion. Annu. Rev. Neurosci. 2023, 46, 211–231. [Google Scholar] [CrossRef]
- Chauhan, P.; Rathawa, A.; Jethwa, K.; Mehra, S. The Anatomy of the Cerebral Cortex. In Cerebral Ischemia; Pluta, R., Ed.; Exon Publications: Brisbane, Australia, 2021; Chapter 1. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574582/ (accessed on 15 February 2025).
- Alizamini, M.M.; Fattahi, M.; Sayehmiri, F.; Haghparast, A.; Liang, J. Regulatory role of PFC corticotropin-releasing factor system in stress-associated depression disorders: A systematic review. Cell. Mol. Neurobiol. 2023, 43, 1785–1797. [Google Scholar] [CrossRef]
- Alexander, L.; Wood, C.M.; Roberts, A.C. The ventromedial prefrontal cortex and emotion regulation: Lost in translation? J. Physiol. 2023, 601, 37–50. [Google Scholar] [CrossRef]
- Rolls, E.T.; Deco, G.; Huang, C.C.; Feng, J. The human orbitofrontal cortex, vmPFC, and anterior cingulate cortex effective connectome: Emotion, memory, and action. Cereb. Cortex 2022, 33, 330–356. [Google Scholar] [CrossRef]
- Izquierdo, I.; Furini, C.R.; Myskiw, J.C. Fear memory. Physiol. Rev. 2016, 96, 695–750. [Google Scholar] [CrossRef]
- Ghosh, A.; Basu, D.; Khandelwal, N.; Ahuja, C.K.; Bn, S.; Rana, D. Risk, reversibility and resilience of brain circuitries linked to opioid dependence: A diffusion tensor imaging study of actively opioid-using subjects and three comparison groups. Asian J. Psychiatr. 2019, 40, 107–115. [Google Scholar] [CrossRef]
- Gardini, S.; Venneri, A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res. Bull. 2012, 87, 205–211. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Pakusch, J.; Ernst, T.M.; Timmann, D. Cerebellum and emotion memory. Adv. Exp. Med. Biol. 2022, 1378, 53–73. [Google Scholar] [CrossRef] [PubMed]
- Laricchiuta, D.; Picerni, E.; Cutuli, D.; Petrosini, L. Cerebellum, embodied emotions, and psychological traits. Adv. Exp. Med. Biol. 2022, 1378, 255–269. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, F.; Orsi, L. Cerebellum and emotion recognition. Adv. Exp. Med. Biol. 2022, 1378, 41–51. [Google Scholar] [CrossRef]
- Clausi, S.; Siciliano, L.; Olivito, G.; Leggio, M. Cerebellum and emotion in social behavior. Adv. Exp. Med. Biol. 2022, 1378, 235–253. [Google Scholar] [CrossRef]
- Hsueh, B.; Chen, R.; Jo, Y.; Tang, D.; Raffiee, M.; Kim, Y.S.; Inoue, M.; Randles, S.; Ramakrishnan, C.; Patel, S.; et al. Cardiogenic control of affective behavioural state. Nature 2023, 615, 292–299. [Google Scholar] [CrossRef]
- Nadeau, S.E. Neural mechanisms of emotions, alexithymia, and depression. Handb. Clin. Neurol. 2021, 183, 299–313. [Google Scholar] [CrossRef]
- Samara, Z.; Evers, E.A.T.; Peeters, F.; Uylings, H.B.M.; Rajkowska, G.; Ramaekers, J.G.; Stiers, P. Orbital and medial prefrontal cortex functional connectivity of major depression vulnerability and disease. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Deng, H.; Xiao, X. The insular cortex: An interface between sensation, emotion and cognition. Neurosci. Bull. 2024, 40, 1763–1773. [Google Scholar] [CrossRef]
- Centanni, S.W.; Janes, A.C.; Haggerty, D.L.; Atwood, B.; Hopf, F.W. Better living through understanding the insula: Why subregions can make all the difference. Neuropharmacology 2021, 198, 108765. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Corzo, J.C.; Cosio van-Hasselt, M.; Escobar, D.; Vázquez-Roque, R.A.; Flores, G. Mirror neurons and empathy-related regions in psychopathy: Systematic review, meta-analysis, and a working model. Soc. Neurosci. 2022, 17, 462–479. [Google Scholar] [CrossRef] [PubMed]
- McBenedict, B.; Petrus, D.; Pires, M.P.; Pogodina, A.; Arrey Agbor, D.B.; Ahmed, Y.A.; Castro Ceron, J.I.; Balaji, A.; Abrahão, A.; Lima Pessôa, B. The role of the insula in chronic pain and associated structural changes: An integrative review. Cureus 2024, 16, e58511. [Google Scholar] [CrossRef]
- Ghahremani, D.G.; Pochon, J.F.; Diaz, M.P.; Tyndale, R.F.; Dean, A.C.; London, E.D. Nicotine dependence and insula subregions: Functional connectivity and cue-induced activation. Neuropsychopharmacology 2023, 48, 936–945. [Google Scholar] [CrossRef]
- Gerosa, M.; Canessa, N.; Morawetz, C.; Mattavelli, G. Cognitive reappraisal of food craving and emotions: A coordinate-based meta-analysis of fMRI studies. Soc. Cogn. Affect. Neurosci. 2024, 19, nsad077. [Google Scholar] [CrossRef]
- Schacht, J.P. Stress, cues, and craving: Does the insula hold the key to understanding stress-induced drinking in alcohol use disorder? Biol. Psychiatry 2024, 95, 200–201. [Google Scholar] [CrossRef]
- Catani, M.; Dell’Acqua, F.; Thiebaut de Schotten, M. A revised limbic system model for memory, emotion and behaviour. Neurosci. Biobehav. Rev. 2013, 37, 1724–1737. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding emotions: Origins and roles of the amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Struct. Funct. 2023, 228, 1201–1257. [Google Scholar] [CrossRef] [PubMed]
- Terranova, J.I.; Yokose, J.; Osanai, H.; Marks, W.D.; Yamamoto, J.; Ogawa, S.K.; Kitamura, T. Hippocampal-amygdala memory circuits govern experience-dependent observational fear. Neuron 2022, 110, 1416–1431.e13. [Google Scholar] [CrossRef] [PubMed]
- Giotakos, O. Neurobiology of emotional trauma. Psychiatriki 2020, 31, 162–171. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Joyce, M.K.P.; Roberts, A.C. The aversive lens: Stress effects on the prefrontal-cingulate cortical pathways that regulate emotion. Neurosci. Biobehav. Rev. 2023, 145, 105000. [Google Scholar] [CrossRef]
- Oane, I.; Barborica, A.; Mindruta, I.R. Cingulate cortex: Anatomy, structural and functional connectivity. J. Clin. Neurophysiol. 2023, 40, 482–490. [Google Scholar] [CrossRef]
- Alexander, W.H.; Brown, J.W. The role of the anterior cingulate cortex in prediction error and signaling surprise. Top. Cogn. Sci. 2019, 11, 119–135. [Google Scholar] [CrossRef]
- Valentinova, K.; Acuña, M.A.; Ntamati, N.R.; Nevian, N.E.; Nevian, T. An amygdala-to-cingulate cortex circuit for conflicting choices in chronic pain. Cell Rep. 2023, 42, 113125. [Google Scholar] [CrossRef]
- Wu, Y.E.; Hong, W. Neural basis of prosocial behavior. Trends Neurosci. 2022, 45, 749–762. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Gong, W.; Qiu, J.; Zhou, C.; Zhang, J.; Lv, W.; Ruan, H.; Wei, D.; Cheng, K. Functional connectivity of the anterior cingulate cortex in depression and in health. Cereb. Cortex 2019, 29, 3617–3630. [Google Scholar] [CrossRef]
- Fornito, A.; Yücel, M.; Dean, B.; Wood, S.J.; Pantelis, C. Anatomical abnormalities of the anterior cingulate cortex in schizophrenia: Bridging the gap between neuroimaging and neuropathology. Schizophr. Bull. 2009, 35, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Fortier, A.; Dumais, A.; Athanassiou, M.; Tikàsz, A.; Potvin, S. Dysconnectivity between the anterior insula and the dorsal anterior cingulate cortex during an emotion go/nogo paradigm is associated with aggressive behaviors in male schizophrenia patients. Psychiatry Res. Neuroimaging. 2023, 328, 111579. [Google Scholar] [CrossRef] [PubMed]
- Pronier, É.; Morici, J.F.; Girardeau, G. The role of the hippocampus in the consolidation of emotional memories during sleep. Trends Neurosci. 2023, 46, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Duman, R. Neuroplasticity in cognitive and psychological mechanisms of depression: An integrative model. Mol. Psychiatry 2020, 25, 530–543. [Google Scholar] [CrossRef]
- Peng, S.; Yang, X.; Meng, S.; Liu, F.; Lv, Y.; Yang, H.; Kong, Y.; Xie, W.; Li, M. Dual circuits originating from the ventral hippocampus independently facilitate affective empathy. Cell Rep. 2024, 43, 114277. [Google Scholar] [CrossRef]
- Shi, H.J.; Wang, S.; Wang, X.P.; Zhang, R.X.; Zhu, L.J. Hippocampus: Molecular, cellular, and circuit features in anxiety. Neurosci. Bull. 2023, 39, 1009–1026. [Google Scholar] [CrossRef]
- Fischer, S. The hypothalamus in anxiety disorders. Handb. Clin. Neurol. 2021, 180, 149–160. [Google Scholar] [CrossRef]
- Menke, A. The HPA axis as target for depression. Curr. Neuropharmacol. 2024, 22, 904–915. [Google Scholar] [CrossRef]
- Osakada, T.; Yan, R.; Jiang, Y.; Wei, D.; Tabuchi, R.; Dai, B.; Wang, X.; Zhao, G.; Wang, C.X.; Liu, J.J.; et al. A dedicated hypothalamic oxytocin circuit controls aversive social learning. Nature 2024, 626, 347–356. [Google Scholar] [CrossRef]
- Liu, Z.; Le, Q.; Lv, Y.; Chen, X.; Cui, J.; Zhou, Y.; Cheng, D.; Ma, C.; Su, X.; Xiao, L.; et al. A distinct D1-MSN subpopulation down-regulates dopamine to promote negative emotional state. Cell Res. 2022, 32, 139–156. [Google Scholar] [CrossRef]
- Choi, T.Y.; Jeon, H.; Jeong, S.; Kim, E.J.; Kim, J.; Jeong, Y.H.; Kang, B.; Choi, M.; Koo, J.W. Distinct prefrontal projection activity and transcriptional state conversely orchestrate social competition and hierarchy. Neuron 2024, 112, 611–627.e8. [Google Scholar] [CrossRef] [PubMed]
- Zinsmaier, A.K.; Dong, Y.; Huang, Y.H. Cocaine-induced projection-specific and cell type-specific adaptations in the nucleus accumbens. Mol. Psychiatry 2022, 27, 669–686. [Google Scholar] [CrossRef] [PubMed]
- Bayassi-Jakowicka, M.; Lietzau, G.; Czuba, E.; Patrone, C.; Kowiański, P. More than addiction—The nucleus accumbens contribution to development of mental disorders and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 2618. [Google Scholar] [CrossRef]
- Noble, K.G.; Hart, E.R.; Sperber, J.F. Socioeconomic disparities and neuroplasticity: Moving toward adaptation, intersectionality, and inclusion. Am. Psychol. 2021, 76, 1486–1495. [Google Scholar] [CrossRef]
- Connor, S.A.; Siddiqui, T.J. Synapse organizers as molecular codes for synaptic plasticity. Trends Neurosci. 2023, 46, 971–985. [Google Scholar] [CrossRef]
- Taylor, K.R.; Monje, M. Neuron-oligodendroglial interactions in health and malignant disease. Nat. Rev. Neurosci. 2023, 24, 733–746. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W.; Scofield, M.D. Astrocyte regulation of synaptic signaling in psychiatric disorders. Neuropsychopharmacology 2023, 48, 21–36. [Google Scholar] [CrossRef]
- Vaidya, N.; Marquand, A.F.; Nees, F.; Siehl, S.; Schumann, G. The impact of psychosocial adversity on brain and behaviour: An overview of existing knowledge and directions for future research. Mol. Psychiatry 2024, 29, 3245–3267. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.H.; Münchau, A.; Mall, V. Neuronale Plastizität und Neuromodulation in der Kinderneurologie [Neuronal plasticity and neuromodulation in pediatric neurology]. Nervenarzt 2018, 89, 1131–1139. (In German) [Google Scholar] [CrossRef]
- François, C.; Ripollés, P.; Ferreri, L.; Muchart, J.; Sierpowska, J.; Fons, C.; Solé, J.; Rebollo, M.; Zatorre, R.J.; Garcia-Alix, A.; et al. Right structural and functional reorganization in four-year-old children with perinatal arterial ischemic stroke predict language production. eNeuro 2019, 6, 0447-18. [Google Scholar] [CrossRef]
- Olulade, O.A.; Seydell-Greenwald, A.; Chambers, C.E.; Turkeltaub, P.E.; Dromerick, A.W.; Berl, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef] [PubMed]
- Craig, B.T.; Hilderley, A.; Kinney-Lang, E.; Long, X.; Carlson, H.L.; Kirton, A. Developmental neuroplasticity of the white matter connectome in children with perinatal stroke. Neurology 2020, 95, e2476–e2486. [Google Scholar] [CrossRef]
- Siffredi, V.; Preti, M.G.; Kebets, V.; Obertino, S.; Leventer, R.J.; McIlroy, A.; Wood, A.G.; Anderson, V.; Spencer-Smith, M.M.; Van De Ville, D. Structural neuroplastic responses preserve functional connectivity and neurobehavioural outcomes in children born without corpus callosum. Cereb. Cortex 2021, 31, 1227–1239. [Google Scholar] [CrossRef]
- Vandormael, C.; Schoenhals, L.; Hüppi, P.S.; Filippa, M.; Borradori Tolsa, C. Language in preterm born children: Atypical development and effects of early interventions on neuroplasticity. Neural Plast. 2019, 2019, 6873270. [Google Scholar] [CrossRef]
- Ver Loren van Themaat, A.H.; Hemager, N.; Korsgaard Johnsen, L.; Klee Burton, B.; Ellersgaard, D.; Christiani, C.; Brandt, J.; Gregersen, M.; Falkenberg Krantz, M.; Søborg Spang, K.; et al. Development of visual attention from age 7 to age 12 in children with familial high risk for schizophrenia or bipolar disorder. Schizophr. Res. 2021, 228, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.C.; Ketchabaw, W.T.; Turkeltaub, P.E. Plasticity of the language system in children and adults. Handb. Clin. Neurol. 2022, 184, 397–414. [Google Scholar] [CrossRef]
- Ismail, F.Y.; Saleem, G.T.; Ljubisavljevic, M.R. Brain data in pediatric disorders of consciousness: Special considerations. J. Clin. Neurophysiol. 2022, 39, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sydnor, V.J.; Larsen, B.; Bassett, D.S.; Alexander-Bloch, A.; Fair, D.A.; Liston, C.; Mackey, A.P.; Milham, M.P.; Pines, A.; Roalf, D.R.; et al. Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron 2021, 109, 2820–2846. [Google Scholar] [CrossRef]
- Higa, G.S.V.; Viana, F.J.C.; Francis-Oliveira, J.; Cruvinel, E.; Franchin, T.S.; Marcourakis, T.; Ulrich, H.; De Pasquale, R. Serotonergic neuromodulation of synaptic plasticity. Neuropharmacology 2024, 257, 110036. [Google Scholar] [CrossRef]
- Puetz, V.B.; Viding, E.; Maguire, E.A.; Mechelli, A.; Armbruster-Genç, D.; Sharp, M.; Rankin, G.; Gerin, M.I.; McCrory, E.J. Functional brain plasticity following childhood maltreatment: A longitudinal fMRI investigation of autobiographical memory processing. Dev. Psychopathol. 2023, 35, 1382–1389. [Google Scholar] [CrossRef]
- Komori, T.; Okamura, K.; Ikehara, M.; Yamamuro, K.; Endo, N.; Okumura, K.; Yamauchi, T.; Ikawa, D.; Ouji-Sageshima, N.; Toritsuka, M.; et al. Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior. Mol. Psychiatry 2024, 29, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.O.K.; Li, W.H.C.; Leung, Y.D.; Cheung, T.C.K.; Chiu, S.Y.; Pong, M.S.Y.; Chan, G.C.F. The feasibility, acceptability, and potential efficacy of a musical training program in promoting neuroplasticity among survivors of pediatric brain tumors: A cohort study. Eur. J. Oncol. Nurs. 2025, 76, 102851. [Google Scholar] [CrossRef] [PubMed]
- Lannon, M.M.; Duda, T.; Martyniuk, A.; Engels, P.T.; Sharma, S.V. Pediatric craniocerebral gunshot injuries: A National Trauma Database study. J. Trauma Acute Care Surg. 2022, 92, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, X.; Zhang, S.; Han, F. Neuroplasticity of children in autism spectrum disorder. Front. Psychiatry 2024, 15, 1362288. [Google Scholar] [CrossRef]
- Rebello, K.; Moura, L.M.; Bueno, A.P.A.; Picon, F.A.; Pan, P.M.; Gadelha, A.; Miguel, E.C.; Bressan, R.A.; Rohde, L.A.; Sato, J.R. Associations between family functioning and maternal behavior on default mode network connectivity in school-age children. Int. J. Environ. Res. Public Health 2022, 19, 6055. [Google Scholar] [CrossRef]
- Çalışkan, G.; Müller, A.; Albrecht, A. Long-term impact of early-life stress on hippocampal plasticity: Spotlight on astrocytes. Int. J. Mol. Sci. 2020, 21, 4999. [Google Scholar] [CrossRef]
- Ho, T.C.; King, L.S. Mechanisms of neuroplasticity linking early adversity to depression: Developmental considerations. Transl. Psychiatry 2021, 11, 517. [Google Scholar] [CrossRef]
- Oswald, L.M.; Dunn, K.E.; Seminowicz, D.A.; Storr, C.L. Early life stress and risks for opioid misuse: Review of data supporting neurobiological underpinnings. J. Pers. Med. 2021, 11, 315. [Google Scholar] [CrossRef]
- Lanza, G.; DelRosso, L.M.; Ferri, R. Sleep and homeostatic control of plasticity. Handb. Clin. Neurol. 2022, 184, 53–72. [Google Scholar] [CrossRef]
- Ugarte, G.; Piña, R.; Contreras, D.; Godoy, F.; Rubio, D.; Rozas, C.; Zeise, M.; Vidal, R.; Escobar, J.; Morales, B. Attention deficit-hyperactivity disorder (ADHD): From abnormal behavior to impairment in synaptic plasticity. Biology 2023, 12, 1241. [Google Scholar] [CrossRef]
- Patra, K.P.; Kumar, R. Screening for Depression and Suicide in Children; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Watson, C.; Ban, S. Body dysmorphic disorder in children and young people. Br. J. Nurs. 2021, 30, 160–164. [Google Scholar] [CrossRef]
- da Silva Chagas, L.; Sandre, P.C.; de Velasco, P.C.; Marcondes, H.; Ribeiro, E.; Ribeiro, N.C.A.; Serfaty, C.A.; Serfaty, C.A.; Ferreira, J.H.; Mauro, L.B.A. Neuroinflammation and brain development: Possible risk factors in COVID-19-infected children. Neuroimmunomodulation 2021, 28, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chagas, L.D.S.; Serfaty, C.A. The influence of microglia on neuroplasticity and long-term cognitive sequelae in long COVID: Impacts on brain development and beyond. Int. J. Mol. Sci. 2024, 25, 3819. [Google Scholar] [CrossRef]
- Lopatina, O.L.; Panina, Y.A.; Malinovskaya, N.A.; Salmina, A.B. Early life stress and brain plasticity: From molecular alterations to aberrant memory and behavior. Rev. Neurosci. 2020, 32, 131–142. [Google Scholar] [CrossRef]
- Phan, T.V.; Sima, D.; Smeets, D.; Ghesquière, P.; Wouters, J.; Vandermosten, M. Structural brain dynamics across reading development: A longitudinal MRI study from kindergarten to grade 5. Hum. Brain Mapp. 2021, 42, 4497–4509. [Google Scholar] [CrossRef]
- Page, C.E.; Biagiotti, S.W.; Alderman, P.J.; Sorrells, S.F. Immature excitatory neurons in the amygdala come of age during puberty. Dev. Cogn. Neurosci. 2022, 56, 101133. [Google Scholar] [CrossRef]
- Mammen, L.; Zlatopolsky, A.; Tu, N. Cochlear implantation in children with single-sided deafness under the age of 5 years: A review of current literature. Curr. Opin. Otolaryngol. Head Neck Surg. 2024, 32, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Mana, S.; Paillère Martinot, M.L.; Martinot, J.L. Brain imaging findings in children and adolescents with mental disorders: A cross-sectional review. Eur. Psychiatry 2010, 25, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Kuperman, S.; Gaffney, G.R.; Hamdan-Allen, G.; Preston, D.F.; Venkatesh, L. Neuroimaging in child and adolescent psychiatry. J. Am. Acad. Child. Adolesc. Psychiatry 1990, 29, 159–172. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Eliez, S.; Reiss, A.L. MRI neuroimaging of childhood psychiatric disorders: A selective review. J. Child Psychol. Psychiatry 2000, 41, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Hulvershorn, L.A.; Cullen, K.; Anand, A. Toward dysfunctional connectivity: A review of neuroimaging findings in pediatric major depressive disorder. Brain Imaging Behav. 2011, 5, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Lowe, V.J.; Weigand, S.D.; Wiste, H.J.; Senjem, M.L.; Knopman, D.S.; Shiung, M.M.; Gunter, J.L.; Boeve, B.F.; Kemp, B.J.; et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: Implications for sequence of pathological events in Alzheimer’s disease. Brain 2009, 132, 1355–1365. [Google Scholar] [CrossRef]
- Meyer, J.H.; Cervenka, S.; Kim, M.J.; Kreisl, W.C.; Henter, I.D.; Innis, R.B. Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 2020, 7, 1064–1074. [Google Scholar] [CrossRef]
- Feldman, H.M.; Yeatman, J.D.; Lee, E.S.; Barde, L.H.; Gaman-Bean, S. Diffusion tensor imaging: A review for pediatric researchers and clinicians. J. Dev. Behav. Pediatr. 2010, 31, 346–356. [Google Scholar] [CrossRef]
- Pavuluri, M.N.; Yang, S.; Kamineni, K.; Passarotti, A.M.; Srinivasan, G.; Harral, E.M.; Sweeney, J.A.; Zhou, X.J. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol. Psychiatry 2009, 65, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J.; Mattiello, J.; LeBihan, D. MR diffusion tensor spectroscopy and imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef]
- Dennis, E.L.; Disner, S.G.; Fani, N.; Salminen, L.E.; Logue, M.; Clarke, E.K.; Haswell, C.C.; Averill, C.L.; Baugh, L.A.; Bomyea, J.; et al. Altered white matter microstructural organization in posttraumatic stress disorder across 3047 adults: Results from the PGC-ENIGMA PTSD consortium. Mol. Psychiatry 2021, 26, 4315–4330. [Google Scholar] [CrossRef]
- Brooks, S.J.; Stein, D.J. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin. Neurosci. 2015, 17, 261–279. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Mayberg, H.S. Neuroimaging-based biomarkers for treatment selection in major depressive disorder. Dialogues Clin. Neurosci. 2014, 16, 479–490. [Google Scholar] [CrossRef]
- Sequeira, S.L.; Silk, J.S.; Ladouceur, C.D.; Hanson, J.L.; Ryan, N.D.; Morgan, J.K.; McMakin, D.L.; Kendall, P.C.; Dahl, R.E.; Forbes, E.E. Association of neural reward circuitry function with response to psychotherapy in youths with anxiety disorders. Am. J. Psychiatry 2021, 178, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Westlund Schreiner, M.; Jacobsen, A.M.; Farstead, B.W.; Miller, R.H.; Jacobs, R.H.; Thomas, L.R.; Bessette, K.L.; Pazdera, M.; Crowell, S.E.; Kaufman, E.A.; et al. Rumination induction task in fMRI: Effects of rumination-focused cognitive behavioral therapy and stability in youth. J. Affect. Disord. 2025, 372, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Villa, L.M.; Goodyer, I.M.; Tait, R.; Kelvin, R.; Reynolds, S.; Wilkinson, P.O.; Suckling, J. Cognitive behavioral therapy may have a rehabilitative, not normalizing, effect on functional connectivity in adolescent depression. J. Affect. Disord. 2020, 268, 1–11. [Google Scholar] [CrossRef]
- Leerssen, J.; Aghajani, M.; Bresser, T.; Rösler, L.; Winkler, A.M.; Foster-Dingley, J.C.; Van Someren, E.J.W. Cognitive, behavioral, and circadian rhythm interventions for insomnia alter emotional brain responses. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2024, 9, 60–69. [Google Scholar] [CrossRef]
- Yang, J.; Lei, D.; Suo, X.; Tallman, M.J.; Qin, K.; Li, W.; Bruns, K.M.; Blom, T.J.; Duran, L.R.P.; Cotton, S.; et al. A preliminary study of the effects of mindfulness-based cognitive therapy on structural brain networks in mood-dysregulated youth with a familial risk for bipolar disorder. Early Interv. Psychiatry 2022, 16, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Piguet, C.; Klauser, P.; Celen, Z.; James Murray, R.; Magnus Smith, M.; Merglen, A. Randomized controlled trial of a mindfulness-based intervention in adolescents from the general population: The Mindfulteen neuroimaging study protocol. Early Interv. Psychiatry 2022, 16, 891–901. [Google Scholar] [CrossRef]
- Chen, F.; Lv, X.; Fang, J.; Li, T.; Xu, J.; Wang, X.; Hong, Y.; Hong, L.; Wang, J.; Wang, W.; et al. Body-mind relaxation meditation modulates the thalamocortical functional connectivity in major depressive disorder: A preliminary resting-state fMRI study. Transl. Psychiatry 2021, 11, 546. [Google Scholar] [CrossRef]
- Joss, D.; Lazar, S.W.; Teicher, M.H. Effects of a mindfulness-based behavioral intervention for young adults with childhood maltreatment history on hippocampal morphometry: A pilot MRI study with voxel-based morphometry. Psychiatry Res. Neuroimaging 2020, 301, 111087. [Google Scholar] [CrossRef]
- Hafeman, D.M.; Ostroff, A.N.; Feldman, J.; Hickey, M.B.; Phillips, M.L.; Creswell, D.; Birmaher, B.; Goldstein, T.R. Mindfulness-based intervention to decrease mood lability in at-risk youth: Preliminary evidence for changes in resting state functional connectivity. J. Affect. Disord. 2020, 276, 23–29. [Google Scholar] [CrossRef]
- Ahmed, S.P.; Bittencourt-Hewitt, A.; Sebastian, C.L. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 2015, 15, 11–25. [Google Scholar] [CrossRef]
- Khafif, T.C.; Rotenberg, L.S.; Nascimento, C.; Beraldi, G.H.; Lafer, B. Emotion regulation in pediatric bipolar disorder: A meta-analysis of published studies. J. Affect. Disord. 2021, 285, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Jadidi, M.; Bell, E. The diagnosis of bipolar disorder in children and adolescents: Past, present and future. Bipolar Disord. 2023, 25, 469–477. [Google Scholar] [CrossRef]
- Post, R.M.; Grunze, H. The challenges of children with bipolar disorder. Medicina 2021, 57, 601. [Google Scholar] [CrossRef]
- Quevedo, K.; Yuan Teoh, J.; Engstrom, M.; Wedan, R.; Santana-Gonzalez, C.; Zewde, B.; Porter, D.; Cohen Kadosh, K. Amygdala circuitry during neurofeedback training and symptoms’ change in adolescents with varying depression. Front. Behav. Neurosci. 2020, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Carvalho, M.; Wu, W.C.; Cummings, K.A.; Clem, R.L. Optogenetic examination of prefrontal-amygdala synaptic development. J. Neurosci. 2017, 37, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Ravindranath, O.; Ordaz, S.J.; Padmanabhan, A.; Foran, W.; Jalbrzikowski, M.; Calabro, F.J.; Luna, B. Influences of affective context on amygdala functional connectivity during cognitive control from adolescence through adulthood. Dev. Cogn. Neurosci. 2020, 45, 100836. [Google Scholar] [CrossRef] [PubMed]
- Linhartová, P.; Látalová, A.; Kóša, B.; Kašpárek, T.; Schmahl, C.; Paret, C. fMRI neurofeedback in emotion regulation: A literature review. Neuroimage 2019, 193, 75–92. [Google Scholar] [CrossRef]
- Ross, M.C.; Heilicher, M.; Cisler, J.M. Functional imaging correlates of childhood trauma: A qualitative review of past research and emerging trends. Pharmacol. Biochem. Behav. 2021, 211, 173297. [Google Scholar] [CrossRef] [PubMed]
- Zaehringer, J.; Ende, G.; Santangelo, P.; Kleindienst, N.; Ruf, M.; Bertsch, K.; Bohus, M.; Schmahl, C.; Paret, C. Improved emotion regulation after neurofeedback: A single-arm trial in patients with borderline personality disorder. Neuroimage Clin. 2019, 24, 102032. [Google Scholar] [CrossRef]
- Himmelmeier, L.; Werheid, K. Neurofeedback training in children with ADHD: A systematic review of personalization and methodological features facilitating training conditions. Clin. EEG Neurosci. 2024, 55, 625–635. [Google Scholar] [CrossRef]
- Kloiber, S.; Rosenblat, J.D.; Husain, M.I.; Ortiz, A.; Berk, M.; Quevedo, J.; Vieta, E.; Maes, M.; Birmaher, B.; Soares, J.C.; et al. Neurodevelopmental pathways in bipolar disorder. Neurosci. Biobehav. Rev. 2020, 112, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Otten, M.; Meeter, M. Hippocampal structure and function in individuals with bipolar disorder: A systematic review. J. Affect. Disord. 2015, 174, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Kiani, I.; Aarabi, M.H.; Cattarinussi, G.; Sambataro, F.; Favalli, V.; Moltrasio, C.; Delvecchio, G. White matter changes in paediatric bipolar disorder: A systematic review of diffusion magnetic resonance imaging studies. J. Affect. Disord. 2025, 373, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Liang, K.; Lu, L.; Gao, Y.; Li, H.; Hu, X.; Xing, H.; Huang, X.; Gong, Q. Efficacy and safety of repetitive transcranial magnetic stimulation in children and adolescents with depression: A systematic review and preliminary meta-analysis. J. Affect. Disord. 2023, 320, 305–312. [Google Scholar] [CrossRef]
- Singh, M.K.; Garrett, A.S.; Chang, K.D. Using neuroimaging to evaluate and guide pharmacological and psychotherapeutic treatments for mood disorders in children. CNS Spectr. 2015, 20, 359–368. [Google Scholar] [CrossRef]
- Kim, S.; Rush, B.S.; Rice, T.R. A systematic review of therapeutic ketamine use in children and adolescents with treatment-resistant mood disorders. Eur. Child Adolesc. Psychiatry 2021, 30, 1485–1501. [Google Scholar] [CrossRef]
- Bruton, A.M.; Wesemann, D.G.; Machingo, T.A.; Majak, G.; Johnstone, J.M.; Marshall, R.D. Ketamine for mood disorders, anxiety, and suicidality in children and adolescents: A systematic review. Eur. Child Adolesc. Psychiatry 2025, 34, 141–157. [Google Scholar] [CrossRef]
- Luciano, M.; Di Vincenzo, M.; Mancuso, E.; Marafioti, N.; Di Cerbo, A.; Giallonardo, V.; Sampogna, G.; Fiorillo, A. Does the brain matter? Cortical alterations in pediatric bipolar disorder: A critical review of structural and functional magnetic resonance studies. Curr. Neuropharmacol. 2023, 21, 1302–1318. [Google Scholar] [CrossRef]
- Özerdem, A.; Ceylan, D.; Can, G. Neurobiology of risk for bipolar disorder. Curr. Treat. Options Psychiatry 2016, 3, 315–329. [Google Scholar] [CrossRef]
- Goodkind, M.; Eickhoff, S.B.; Oathes, D.J.; Jiang, Y.; Chang, A.; Jones-Hagata, L.B.; Ortega, B.N.; Zaiko, Y.V.; Roach, E.L.; Korgaonkar, M.S.; et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015, 72, 305–315. [Google Scholar] [CrossRef]
- Raschle, N.; Zuk, J.; Ortiz-Mantilla, S.; Sliva, D.D.; Franceschi, A.; Grant, P.E.; Benasich, A.A.; Gaab, N. Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Ann. N. Y Acad. Sci. 2012, 1252, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; Moeller, S.J.; Ali, F.; DeLorenzo, C.; Domschke, K.; Horga, G.; Jutla, A.; Kotov, R.; Paulus, M.P.; Rubio, J.M.; et al. Candidate biomarkers in psychiatric disorders: State of the field. World Psychiatry 2023, 22, 236–262. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R.; Garza, A.; Church, J.A. Key considerations for child and adolescent MRI data collection. Front. Neuroimaging 2022, 1, 981947. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.C. Brain Development Cooperative Group. The NIH MRI Study of Normal Brain Development. NeuroImage 2006, 30, 184–202. [Google Scholar] [CrossRef]
- Ayling, E.; Aghajani, M.; Fouche, J.P.; van der Wee, N. Diffusion tensor imaging in anxiety disorders. Curr. Psychiatry Rep. 2012, 14, 197–202. [Google Scholar] [CrossRef]
- Young, K.D.; Siegle, G.J.; Zotev, V.; Phillips, R.; Misaki, M.; Yuan, H.; Drevets, W.C.; Bodurka, J. Randomized clinical trial of real-time fMRI amygdala neurofeedback for major depressive disorder: Effects on symptoms and autobiographical memory recall. Am. J. Pschiatry 2017, 174, 748–755. [Google Scholar] [CrossRef]
- Marano, G.; Rossi, S.; Sfratta, G.; Traversi, G.; Lisci, F.M.; Anesini, M.B.; Pola, R.; Gasbarrini, A.; Gaetani, E.; Mazza, M. Gut Microbiota: A New Challenge in Mood Disorder Research. Life 2025, 15, 593. [Google Scholar] [CrossRef]
- Hortensius, L.M.; van den Hooven, E.H.; Dudink, J.; Tataranno, M.L.; van Elburg, R.M.; Benders, M.J.N.L. NutriBrain: Protocol for a randomised, double-blind, controlled trial to evaluate the effects of a nutritional product on brain integrity in preterm infants. BMC Pediatr. 2021, 21, 132. [Google Scholar] [CrossRef]
- Marano, G.; Traversi, G.; Nannarelli, C.; Pitrelli, S.; Mazza, S.; Mazza, M. Functional neuroimaging: Points of intersection between biology and psychotherapy. Clin. Ter. 2012, 163, e443–e456. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).