Functional and Structural Alterations in Pediatric Multiple Sclerosis: A Systematic Review and a Preliminary Activation Likelihood Estimation Functional Magnetic Resonance Imaging Meta-Analysis
Abstract
1. Introduction
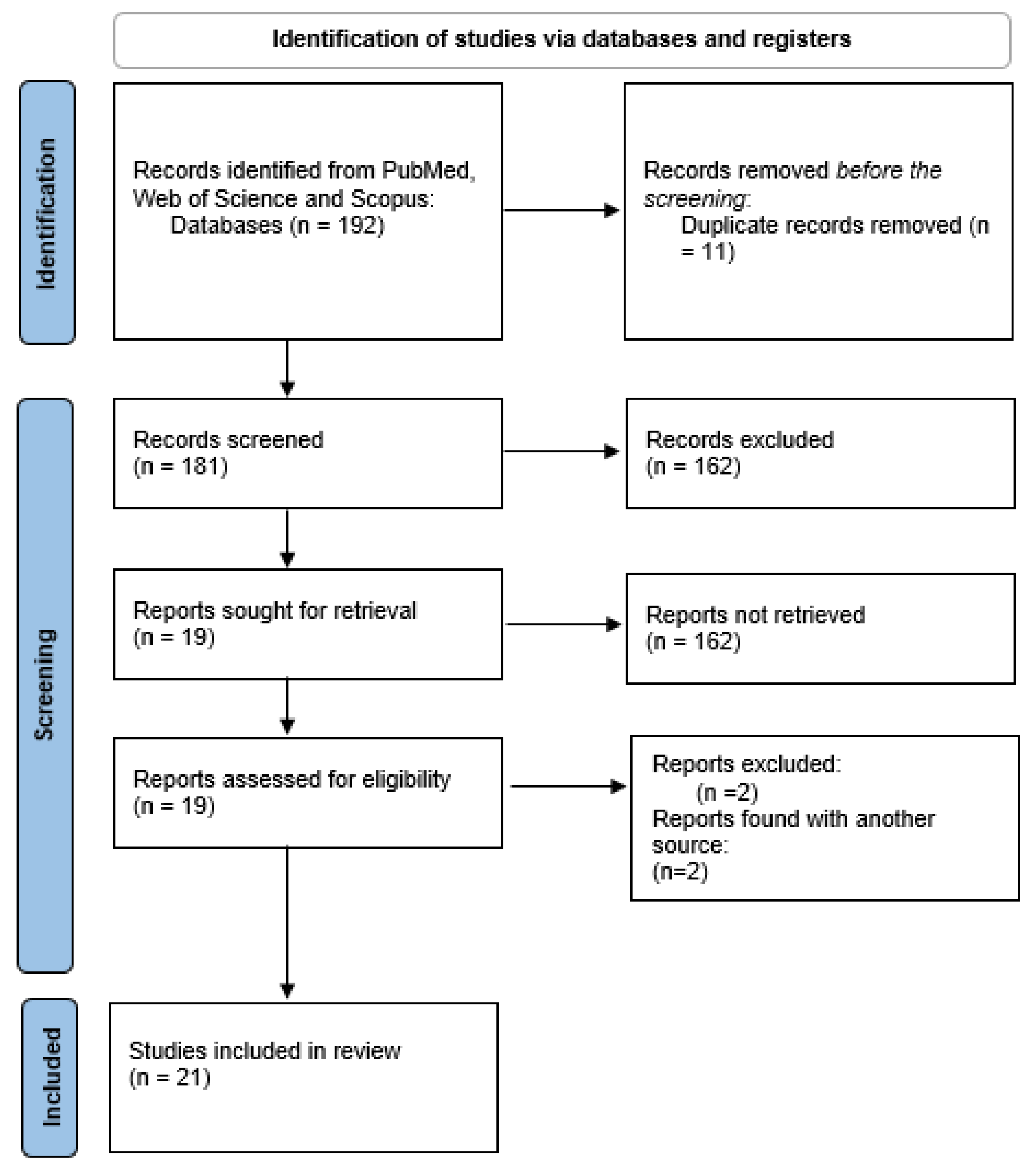
2. Materials and Methods
3. Results
3.1. Characteristics of the Included Studies
3.2. Neuropsychological Results
3.3. Structural Imaging Results
3.4. Functional MRI Studies and ALE Meta-Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Neuropsychological Assessment as Reported in the Included Studies
| Source | Tests/Batteries | Cognitive Domains | Results |
| Cirillo et al. 2016 [24] | Neuropsychological Battery for Pediatric Patients Affected by MS. | - | 19% of patients were cognitively impaired. |
| De Meo et al., 2017 [26] | Modified Card Sorting Test; Oral Denomination Test; Phrase Comprehension Test; Symbol Digit Modalities Test; Spatial Recall Test; Spatial Recall Test Delayed; Selective Reminding Test Consistent Long-Term Retrieval; Selective Reminding Test Delayed; Selective Reminding Test Long-Term Storage; Trail Making Test A/B. | Abstract and conceptual Reasoning Memory Language Attention | 23% of MS patients were classified as cognitively impaired. 36% of the cognitively impaired patients and none of the cognitively preserved patients had impairment on attention tests. |
| Fuentes et al., 2012 [27] | The Test of Memory and Learning—Second Edition (TOMAL-2) Subscales: Word Selective Reminding, Memory for Stories, Abstract Visual Memory, and Facial Memory. | Memory | Overall, lower memory scores in the MS group compared to the HC. All of the patients performed at least within the age-expected level on a measure of delayed, unaided recall of a word list, suggesting that verbal information was well-consolidated for later retrieval. |
| Hubacher et al., 2015 [36] | Corsi Blocks Backward; Digit Span Backward; Symbol Digit Modalities Test; Test Battery for Attention Performance (TAP). | Working memory functions and basic attention | No working memory deficits compared with normative data. |
| Rocca et al., 2014 [29] | Brief Neuropsychological Battery for Children (BNBC). Selective Reminding Test (SRT, SRT-Delayed); Spatial Recall Test (SPART, SPART-Delayed); Symbol Digit Modalities Test; Trail Making Test (TMT-A/B); Modified Card Sorting Test; Semantic and Phonemic Verbal Fluency Test; Oral Denomination Test; Token Test, the Indication of Pictures; Battery for the Analysis of Aphasic Deficits. | Memory Language, sustained attention Abstract reasoning | 45% of patients were classified as cognitively impaired. The cognitive domains most frequently involved were spatial and verbal memory, language, and attention. |
| Rocca et al., 2014 [31] | Brief Neuropsychological Battery for Children (BNBC). | Global cognitive functioning, verbal learning and delayed recall, visuospatial learning and delayed recall, sustained attention and concentration, abstract reasoning, expressive language, and receptive language | 25 pediatric MS patients were CP. |
| Margoni et al., 2020 [37] | Trail Making Test—Part B (TMT-B); Symbol Digit Modalities Test—oral version (SDMT). | Executive functioning, cognitive flexibility | Normal performance on neuropsychological tests. |
| Weier et al., 2016 [38] | Wechsler Abbreviated Scale of Intelligence (WASI FSIQ); Trail Making Test—Part B (TMT-B); Symbol Digit Modalities Test—Oral Version (SDMT); Beery Visual Motor Integration (VMI); Vocabulary Subtest of the WASI; Grooved Pegboard Test. | Intelligence, cognitive flexibility, language, and motor skills | MS patients showed impaired performance (defined as performance falling ≥ 1.5 SD below normative values) in the following subtests: TMT B, VMI, SDMT, and the Pegboard Test. |
| Cacciaguerra et al., 2024 [41] | Wechsler Intelligence Scale for children (WISC); Wechsler Adult Intelligence Scale (WAIS); Neuropsychological Battery for Children; Symbol Digit Modalities Test (SDMT); Coding Design (CD); Trail Making Test A-B (TMT-A/TMT-B); Semantic Verbal Fluency Test (SVFT); Phonemic Verbal Fluency Test (PVFT). | IQ, executive speed, and expressive language | 21.1% of patients failed the CD, 15.8% the TMT-B, 10.5% the TMT-A, 9.2% the SDMT, 3.9% the PVFT, 6.6% the BD, and 1.3% the SVFT. |
| Green et al., 2018 [40] | Behavior Assessment System for Children, Second Edition: Parent Rating Scale (BASC-2 PRS): Social Skills and Functional Communication Subscales; Wechsler Abbreviated Scale of Intelligence (WASI); Test of Memory and Learning—2nd Edition (TOMAL-2) Subscales: Memory for Stories, Word Selective Reminding, Facial Memory, and Abstract Visual Memory; Children’s Depression Inventory (CDI); Fatigue Severity Scale (FSS); Selective Reminding Test (SRT—Consistent Long-Term Retrieval and SRT—Long-Term Storage); Spatial Recall Test (SPART and SPART-Delayed); Symbol Digit Modalities Test (SDMT); Trail Making Test A-B (TMT-A/TMT-B); Modified Card Sorting Test (MCST); Indication of Pictures Test (IPT); Phrase Comprehension Test (PCT). | Social skills and functional communication Verbal and nonverbal socialization Expressive and receptive communication skills Verbal memory (delayed condition and delayed recall) and visual–spatial memory function | Full-scale IQ was higher in the HC group relative to the MS group, although groups fell within the broad range of average for IQ. Relative to the HC group, patients performed significantly worse on the MFS-D and AVM subtests of the TOMAL-2. They were reported as having poorer functional communication skills on the BASC-2. Performance fell in the impaired range in 9.4% of patients in the MFS-D subtest and only 3.1% of patients in the AVM subtest. Clinically elevated scores were observed in 12.5% of patients regarding functional communication and 3.1% on the Social Skills BASC-2 scale. |
| Rocca et al., 2016 [32] | Selective Reminding Test (SRT); Spatial Recall Test (SPART) | Long-term storage, consistent long-term retrieval and delayed visuospatial–spatial recall | 22.6% of patients were CI. Two patients scored < 70 in IQ, and twelve scored in the inferior range (<90). The cognitive domains most frequently involved were spatial memory, verbal memory, language abilities, attention, and concentration functions. |
References
- Waldman, A.; Ghezzi, A.; Bar-Or, A.; Mikaeloff, Y.; Tardieu, M.; Banwell, B. Multiple sclerosis in children: An update on clinical diagnosis, therapeutic strategies, and research. Lancet Neurol. 2014, 13, 936–948. [Google Scholar] [CrossRef]
- Narula, S. Pediatric multiple sclerosis: Updates in epidemiology, clinical features and management. Neurodegener Dis. Manag. 2016, 6, 3–7. [Google Scholar] [CrossRef]
- Yan, K.; Balijepalli, C.; Desai, K.; Gullapalli, L.; Druyts, E. Epidemiology of pediatric multiple sclerosis: A systematic literature review and meta-analysis. Mult. Scler. Relat. Disord. 2020, 44, 102260. [Google Scholar] [CrossRef]
- Banwell, B.; Ghezzi, A.; Bar-Or, A.; Mikaeloff, Y.; Tardieu, M. Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007, 6, 887–902. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Altmann, D.R.; Alves Da Mota, P.; Swanton, J.K.; Miszkiel, K.A.; Wheeler-Kingshott, C.G.; Ciccarelli, O.; Miller, D.H. Association of asymptomatic spinal cord lesions and atrophy with disability 5 years after a clinically isolated syndrome. Mult. Scler. J. 2017, 23, 665–674. [Google Scholar] [CrossRef]
- Palavra, F.; Silva, D.; Fernandes, C.; Faustino, R.; Vasconcelos, M.; Pereira, C.; Costa, C.; Ribeiro, J.A.; Amaral, J.; Robalo, C. Clinical predictors of NEDA-3 one year after diagnosis of pediatric multiple sclerosis: An exploratory single-center study. Front. Neurosci. 2023, 17, 1259306. [Google Scholar] [CrossRef]
- De Carvalho, S.; Pinto, J.; Correia, I.; Faustino, R.; Vasconcelos, M.; Sousa, L.; Palavra, F. Inflammatory Activity and Treatment Response in Pediatric Compared to Adult Multiple Sclerosis: A Pilot, Retrospective and Observational Study of the First Year After Diagnosis. Acta Med. Port. 2021, 34, 28–34. [Google Scholar] [CrossRef]
- Mikaeloff, Y.; Caridade, G.; Assi, S.; Suissa, S.; Tardieu, M. Prognostic factors for early severity in a childhood multiple sclerosis cohort. Pediatrics 2006, 118, 1133–1139. [Google Scholar] [CrossRef]
- Iaffaldano, P.; Simone, M.; Lucisano, G.; Ghezzi, A.; Coniglio, G.; Brescia Morra, V.; Salemi, G.; Patti, F.; Lugaresi, A.; Izquierdo, G.; et al. Prognostic indicators in pediatric clinically isolated syndrome. Ann. Neurol. 2017, 81, 729–739. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Rocca, M.A.; Schoonheim, M.M.; Valsasina, P.; Geurts, J.J.G.; Filippi, M. Task- and resting-state fMRI studies in multiple sclerosis: From regions to systems and time-varying analysis. Current status and future perspective. Neuroimage Clin. 2022, 35, 103076. [Google Scholar] [CrossRef]
- Laura, G.; Silvia, T.; Nikolaos, P.; Patrizia, P. The Role of fMRI in the Assessment of Neuroplasticity in MS: A Systematic Review. Neural. Plast. 2018, 2018, 3419871. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. bmj 2021, 372, n71. [Google Scholar] [CrossRef]
- Cera, N.; Vargas-Cáceres, S.; Oliveira, C.; Monteiro, J.; Branco, D.; Pignatelli, D.; Rebelo, S. How relevant is the systemic oxytocin concentration for human sexual behavior? A systematic review. Sex. Med. 2021, 9, 100370. [Google Scholar] [CrossRef]
- Vargas-Cáceres, S.; Cera, N.; Nobre, P.; Ramos-Quiroga, J.A. The impact of psychosis on sexual functioning: A systematic review. J. Sex. Med. 2021, 18, 457–466. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A.; Ciccarelli, O.; De Stefano, N.; Evangelou, N.; Kappos, L.; Rovira, A.; Sastre-Garriga, J.; Tintorè, M.; Frederiksen, J.L.; et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 2016, 15, 292–303. [Google Scholar] [CrossRef]
- Krupp, L.B.; Tardieu, M.; Amato, M.P.; Banwell, B.; Chitnis, T.; Dale, R.C.; Ghezzi, A.; Hintzen, R.; Kornberg, A.; Pohl, D.; et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: Revisions to the 2007 definitions. Mult. Scler. J. 2013, 19, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. Neuroimage 2012, 59, 2349–2361. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Cykowski, M.D.; McKay, D.R.; Kochunov, P.V.; Fox, P.T.; Rogers, W.; Toga, A.W.; Zilles, K.; Amunts, K.; Mazziotta, J. Anatomical global spatial normalization. Neuroinformatics 2010, 8, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.L.; Laird, A.R.; Eickhoff, S.B.; Martinez, M.J.; Fox, P.M.; Fox, P.T. Automated regional behavioral analysis for human brain images. Front. Neuroinform. 2012, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Aubert-Broche, B.; Fonov, V.; Ghassemi, R.; Narayanan, S.; Arnold, D.L.; Banwell, B.; Sled, J.G.; Collins, D.L. Regional brain atrophy in children with multiple sclerosis. Neuroimage 2011, 58, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, S.; Rocca, M.A.; Ghezzi, A.; Valsasina, P.; Moiola, L.; Veggiotti, P.; Amato, M.P.; Comi, G.; Falini, A.; Filippi, M. Abnormal cerebellar functional MRI connectivity in patients with paediatric multiple sclerosis. Mult. Scler. J. 2016, 22, 292–301. [Google Scholar] [CrossRef]
- Rocca, M.A.; Absinta, M.; Ghezzi, A.; Moiola, L.; Comi, G.; Filippi, M. Is a preserved functional reserve a mechanism limiting clinical impairment in pediatric MS patients? Hum. Brain Mapp. 2009, 30, 2844–2851. [Google Scholar] [CrossRef]
- De Meo, E.; Moiola, L.; Ghezzi, A.; Veggiotti, P.; Capra, R.; Amato, M.P.; Pagani, E.; Fiorino, A.; Pippolo, L.; Pera, M.C.; et al. MRI substrates of sustained attention system and cognitive impairment in pediatric MS patients. Neurology 2017, 89, 1265–1273. [Google Scholar] [CrossRef]
- Fuentes, A.; Collins, D.L.; Garcia-Lorenzo, D.; Sled, J.G.; Narayanan, S.; Arnold, D.L.; Banwell, B.L.; Till, C. Memory performance and normalized regional brain volumes in patients with pediatric-onset multiple sclerosis. J. Int. Neuropsychol. Soc. 2012, 18, 471–480. [Google Scholar] [CrossRef]
- Rocca, M.A.; Absinta, M.; Moiola, L.; Ghezzi, A.; Colombo, B.; Martinelli, V.; Comi, G.; Filippi, M. Functional and structural connectivity of the motor network in pediatric and adult-onset relapsing-remitting multiple sclerosis. Radiology 2010, 254, 541–550. [Google Scholar] [CrossRef]
- Rocca, M.A.; Absinta, M.; Amato, M.P.; Moiola, L.; Ghezzi, A.; Veggiotti, P.; Capra, R.; Portaccio, E.; Fiorino, A.; Pippolo, L.; et al. Posterior brain damage and cognitive impairment in pediatric multiple sclerosis. Neurology 2014, 82, 1314–1321. [Google Scholar] [CrossRef]
- Rocca, M.A.; Sonkin, M.; Copetti, M.; Pagani, E.; Arnold, D.L.; Narayanan, S.; Sled, J.G.; Banwell, B.; Filippi, M. Diffusion tensor magnetic resonance imaging in very early onset pediatric multiple sclerosis. Mult. Scler. J. 2016, 22, 620–627. [Google Scholar] [CrossRef]
- Rocca, M.A.; Valsasina, P.; Absinta, M.; Moiola, L.; Ghezzi, A.; Veggiotti, P.; Amato, M.P.; Horsfield, M.A.; Falini, A.; Comi, G.; et al. Intranetwork and internetwork functional connectivity abnormalities in pediatric multiple sclerosis. Hum. Brain Mapp. 2014, 35, 4180–4192. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Morelli, M.E.; Amato, M.P.; Moiola, L.; Ghezzi, A.; Veggiotti, P.; Capra, R.; Pagani, E.; Portaccio, E.; Fiorino, A.; et al. Regional hippocampal involvement and cognitive impairment in pediatric multiple sclerosis. Mult. Scler. J. 2016, 22, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, A.; Rocca, M.A.; Perego, E.; Moiola, L.; Ghezzi, A.; Martinelli, V.; Comi, G.; Filippi, M. Deep grey matter T2 hypo-intensity in patients with paediatric multiple sclerosis. Mult. Scler. J. 2011, 17, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Bartels, F.; Nobis, K.; Cooper, G.; Wendel, E.; Cleaveland, R.; Bajer-Kornek, B.; Blaschek, A.; Schimmel, M.; Blankenburg, M.; Baumann, M.; et al. Childhood multiple sclerosis is associated with reduced brain volumes at first clinical presentation and brain growth failure. Mult. Scler. J. 2019, 25, 927–936. [Google Scholar] [CrossRef]
- De Meo, E.; Bonacchi, R.; Moiola, L.; Colombo, B.; Sangalli, F.; Zanetta, C.; Amato, M.P.; Martinelli, V.; Rocca, M.A.; Filippi, M. Early Predictors of 9-Year Disability in Pediatric Multiple Sclerosis. Ann. Neurol. 2021, 89, 1011–1022. [Google Scholar] [CrossRef]
- Hubacher, M.; DeLuca, J.; Weber, P.; Steinlin, M.; Kappos, L.; Opwis, K.; Penner, I.K. Cognitive rehabilitation of working memory in juvenile multiple sclerosis-effects on cognitive functioning, functional MRI and network related connectivity. Restor. Neurol. Neurosci. 2015, 33, 713–725. [Google Scholar] [CrossRef]
- Margoni, M.; Franciotta, S.; Poggiali, D.; Riccardi, A.; Rinaldi, F.; Nosadini, M.; Sartori, S.; Anglani, M.G.; Causin, F.; Perini, P.; et al. Cerebellar gray matter lesions are common in pediatric multiple sclerosis at clinical onset. J. Neurol. 2020, 267, 1824–1829. [Google Scholar] [CrossRef]
- Weier, K.; Till, C.; Fonov, V.; Yeh, E.A.; Arnold, D.L.; Banwell, B.; Collins, D.L. Contribution of the cerebellum to cognitive performance in children and adolescents with multiple sclerosis. Mult. Scler. J. 2016, 22, 599–607. [Google Scholar] [CrossRef]
- Kerbrat, A.; Aubert-Broche, B.; Fonov, V.; Narayanan, S.; Sled, J.G.; Arnold, D.A.; Banwell, B.; Collins, D.L. Reduced head and brain size for age and disproportionately smaller thalami in child-onset MS. Neurology 2012, 78, 194–201. [Google Scholar] [CrossRef]
- Green, R.; Adler, A.; Banwell, B.L.; Fabri, T.L.; Yeh, E.A.; Collins, D.L.; Sled, J.G.; Narayanan, S.; Till, C. Involvement of the Amygdala in Memory and Psychosocial Functioning in Pediatric-Onset Multiple Sclerosis. Dev. Neuropsychol. 2018, 43, 524–534. [Google Scholar] [CrossRef]
- Cacciaguerra, L.; Curatoli, C.; Vizzino, C.; Valsasina, P.; Filippi, M.; Rocca, M.A. Functional correlates of cognitive abilities vary with age in pediatric multiple sclerosis. Mult. Scler. Relat. Disord. 2024, 82, 105404. [Google Scholar] [CrossRef] [PubMed]
- Waubant, E.; Chabas, D.; Okuda, D.T.; Glenn, O.; Mowry, E.; Henry, R.G.; Strober, J.B.; Soares, B.; Wintermark, M.; Pelletier, D. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch. Neurol. 2009, 66, 967–971. [Google Scholar] [CrossRef] [PubMed]
- De Meo, E.; Storelli, L.; Moiola, L.; Ghezzi, A.; Veggiotti, P.; Filippi, M.; Rocca, M.A. In vivo gradients of thalamic damage in paediatric multiple sclerosis: A window into pathology. Brain 2021, 144, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Portaccio, E.; Goretti, B.; Lori, S.; Zipoli, V.; Centorrino, S.; Ghezzi, A.; Patti, F.; Bianchi, V.; Comi, G.; Trojano, M.; et al. The brief neuropsychological battery for children: A screening tool for cognitive impairment in childhood and juvenile multiple sclerosis. Mult. Scler. J. 2009, 15, 620–626. [Google Scholar] [CrossRef]
- Penner, I.K.; Raselli, C.; Stöcklin, M.; Opwis, K.; Kappos, L.; Calabrese, P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): Validation of a new instrument to assess multiple sclerosis-related fatigue. Mult. Scler. J. 2009, 15, 1509–1517. [Google Scholar] [CrossRef]
- Vogt, A.; Kappos, L.; Calabrese, P.; Stöcklin, M.; Gschwind, L.; Opwis, K.; Penner, I.K. Working memory training in patients with multiple sclerosis–comparison of two different training schedules. Restor. Neurol. Neurosci. 2009, 27, 225–235. [Google Scholar] [CrossRef]
- Baddeley, A.D. Working memory: Some recent developments. Int. J. Psychol. 2000, 35, 203. [Google Scholar]
- Ghezzi, A.; Deplano, V.; Faroni, J.; Grasso, M.G.; Liguori, M.; Marrosu, G.; Pozzilli, C.; Simone, I.L.; Zaffaroni, M. Multiple sclerosis in childhood: Clinical features of 149 cases. Mult. Scler. J. 1997, 3, 43–46. [Google Scholar] [CrossRef]
- Sacks, D.; Society, C.P.; Adolescent Health Committee. Age limits and adolescents. Paediatr. Child Health 2003, 8, 577–578. [Google Scholar] [CrossRef]
- Mikaeloff, Y.; Suissa, S.; Vallée, L.; Lubetzki, C.; Ponsot, G.; Confavreux, C.; Tardieu, M.; KIDMUS Study Group. First episode of acute CNS inflammatory demyelination in childhood: Prognostic factors for multiple sclerosis and disability. J. Pediatr. 2004, 144, 246–252. [Google Scholar] [CrossRef]
- Ekmekci, O. Pediatric multiple sclerosis and cognition: A review of clinical, neuropsychologic, and neuroradiologic features. Behav. Neurol. 2017, 2017, 1463570. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.; Serafin, D.; Charvet, L.; Ackerson, J.; Benedict, R.; Braaten, E.; Brown, T.; O’Donnell, E.; Parrish, J.; Preston, T.; et al. Cognitive impairment occurs in children and adolescents with multiple sclerosis: Results from a United States network. J. Child Neurol. 2013, 28, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.H.; Li, D.; Traboulsee, A.; Coyle, P.K.; Arnold, D.L.; Barkhof, F.; Frank, J.A.; Grossman, R.; Paty, D.W.; Radue, E.W.; et al. Standardized MR imaging protocol for multiple sclerosis: Consortium of MS Centers consensus guidelines. AJNR Am. J. Neuroradiol. 2006, 27, 455–461. [Google Scholar] [PubMed]
- Riva, D.; Cazzaniga, F.; Esposito, S.; Bulgheroni, S. Executive functions and cerebellar development in children. Appl. Neuropsychol. Child 2013, 2, 97–103. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Disorders of the cerebellum: Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 367–378. [Google Scholar] [CrossRef]
- Timmann, D.; Drepper, J.; Frings, M.; Maschke, M.; Richter, S.; Gerwig, M.; Kolb, F.P. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 2010, 46, 845–857. [Google Scholar] [CrossRef]
- Sereno, M.I.; Diedrichsen, J.; Tachrount, M.; Testa-Silva, G.; d’Arceuil, H.; De Zeeuw, C. The human cerebellum has almost 80% of the surface area of the neocortex. Proc. Natl. Acad. Sci. USA 2020, 117, 19538–19543. [Google Scholar] [CrossRef]
- Rondi-Reig, L.; Paradis, A.L.; Fallahnezhad, M. A liaison brought to light: Cerebellum-hippocampus, partners for spatial cognition. Cerebellum 2022, 21, 826–837. [Google Scholar] [CrossRef]
- Tedesco, A.M.; Chiricozzi, F.R.; Clausi, S.; Lupo, M.; Molinari, M.; Leggio, M.G. The cerebellar cognitive profile. Brain 2011, 134, 3672–3686. [Google Scholar] [CrossRef]
- Tervo-Clemmens, B.; Calabro, F.J.; Parr, A.C.; Fedor, J.; Foran, W.; Luna, B. A canonical trajectory of executive function maturation from adolescence to adulthood. Nat. Commun. 2023, 14, 6922. [Google Scholar] [CrossRef]
- Tiemeier, H.; Lenroot, R.K.; Greenstein, D.K.; Tran, L.; Pierson, R.; Giedd, J.N. Cerebellum development during childhood and adolescence: A longitudinal morphometric MRI study. Neuroimage 2010, 49, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Yassa, M.A.; Stark, C.E. Pattern separation in the hippocampus. Trends Neurosci. 2011, 34, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.E.; Augustinack, J.C.; Nguyen, K.; Player, C.M.; Player, A.; Wright, M.; Roy, N.; Frosch, M.P.; McKee, A.C.; Wald, L.L.; et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage 2015, 115, 117–137. [Google Scholar] [CrossRef]
- Mu, S.H.; Yuan, B.K.; Tan, L.H. Effect of Gender on Development of Hippocampal Subregions From Childhood to Adulthood. Front. Hum. Neurosci. 2020, 14, 611057. [Google Scholar] [CrossRef]
- Rocca, M.A.; Barkhof, F.; De Luca, J.; Frisén, J.; Geurts, J.J.; Hulst, H.E.; Sastre-Garriga, J.; Filippi, M.; Yousry, T.A. The hippocampus in multiple sclerosis. Lancet Neurol. 2018, 17, 918–926. [Google Scholar] [CrossRef]
- Wierenga, L.; Langen, M.; Ambrosino, S.; van Dijk, S.; Oranje, B.; Durston, S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage 2014, 96, 67–72. [Google Scholar] [CrossRef]
- LaBerge, D.; Buchsbaum, M.S. Positron emission tomographic measurements of pulvinar activity during an attention task. J. Neurosci. 1990, 10, 613–619. [Google Scholar] [CrossRef]
- Minagar, A.; Barnett, M.H.; Benedict, R.H.; Pelletier, D.; Pirko, I.; Sahraian, M.A.; Frohman, E.; Zivadinov, R. The thalamus and multiple sclerosis: Modern views on pathologic, imaging, and clinical aspects. Neurology 2013, 80, 210–219. [Google Scholar] [CrossRef]
- Harrison, D.M.; Oh, J.; Roy, S.; Wood, E.T.; Whetstone, A.; Seigo, M.A.; Jones, C.K.; Pham, D.; van Zijl, P.; Reich, D.S.; et al. Thalamic lesions in multiple sclerosis by 7T MRI: Clinical implications and relationship to cortical pathology. Mult. Scler. J. 2015, 21, 1139–1150. [Google Scholar] [CrossRef]
- Rushworth, M.F.; Buckley, M.J.; Behrens, T.E.; Walton, M.E.; Bannerman, D.M. Functional organization of the medial frontal cortex. Curr. Opin. Neurobiol. 2007, 17, 220–227. [Google Scholar] [CrossRef]
- Margulies, D.S.; Kelly, A.C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage 2007, 37, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the brain network: A review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Shenhav, A.; Cohen, J.D.; Botvinick, M.M. Dorsal anterior cingulate cortex and the value of control. Nat. Neurosci. 2016, 19, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Cera, N.; Esposito, R.; Cieri, F.; Tartaro, A. Altered cingulate cortex functional connectivity in normal aging and mild cognitive impairment. Front. Neurosci. 2019, 13, 857. [Google Scholar] [CrossRef]
- Han, S.W.; Eaton, H.P.; Marois, R. Functional fractionation of the cingulo-opercular network: Alerting insula and updating cingulate. Cereb. Cortex 2019, 29, 2624–2638. [Google Scholar] [CrossRef]
- Silveira, S.; Boney, S.; Tapert, S.F.; Mishra, J. Developing functional network connectivity of the dorsal anterior cingulate cortex mediates externalizing psychopathology in adolescents with child neglect. Dev. Cogn. Neurosci. 2021, 49, 100962. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef]
- Simmonds, D.J.; Hallquist, M.N.; Asato, M.; Luna, B. Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. Neuroimage 2014, 92, 356–368. [Google Scholar] [CrossRef]
- Petanjek, Z.; Judaš, M.; Šimić, G.; Rašin, M.R.; Uylings, H.B.; Rakic, P.; Kostović, I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 13281–13286. [Google Scholar] [CrossRef]
- Faustino, R.; Lopes, C.; Jantarada, A.; Mendonça, A.; Raposo, R.; Ferrão, C.; Freitas, J.; Mateus, C.; Pinto, A.; Almeida, E.; et al. Neuroimaging characterization of multiple sclerosis lesions in pediatric patients: An exploratory radiomics approach. Front. Neurosci. 2024, 18, 1294574. [Google Scholar] [CrossRef]
- Raichle, M.E. The restless brain. Brain Connect. 2011, 1, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Carroll, D.C. Self-projection and the brain. Trends Cogn. Sci. 2007, 11, 49–57. [Google Scholar] [CrossRef]
- Schilbach, L.; Eickhoff, S.B.; Rotarska-Jagiela, A.; Fink, G.R.; Vogeley, K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008, 17, 457–467. [Google Scholar] [CrossRef]
- Brüne, M.; Brüne-Cohrs, U. Theory of mind—Evolution, ontogeny, brain mechanisms and psychopathology. Neurosci. Biobehav. Rev. 2006, 30, 437–455. [Google Scholar] [CrossRef]
- Charvet, L.E.; Cleary, R.E.; Vazquez, K.; Belman, A.L.; Krupp, L.B.; USNetwork for Pediatric, M.S. Social cognition in pediatric-onset multiple sclerosis (MS). Mult. Scler. J. 2014, 20, 1478–1484. [Google Scholar] [CrossRef]
- Isernia, S.; Rossetto, F.; Castelli, F.; Rovaris, M.; Blasi, V.; Baglio, F. Beyond the simplicity of theory of mind deficit in multiple sclerosis: From kinetic perception to socio-emotional abstraction and mentalizing. Mult. Scler. Relat. Disord. 2023, 77, 104894. [Google Scholar] [CrossRef]
- Massano, C.; Lima, M.; Monteiro, I.; Machado, R.; Correia, I.; Nunes, C.C.; Macário, C.; Sousa, L.; Santana, I.; Batista, S. Outcomes on Social and Classic Cognition in adults with Pediatric-onset Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 53, 103071. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Design | |
| Experimental studies Case–control studies | Other designs Systematic reviews |
| Population | |
| Children Adolescents | Animals Adults |
| Intervention | |
| Neuroimaging techniques Emotional task Cognitive task Resting state Structural MRI | EEG Other imaging techniques |
| Topic | |
| Brain activation Brain activity Brain connectivity Gray matter alteration | Brain activation, brain activity, brain connectivity, and gray matter alteration in adult MS patients or adult MS patients with pediatric-onset |
| Hits | Selection | Comparability | Exposure |
|---|---|---|---|
| Aubert Broche et al., 2011 [23] | **** | * | ** |
| Cirillo et al., 2016 [24] | **** | * | ** |
| Rocca et al., 2009 [25] | **** | ** | *** |
| De Meo et al., 2017 [26] | *** | ** | ** |
| Fuentes et al., 2012 [27] | **** | ** | *** |
| Rocca et al., 2010 [28] | **** | ** | *** |
| Rocca et al., 2014 [29] | *** | ** | * |
| Rocca et al., 2016 [30] | ** | ** | ** |
| Rocca et al., 2014 [31] | *** | * | ** |
| Rocca et al., 2016 [32] | *** | ** | ** |
| Ceccarelli et al., 2011 [33] | *** | * | ** |
| Bartels et al., 2019 [34] | *** | ** | *** |
| De Meo et al., 2021 [35] | ** | - | * |
| Hubacher et al., 2015 [36] | ** | - | * |
| Margoni et al., 2020 [37] | ** | - | * |
| Weier et al., 2016 [38] | **** | ** | ** |
| Kerbrat et al., 2012 [39] | **** | ** | ** |
| Green et al., 2018 [40] | **** | ** | *** |
| Cacciaguerra et al., 2024 [41] | *** | ** | *** |
| Waubant et al., 2009 [42] | ** | ** | *** |
| De Meo et al., 2021 [43] | *** | ** | *** |
| Source | N/Age | Neurological Assessment | Magnetic Field/MRI Sequence | Analytical Techniques | Tasks | Lesion Volume | Principal Results |
|---|---|---|---|---|---|---|---|
| Aubert—Broche et al., 2011 [23] | MS RR: N = 30 (24 girls); mean age = 15.4 ± 2.1 years. HCs: N = 29 (23 girls); mean age = 15.5 ± 2 years | EDSS = Median: 1 (0.0–4.5) | MRI; 1.5 T; 3D T1w RF-spoiled gradient-recalled echo sequence; 2D multislice proton density-weighted (PDw)/T2w fast spin echo sequence | Preprocessing, spatial normalization, and Jacobian calculation GLM | -- | Median 3303 mm3 | Significant volume loss in the MS group in the pulvinar, right anterior nuclei, splenium of the corpus callosum, and globus pallidus. Volume expansion significant in the foramen of Monroe and left lateral ventricle. Regression against disease duration showed significantly correlated volume reductions in the left and right globus pallidus and within the optic tract, from the optic chiasm to the lateral geniculate nucleus and anterior part of the optic radiations |
| Ceccarelli et al., 2011 [24] | 10 CIS and 35 RRMS (27 girls and 18 boys); mean age (SD) 14.7 (2.4) years. 14 HCs (7 girls and 7 boys); mean age (SD) 16.4 (4.7) years | EDSS = Median: 1.0 (0.0–6.5) | 1.5 T; dual-echo turbo SE TR = 3300 ms, TE = 16/98 ms | T2 intensity in deep gray matter structures | -- | Mean (ml) = 5.2 SD = 7 | T2 intensity of the head of the left caudate nucleus differed between pediatric MS patients and HCs (p = 0.001) |
| Fuentes et al., 2012 [27] | Pediatric MS: N = 32 (25 girls); age 16.3. HCs: N = 26 (21 girls); age = 16.4 | EDSS = Median: 1.0 (0.0–4.0); neuropsychological assessment | 1.5T 3D T1-weighted radiofrequency-spoiled gradient-recalled echo sequence with 1.5 mm thick sagittal partitions, TR = 22 ms, TE = 8 ms. 2D multislice proton density-weighted /T2-weighted fast spin echo sequence with an echo train Length = 58, TR: 3500 ms | Segmentation of brain structures. ANOVA | -- | Log T2-LV total brain mean = 3.68 SD = 0.54 | MS patients > HCs = Lower whole brain volume Lower normalized volumes in the amygdala (total and left side) and thalamus (left, right, and total) |
| Rocca et al., 2014 [29] | Pediatric RRMS CP patients: N = 35 (21 girls); age = 15.4. Pediatric RRMS CI patients: N = 16 (9 girls); age = 15.2. HCs: N = 16 (9 girls); age = 14.3 | EDSS = Median: 1.5 (0–3.5), neuropsychological assessment | 3T fMRI:T2*-weighted single-shot EPI; dual-echo turbo SE; 3D T1-weighted FFE; and pulsed-gradient SE EPI with diffusion gradients applied in 35 noncollinear directions | Functional connectivity analysis. Segmentation. Voxel brain morphometry, | RS | Mean (mL) = 4.8 SD = 6.7 | CI patients had an increased probability of harboring lesions in the right thalamus, middle and posterior cingulate cortex, and bilateral parieto-occipital white matter. GM atrophy: CI patients had atrophy of the right precuneus and left middle temporal gyrus. MS patients had decreased FC of the posterior regions of the DMN (Right precuneus). CP patients vs. HCs had decreased RS FC of the right angular gyrus. CI patients vs. both HCs and CP patients had decreased RS FC of the right precuneus. CP patients experienced an increased RS FC of the anterior cingulate cortex |
| Rocca et al., 2014 [31] | MS CP: N = 28 (19 girls); age = 14.9. MS CI: N = 16 (9 girls); age = 15.7. HCs: N = 27; females: 16; age = 15.3 | EDSS = Median: 1.5 (0.0–4.0), neuropsychological assessment | 3T fMRI: T-2 *W single-shot EPI: TR = 3000 ms, TE = 35 ms. Dual-echo turbo SE. 3D-T1-W FFE. Pulsed-gradient SE EPI, 35 noncollinear direction | Independent component analysis | RS | Mean (mL) = 4.8 SD = 6.7 | Compared to HCs, MS patients showed increased FC between the sensorimotor II network and the DMN and between the WMN and the attention network. They also showed a decreased FC between the WMN and the DMN. The decreased FC between WMN and DMN was corrected for GM atrophy. No differences in FC were found between CP and CI MS patients. |
| Weier et al., 2016 [38] | MS: N = 28 (21 girls); age = 16.3 ± 2.2. HCs: N = 33 (26 girls); age = 15.5 ± 2.7 | EDSS Median: 1.25 (0–4); neuropsychological assessment | 1.5T 3D T1-W RF-spoiled gradient-recalled echo; 2D proton density-T2-W fast SE | Segmentation of the human cerebellum and its lobules, combining patch-based label fusion and a template library of manually labeled cerebella of the controls | -- | Cerebellar lesion (infratentorial): Median 0.1 (0–4.4) Supratentorial lesion: Median: 4.6 (0.3–35.6) | Cerebral volume was reduced in MS patients. Cerebellar volumes did not differ. Correlation between cerebellar volume and neuropsychological results |
| Hubacher et al., 2015 [36] | 5 patients between 12 and 18 yrs. Case series study | EDSS = NA; neuropsychological assessment | 3T; MPRAGE; FLAIR; fMRI: EPI—TR/TE = 2000/23 ms, | GLM; FC | N-back | Mean (mL): Case 1 = 4.2; Case 2 = 5.3; Case 3 = 5.9; Case 4 = 0.85; Case 5 = 9.0 | FC between the ventral and the dorsal DMN increased in 2 patients. No differences in the other patients |
| Cirillo et al., 2016 [24] | RRMS: N = 48; (31 girls); age: 14.9. CP MS: N = 39 (22 girls); age: 14.9. CI MS: N = 8 (5 girls); age: 15.8 | EDSS: Median: 1.5 (0–4.0) 9-HPT: nine-hole peg test | 3T. fMRI: EPI-TR = 3000 ms, TE = 35 ms; dual-echo turbo spin echo TR/ TE = 2599/16.80 ms. MRI: T1-weighted FFE TR = 25 ms, TE = 4.6 ms | Resting state; seed-based FC; seeds: left and right dentate nucleus of the cerebellum | RS | Mean (mL) = 5.3 SD = 6.7 | In MS infratentorial, the T2 lesion volume was approximately 10% of the total T2 lesion volume. Compared with controls, MS patients also showed an increased rest FC between the bilateral dentate nucleus and the left pre- and postcentral gyri |
| Rocca et al., 2016 [30] | MS patients: N = 11 (9 girls); age = 11.1. HCs: N = 13 (11 girls); age = 12.2 | EDSS = Median: 1.0 (0–3.0) | 1.5 T, dual-echo fast SE TR = 3500 ms, TE = 15/63 ms. 3D T1-W TR = 22 ms, TE = 8 ms, pulsed gradient spin echo single-shot echo planar imaging, 25 noncollinear directions. | FA and MD | -- | Mean (mL) = 2.5 SD = 4.7 Mean (mL) = 7.1 SD = 3.6 | MS patients had significantly reduced FA and increased MD in the left and right superior longitudinal fasciculus and corpus callosum |
| Rocca et al., 2016 [32] | MS CP: N = 41 (26 girls); age = 15.1. MS CI N = 12 (6 girls), age = 15.0. HCs: N = 18 (10 girls); age = 14.0 | EDSS = Median: 1.5 (0.0–4.0); neuropsychological assessment | 3T; brain dual-echo, turbo SE, and brain 3D T1-weighted FFE | Radial mapping analysis | -- | Mean (mL) = 6.3 SD = 7.5 | Global hippocampal volume was reduced in MS patients compared to HCs. Compared to HCs, MS patients showed atrophy of the cornu ammonis, subiculum, and dentate gyrus subfields and radial hypertrophy of the dentate gyrus subfield. Regional hippocampal volume modifications correlated with brain T2 lesion volume, attention, and language abilities. Compared to CP patients, CI patients had atrophy of the right subiculum and dentate gyrus subfields |
| De Meo et al., 2017 [26] | MS CP: N = 44 (30 girls); age: 15.2. MS CI: N = 13 (6 girls); age: 15.1. HCs: N = 14 (8 girls); age: 13.6 | EDSS: Median: 1.0 (0.0–4.0); neuropsychological assessment | T2*-weighted single-shot echo-planar imaging scan during the task; DT MRI scan, dual-echo turbo spin echo scan, and 3-dimensional T1-weighted FFE | GLM | CCPT | Mean (mL) = 6.0 SD = 7.8 | CI vs. CP patients: lower normalized brain volume, lower WM volume, and lower FA values in the tracts connecting the left anterior insula to the anterior cingulate cortex (ACC) and precuneus. CCPT load condition: CP patients > HCs. Increased activation of the left anterior insula and thalamus and decreased deactivation of the ACC and right inferior frontal gyrus. Increased deactivation of the right precuneus and superior parietal lobule. CI > CP patients decreased activation of the right postcentral gyrus and increased deactivation of bilateral precuneus. CP > CI patients with pediatric MS had increased activity in the parietal and occipital lobes and cerebellum. No significant correlations |
| Bartels et al., 2019 [34] | MS: N = 37 (18 girls); age = 15; multicenter study—2-year follow-up | EDSS = Median: 0(0–2) | 3T; 3D T1-weighted (MPRAGE) | NA | -- | Total lesion count: median = 4; range = 1–10 | MS patients: significant brain volume loss, GM, and WM volume associated with increased ventricular volume at the first clinical presentation. Patients continue to have further brain volume loss at follow-up (2 years). |
| De Meo et al., 2021 [35] | N = 123 (brain MRI); N = 115 (cervical cord MRI); 89 girls; age: 14.4 yr. 1-year and 2-year follow-ups | EDSS = Median: 1.5 (0.0–6.0) | 1.5 T; BRAIN: FLAIR T2-W, and/or CERVICAL CORD: STIR, T2-W | NA | -- | Nr of T2 lesions: mean (range): 30.5 (3–180) | Optic neuritis was observed in 74% of patients. No significant differences in demographic, clinical, and MRI features were observed. Significant nr. of lesions at the brain stem and cervical cord (baseline); 1 yr follow-up: no significant nr. of lesions vs. baseline; 2 yr follow-up: significant nr. of new lesions. |
| Margoni et al., 2020 [37] | MS = 15 (12 girls); age = 14.1 ± 2.3 | EDSS = Median: 1.5 (IQR: 1.0–2.25); neuropsychological assessment | 3 T; DIR = resolution 1 × 1 × 3 mm, FOV 230 × 200 mm, TR 13,000 ms, TE 10 ms, TI 3400/325 ms, slices n40, time 3.5 min; PSIR: resolution 1 × 1 × 3 mm, FOV 230 × 200 mm, TR 7000 ms, TE 13 ms, TI 400 ms, slices = 40, and time 7 min. | NA | -- | DIR WM lesion, mean nr = 1; PSIR WML, mean nr = 1.6; DIR cortical L, mean nr = 1.1; PSIR cortical mean: nr = 2.3 | WM and/or GM lesions were found in the cerebellum in patients. PSIR allowed the identification of a significantly higher number of GM lesions than DIR. Patients had no symptoms of cerebellar dysfunction. The number of supratentorial lesions did not correlate with the number of cerebellar lesions |
| De Meo, 2021 [43] | MS: N = 70 (44 girls); median age = 15.6. HCs: N = 26 (16 girls); median age = 15.7 | Patients’ EDSS = Median: 1.5 (Range: 0.0–4.0) | 3T; 3D T1-weighted | MRI analysis Cortical surface reconstruction T1/T2 ratio image reconstruction DTI analysis—FA and MD | -- | Median T2 LV, mL (IQR) = 2.9 (1.3–6.3) Median T1 LV, mL (IQR) = 1.7 (0.7–3.8) Median T2 thalamic LV (normalized for thalamic volume), ml (IQR) = 0.0 (0.0–0.8) | Compared to healthy controls, patients had significantly increased fractional anisotropy in the whole thalamus and increased mean diffusivity in thalamic white matter with a trend toward a reduced thalamic volume. In patients, significant fractional anisotropy abnormalities were detected in bands nearest to CSF and in those closest to white matter, while significant mean diffusivity and T1/T2-weighted ratio abnormalities were found in thalamic bands closest to CSF |
| Cacciaguerra, 2024 [41] | MS: N = 76 (46 girls); age: (median) 14.8 (7.0–17.8). HCs: N = 22 (11 girls); age: 13.8 (8.3–17.9) | EDSS = Median: 1.5 (0.0–4.0) Neuropsychological assessment | 3T: T2*-weighted single-shot echo planar imaging (EPI)-T1W | FC | Resting state | Mean = 6.9 SD = 8.5 mL | PedMS showed reduced RS FC in all networks compared to controls, especially in the basal ganglia. In younger patients, reduced RS FC in the basal ganglia, language, and sensorimotor networks is associated with poorer cognitive performance. Older patients showed increased RS FC in the basal ganglia, DMN. In both groups, lower RS FC of the caudate nucleus was associated with poorer executive speed |
| Rocca, 2009 [25] | MS: N = 17 (11 girls); age: 14.6. HCs: N = 9 (6 girls); age 15.6 | EDSS = Median: 1.0, range 5 0.0–3.0 | 1.5T T2*-W EPI; dual-echo turbo spin echo sequence (TSE); MPRAGE | FC | Finger tapping + rest | Mean = 9.4 ± 13.3 mL | MS patients > HCs increased recruitment of the left (L) primary sensorimotor cortex (SMC). They also showed reduced FC between the L primary SMC and the L thalamus, the L insula and the L secondary sensorimotor cortex, the L thalamus and the L insula, and the L thalamus and the L SII |
| Green, 2018 [40] | MS: N = 32; 78% girls; age: 16.28. HCs: N = 30; 80% girls; age: 16.01 | EDSS = Median: 1.0, range 0.0–4.0 Neuropsychological assessment | --- | -- | -- | -- | Greater amygdala volume in patients correlated with parent-reported functional communication and social skills. The right amygdala volume was positively associated with visual memory; the left amygdala volume was a stronger predictor of parent-reported social skills |
| Waubant, 2009 [42] | MS: N = 41 (51.1% girls); age, mean (SD) 11.4 (4.4). | -- | 1.5T/3T; T2-weighted FLAIR | Mann- Whitney Multivariate logistic regression | -- | Number of T2 LV = 21 | Pediatric MS patients showed significant lesions in the juxtacortical, cerebellar, brainstem, and periventricular areas |
| Kerbrat, 2012 [39] | MS: N = 38; (29 girls); age: 15.2. HCs: N = 33 (28 girls); age: 15.6 | -- | 1.5 T; T1-W gradient-recalled echo; 2-dimensional multislice proton density-weighted/T2-W fast spin echo | -- | -- | Mean = 6.3 ± 8.1 mL | The intracranial volume z-score was significantly lower in MS patients compared with the HC participants. Patients with MS also demonstrated significant decreases in normalized brain volume z scores. After correction for global brain volume, thalamic volumes in the MS population remained lower than those of HCs |
| Rocca, 2010 [28] | Pediatric MS: N = 17 (11 girls); age: 14.6. HCs: N = 10 (6 girls); age 15.8. Adult patients/Adult HCs | EDSS = 1.0 (0–3.0) | 1.5 T; T2*-W single-shot EPI | Dynamic causal modeling | Finger tapping | Mean = 9.4 ± 13.3 | The connectivity of the sensorimotor network was similar in control subjects and pediatric MS patients |
| Clusters | Hemisphere | BA | x | Y | z | ALE | Z | p-Value |
|---|---|---|---|---|---|---|---|---|
| Dorsal anterior cingulate cortex | R | 32 | 2 | 12 | 46 | 0.013638 | 5.568491 | 0.00000 |
| Supplementary motor cortex | L | 6 | −4 | −2 | 58 | 0.005488 | 2.99942 | 0.00135 |
| Superior parietal Lobe | R | 7 | 26 | −70 | 34 | 0.011553 | 5.012381 | 0.00000 |
| Angular gyrus | R | 39 | 42 | −58 | 38 | 0.010642 | 4.790189 | 0.00000 |
| Angular gyrus | L | 39 | −44 | −72 | 34 | 0.00792 | 3.794148 | 0.00007 |
| Angular gyrus inferior part | L | 39 | −50 | −64 | 26 | 0.005742 | 3.196908 | 0.00069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cera, N.; Pinto, J.; Faustino, R. Functional and Structural Alterations in Pediatric Multiple Sclerosis: A Systematic Review and a Preliminary Activation Likelihood Estimation Functional Magnetic Resonance Imaging Meta-Analysis. Pediatr. Rep. 2025, 17, 57. https://doi.org/10.3390/pediatric17030057
Cera N, Pinto J, Faustino R. Functional and Structural Alterations in Pediatric Multiple Sclerosis: A Systematic Review and a Preliminary Activation Likelihood Estimation Functional Magnetic Resonance Imaging Meta-Analysis. Pediatric Reports. 2025; 17(3):57. https://doi.org/10.3390/pediatric17030057
Chicago/Turabian StyleCera, Nicoletta, Joana Pinto, and Ricardo Faustino. 2025. "Functional and Structural Alterations in Pediatric Multiple Sclerosis: A Systematic Review and a Preliminary Activation Likelihood Estimation Functional Magnetic Resonance Imaging Meta-Analysis" Pediatric Reports 17, no. 3: 57. https://doi.org/10.3390/pediatric17030057
APA StyleCera, N., Pinto, J., & Faustino, R. (2025). Functional and Structural Alterations in Pediatric Multiple Sclerosis: A Systematic Review and a Preliminary Activation Likelihood Estimation Functional Magnetic Resonance Imaging Meta-Analysis. Pediatric Reports, 17(3), 57. https://doi.org/10.3390/pediatric17030057







