The Penguin Study: A Randomised, Double-Blinded, Equivalence Trial on the Safety and Suitability of an Infant Formula with Partially Hydrolysed 100% Whey Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Centres
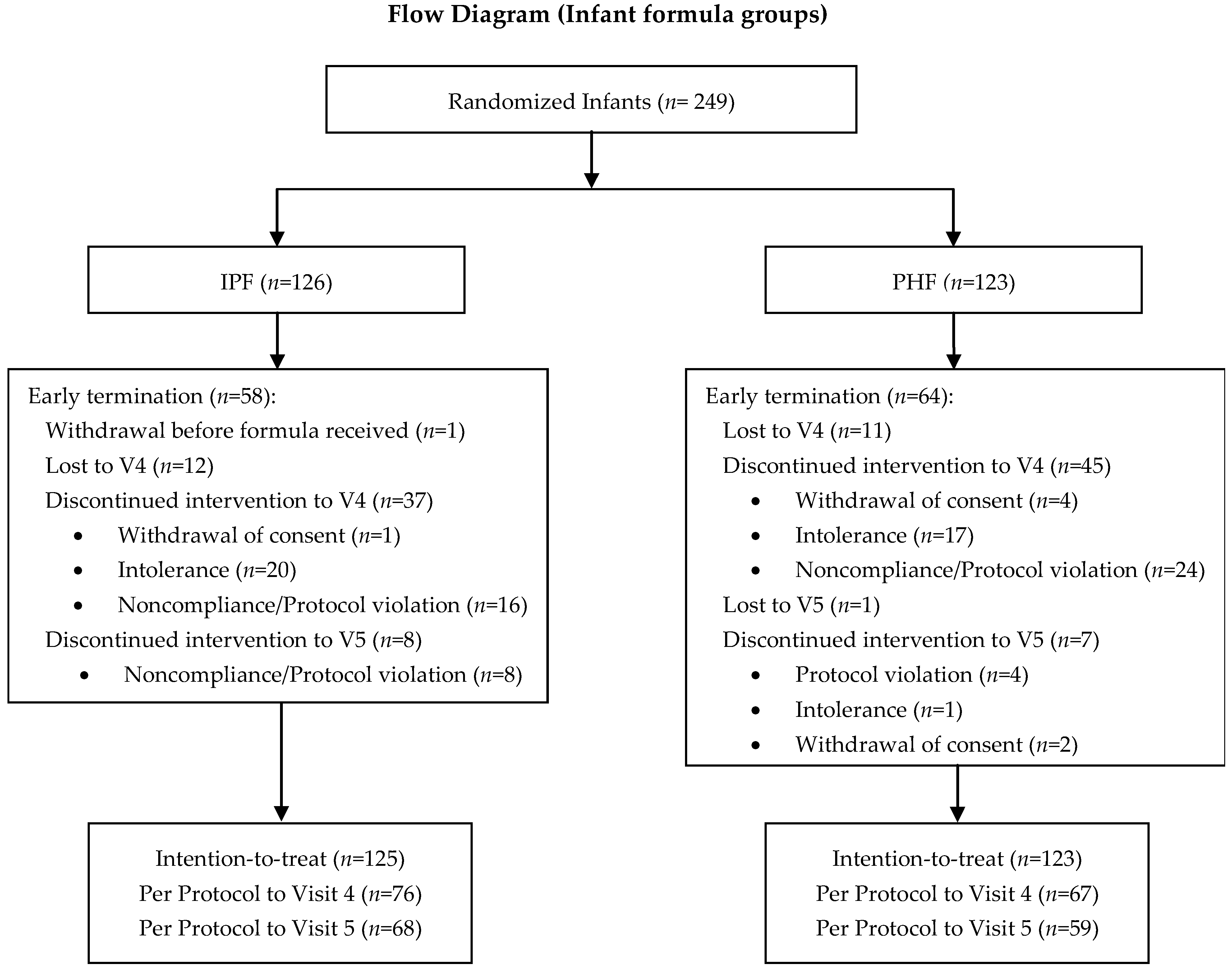
2.2. Study Design and Population
2.3. Study Product
2.4. Anthropometric Measurements
2.5. Formula Intake
2.6. Overall Health and Adverse Events
2.7. Statistics
3. Results
3.1. Study Population Characteristics
3.2. Growth
3.2.1. Primary Objective: Equivalence in Weight Gain
3.2.2. Secondary Objectives: Further Growth Parameters
3.3. Formula Intake
3.4. Behaviour and Tolerance
3.5. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieto, A.; Wahn, U.; Bufe, A.; Eigenmann, P.; Halken, S.; Hedlin, G.; Høst, A.; Hourihane, J.; Just, J.; Lack, G.; et al. Allergy and asthma prevention 2014. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2014, 25, 516–533. [Google Scholar] [CrossRef]
- Bührer, C.; Genzel-Boroviczény, O.; Jochum, F.; Kauth, T.; Kersting, M.; Koletzko, B.; Mihatsch, W.; Przyrembel, H.; Reinehr, T.; Zimmer, P.; et al. Ernährung gesunder Säuglinge. Monatsschr. Kinderheilkd. 2014, 162, 527–538. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Latiff, A.H.A.; Fleischer, D.M.; Gutiérrez-Castrellón, P.; Miqdady, M.-I.S.; Smith, P.K.; von Berg, A.; Greenhawt, M.J. Partially hydrolyzed formula in non-exclusively breastfed infants: A systematic review and expert consensus. Nutrition 2019, 57, 268–274. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef]
- Schäfer, T.; Bauer, C.-P.; Beyer, K.; Bufe, A.; Friedrichs, F.; Gieler, U.; Gronke, G.; Hamelmann, E.; Hellermann, M.; Kleinheinz, A.; et al. S3-Guideline on allergy prevention: 2014 update: Guideline of the German Society for Allergology and Clinical Immunology (DGAKI) and the German Society for Pediatric and Adolescent Medicine (DGKJ). Allergo J. Int. 2014, 23, 186–199. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation (EU) 2016/127 of 25 September 2015 supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as regards the specific compositional and information requirements for infant formula and follow-on formula and as regards requirements on information relating to infant and young child feeding (Text with EEA relevance). Off. J. Eur. Union 2016, 25, 1–29. [Google Scholar]
- WHO Breastfeeding. Available online: https://www.who.int/westernpacific/health-topics/breastfeeding (accessed on 17 March 2022).
- EFSA Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [CrossRef]
- American Academy of Pediatrics, Committee on Nutrition Task Force. Clinical Testing of Infant Formulas with Respect to Nutritional Suitability for Term Infants. Available online: https://wayback.archive-it.org/7993/20170722090324/https://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm170649.htm (accessed on 26 April 2022).
- Fields, D.; Czerkies, L.; Sun, S.; Storm, H.; Saavedra, J.; Sorensen, R. A randomized controlled trial assessing growth of infants fed a 100% whey extensively hydrolyzed formula compared with a casein-based extensively hydrolyzed formula. Glob. Pediatr. Health 2016, 3, 2333794X16636613. [Google Scholar] [CrossRef]
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631. [Google Scholar] [CrossRef]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Available online: https://www.who.int/tools/child-growth-standards/standards (accessed on 17 March 2022).
- Czerkies, L.A.; Kineman, B.D.; Cohen, S.S.; Reichert, H.; Carvalho, R.S. A Pooled Analysis of Growth and Tolerance of Infants Exclusively Fed Partially Hydrolyzed Whey or Intact Protein-Based Infant Formulas. Int. J. Pediatr. 2018, 2018, 4969576. [Google Scholar] [CrossRef]
- Kantaras, P.; Kokkinopoulou, A.; Hageman, J.H.J.; Hassapidou, M.; Androutsos, O.; Kanaki, M.; Bovee-Oudenhoven, I.; Karaglani, E.; Kontochristopoulou, A.-M.; Bos, R.; et al. Growth and gut comfort of healthy term infants exclusively fed with a partially hydrolysed protein-based infant formula: A randomized controlled double-blind trial. Front. Pediatr. 2024, 12, 1328709. [Google Scholar] [CrossRef]
- Karaglani, E.; Thijs-Verhoeven, I.; Gros, M.; Chairistanidou, C.; Zervas, G.; Filoilia, C.; Kampani, T.-M.; Miligkos, V.; Matiatou, M.; Valaveri, S.; et al. A Partially Hydrolyzed Whey Infant Formula Supports Appropriate Growth: A Randomized Controlled Non-Inferiority Trial. Nutrients 2020, 12, 3056. [Google Scholar] [CrossRef] [PubMed]
- Picaud, J.-C.; Pajek, B.; Arciszewska, M.; Tarczón, I.; Escribano, J.; Porcel, R.; Adelt, T.; Hassink, E.; Rijnierse, A.; Abrahamse-Berkeveld, M.; et al. An Infant Formula with Partially Hydrolyzed Whey Protein Supports Adequate Growth and Is Safe and Well-Tolerated in Healthy, Term Infants: A Randomized, Double-Blind, Equivalence Trial. Nutrients 2020, 12, 2072. [Google Scholar] [CrossRef] [PubMed]
- Rigo, J.; Schoen, S.; Verghote, M.; van Overmeire, B.; Marion, W.; Abrahamse-Berkeveld, M.; Alliet, P. Partially Hydrolysed Whey-Based Formulae with Reduced Protein Content Support Adequate Infant Growth and Are Well Tolerated: Results of a Randomised Controlled Trial in Healthy Term Infants. Nutrients 2019, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential Growth Patterns Among Healthy Infants Fed Protein Hydrolysate or Cow-Milk Formulas. Pediatrics 2011, 127, 110–118. [Google Scholar] [CrossRef]
- Mennella, J.A.; Griffin, C.E.; Beauchamp, G.K. Flavor Programming During Infancy. Pediatrics 2004, 113, 840–845. [Google Scholar] [CrossRef]
- Ahrens, B.; Hellmuth, C.; Haiden, N.; Olbertz, D.; Hamelmann, E.; Vusurovic, M.; Fleddermann, M.; Roehle, R.; Knoll, A.; Koletzko, B.; et al. Hydrolyzed Formula with Reduced Protein Content Supports Adequate Growth: A Randomized Controlled Noninferiority Trial. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 822–830. [Google Scholar] [CrossRef]
- Berendzen, J.A.; Howard, B.C. Association between cesarean delivery rate and body mass index. Tenn. Med. J. Tenn. Med. Assoc. 2013, 106, 35–37, 42. [Google Scholar]
- Davie, P.; Bick, D.; Chilcot, J. To what extent does maternal body mass index predict intentions, attitudes, or practices of early infant feeding? Matern. Child. Nutr. 2019, 15, e12837. [Google Scholar] [CrossRef]
- Flores, T.R.; Mielke, G.I.; Wendt, A.; Nunes, B.P.; Bertoldi, A.D. Prepregnancy weight excess and cessation of exclusive breastfeeding: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2018, 72, 480–488. [Google Scholar] [CrossRef]
- Ziegler, E.E. Growth of Breast-Fed and Formula-Fed Infants. In Protein and Energy Requirements in Infancy and Childhood; S.Karger AG: Basel, Switzerland, 2006; Volume 58, pp. 51–64. [Google Scholar] [CrossRef]
- Botton, J.; Heude, B.; Maccario, J.; Borys, J.-M.; Lommez, A.; Ducimetière, P.; Charles, M.A.; FLVS Study Group. Parental body size and early weight and height growth velocities in their offspring. Early Hum. Dev. 2010, 86, 445–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- CDC Results: Breastfeeding and Infant Feeding Practices. Available online: https://www.cdc.gov/breastfeeding-data/studies/methods-results-ifps.html?CDC_AAref_Val=https://www.cdc.gov/breastfeeding/data/ifps/results.htm (accessed on 17 March 2022).
- Jia, N.; Gu, G.; Zhao, L.; He, S.; Xiong, F.; Chai, Y.; Quan, L.; Hou, H.; Dai, Y. Longitudinal study of breastfeeding and growth in 0–6 month infants. Asia Pac. J. Clin. Nutr. 2018, 27, 1294–1301. [Google Scholar] [CrossRef] [PubMed]

| Content per 100 mL | PHF | IPF |
|---|---|---|
| Energy (kcal/kJ) | 64/269 | 64/269 |
| Fat (g) * | 3.26 | 3.26 |
| Saturated (g) | 1.4 | 1.4 |
| Monounsaturated (g) | 1.2 | 1.2 |
| Polyunsaturated (g) | 0.59 | 0.59 |
| Linoleic acid (mg) | 422.4 | 422.4 |
| Alpha linolenic acid (mg) | 57.0 | 57.0 |
| Arachidonic acid (mg) | 15.4 | 15.4 |
| Docosahexaenoic acid (mg) | 15.4 | 15.4 |
| Protein (g) | 1.3 | 1.3 |
| Whey–Casein | 100:0 | 60:40 |
| Carbohydrate (g) † | 7.28 | 7.28 |
| scGOS (g) | 0.54 | 0.54 |
| Parameter | IPF (n = 76) | PHF (n = 67) | Breastfed (n = 45) |
|---|---|---|---|
| Sex, girls | 35 (46.1) | 33 (49.3) | 24 (53.3) |
| Birth characteristics | |||
| Caesarean section | 38 (50.7) | 40 (59.7) | 17 (38.6) |
| Complications | 4 (5.3) | 0 (0) | 0 (0) |
| Gestational age (weeks) | 38.8 ± 1.02 | 38.9 ± 0.99 | 39.0 ± 1.04 |
| Weight (g) | 3285.2 ± 448.74 | 3278.8 ± 381.85 | 3421.9 ± 346.18 |
| Length (cm) | 50.3 ± 2.14 | 50.6 ± 2.30 | 51.5 ± 2.05 |
| Head circumference (cm) | 34.7 ± 1.42 | 34.5 ± 1.33 | 34.7 ± 1.34 |
| Baseline characteristics | |||
| Age (days) | 9.3 ± 9.21 | 8.9 ± 8.38 | 7.9 ± 6.78 |
| Weight (g) | 3339.7 ± 501.79 | 3303.8 ± 457.00 | 3433.9 ± 382.42 |
| Length (cm) | 50.6 ± 2.14 | 50.8 ± 2.18 | 52.1 ± 2.22 |
| Head circumference (cm) | 34.9 ± 1.48 | 34.7 ± 1.30 | 35.0 ± 1.38 |
| Parental characteristics | |||
| Weight of mother (kg) | 75.1 ± 17.11 | 74.8 ± 14.18 | 68.6 ± 15.32 |
| Height of mother (cm) | 165.3 ± 6.95 | 166.4 ± 6.77 | 166.3 ± 6.33 |
| BMI of mother (kg/m2) | 27.5 ± 6.16 | 27.0 ± 5.26 | 24.8 ± 5.16 |
| Age of mother (years) | 30.5 ± 5.65 | 29.7 ± 5.84 | 31.9 ± 4.42 |
| Weight of father (kg) | 88.4 ± 15.47 | 90.2 ± 17.44 | 86.5 ± 10.79 |
| Height of father (cm) | 178.7 ± 6.09 | 177.8 ± 7.70 | 181.0 ± 7.15 |
| BMI of father (kg/m2) | 27.6 ± 4.36 | 28.5 ± 5.14 | 26.4 ± 3.23 |
| Age of father (years) | 34.0 ± 7.02 | 33.2 ± 7.68 | 34.2 ± 4.78 |
| Country | |||
| Germany | 40 (52.6) | 34 (50.8) | 21 (46.7) |
| Bulgaria | 36 (47.4) | 33 (49.3) | 24 (53.3) |
| Population | Country | Group | N | Weight Gain (g/d) V1 to V4. LSM (SEM) | Difference Between Groups. Estimate (95% CI) | Sex p-Value | Country p-Value | Group p-Value |
|---|---|---|---|---|---|---|---|---|
| Partial η2 | Partial η2 | Partial η2 | ||||||
| PP | All | PHF | 67 | 34.9 (0.77) | 2.4 (0.3–4.5) | 0.002 | 0.038 | 0.027 |
| IPF | 76 | 32.5 (0.73) | 0.07 | 0.03 | 0.04 | |||
| Germany | PHF | 34 | 35.3 (1.10) | 0.9 (−2.0–3.9) | ||||
| IPF | 40 | 34.4 (1.02) | ||||||
| Bulgaria | PHF | 33 | 34.5 (1.11) | 3.9 (0.9–7.0) | ||||
| IPF | 36 | 30.5 (1.08) | ||||||
| ITT | All | PHF | 123 | 34.6 (0.70) | 1.9 (−0.0–3.9) | <0.001 | 0.092 | 0.059 |
| IPF | 125 | 32.7 (0.69) | 0.08 | 0.02 | 0.02 | |||
| Germany | PHF | 76 | 35.0 (1.00) | 0.79 (−1.91–3.49) | ||||
| IPF | 75 | 34.2 (0.96) | ||||||
| Bulgaria | PHF | 47 | 34.3 (1.01) | 3.05 (0.23–5.86) | ||||
| IPF | 50 | 31.2 (1.02) |
| Variable | Visit | IPF (n = 68) | PHF (n = 59) | Breastfed (n = 45) | ||
|---|---|---|---|---|---|---|
| LSM | SEM | LSM | SEM | Mean | ||
| Weight (g) a,c,e,f | V1 | 3340.9 | 76.36 | 3284.8 | 81.27 | 3433.9 |
| V2 | 4421.4 | 4438.3 | 4506.6 | |||
| V3 | 5434.0 | 5491.7 | 5496.8 | |||
| V4 | 6305.0 | 6447.0 | 6232.7 | |||
| V5 | 7920.0 | 8207.9 | 7714.4 | |||
| Length (cm) a,b,c,e | V1 | 50.6 | 0.34 | 50.7 | 0.37 | 52.1 |
| V2 | 54.0 | 54.2 | 55.6 | |||
| V3 | 57.2 | 56.8 | 59.3 | |||
| V4 | 60.0 | 60.3 | 62.5 | |||
| V5 | 66.6 | 67.1 | 67.3 | |||
| Head circumference (cm) a,b,e | V1 | 34.8 | 0.17 | 34.6 | 0.18 | 35.0 |
| V2 | 37.0 | 36.8 | 37.2 | |||
| V3 | 38.7 | 38.7 | 39.0 | |||
| V4 | 40.0 | 39.9 | 40.4 | |||
| V5 | 43.4 | 43.2 | 43.0 | |||
| Visit | IPF | PHF | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | LSM | SEM | N | LSM | SEM | ||
| V2 | 63 | 161.6 | 3.60 | 58 | 162.8 | 3.78 | 0.823 |
| V3 | 63 | 143.51 | 3.59 | 58 | 144.5 | 3.78 | 0.845 |
| V4 | 67 | 129.0 | 3.49 | 54 | 132.7 | 3.88 | 0.475 |
| V5 | 65 | 101.8 | 3.54 | 56 | 94.5 | 3.88 | 0.164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otten, L.; Nomayo, A.; Gunn, C.; Fuad, M.; Kuhn-Sherlock, B.; Gallier, S.; Schelker, E.; Foster, J.; Jochum, F. The Penguin Study: A Randomised, Double-Blinded, Equivalence Trial on the Safety and Suitability of an Infant Formula with Partially Hydrolysed 100% Whey Protein. Pediatr. Rep. 2025, 17, 45. https://doi.org/10.3390/pediatric17020045
Otten L, Nomayo A, Gunn C, Fuad M, Kuhn-Sherlock B, Gallier S, Schelker E, Foster J, Jochum F. The Penguin Study: A Randomised, Double-Blinded, Equivalence Trial on the Safety and Suitability of an Infant Formula with Partially Hydrolysed 100% Whey Protein. Pediatric Reports. 2025; 17(2):45. https://doi.org/10.3390/pediatric17020045
Chicago/Turabian StyleOtten, Lindsey, Antonia Nomayo, Caroline Gunn, Maher Fuad, Barbara Kuhn-Sherlock, Sophie Gallier, Elisabeth Schelker, Janine Foster, and Frank Jochum. 2025. "The Penguin Study: A Randomised, Double-Blinded, Equivalence Trial on the Safety and Suitability of an Infant Formula with Partially Hydrolysed 100% Whey Protein" Pediatric Reports 17, no. 2: 45. https://doi.org/10.3390/pediatric17020045
APA StyleOtten, L., Nomayo, A., Gunn, C., Fuad, M., Kuhn-Sherlock, B., Gallier, S., Schelker, E., Foster, J., & Jochum, F. (2025). The Penguin Study: A Randomised, Double-Blinded, Equivalence Trial on the Safety and Suitability of an Infant Formula with Partially Hydrolysed 100% Whey Protein. Pediatric Reports, 17(2), 45. https://doi.org/10.3390/pediatric17020045






