Energy Utilization in Premature Neonates Undergoing Screening for Retinopathy of Prematurity
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Enrollment
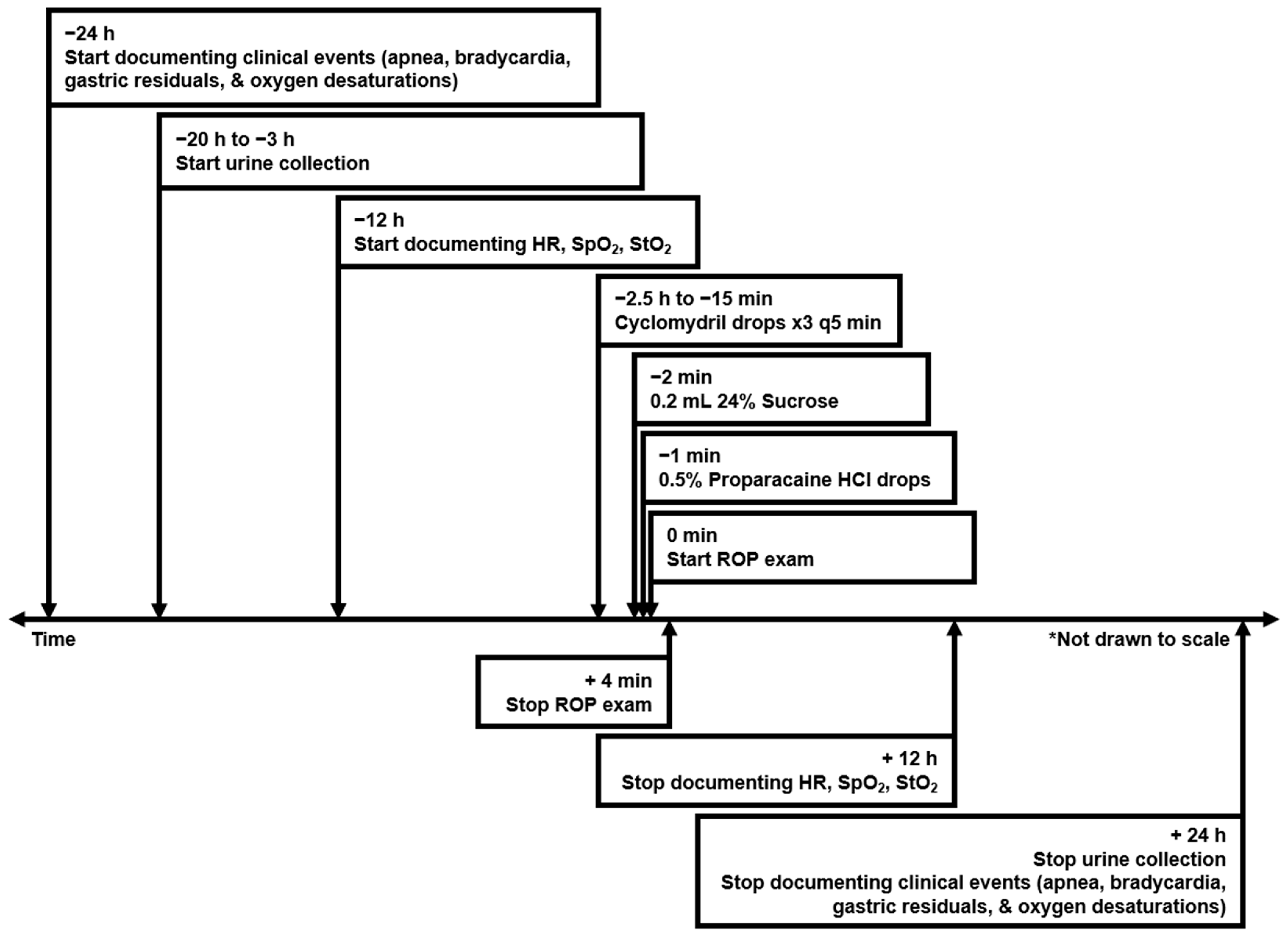
2.2. ROP Screening Procedure and Documentation of Clinical Data
2.3. Measurement of Urinary Uric Acid-to-Creatinine Concentration Ratios
2.4. Statistical Analysis
3. Results
3.1. Subject Demographics
3.2. Urinary Uric Acid-to-Creatinine Concentration Ratios Before and After ROP Exam
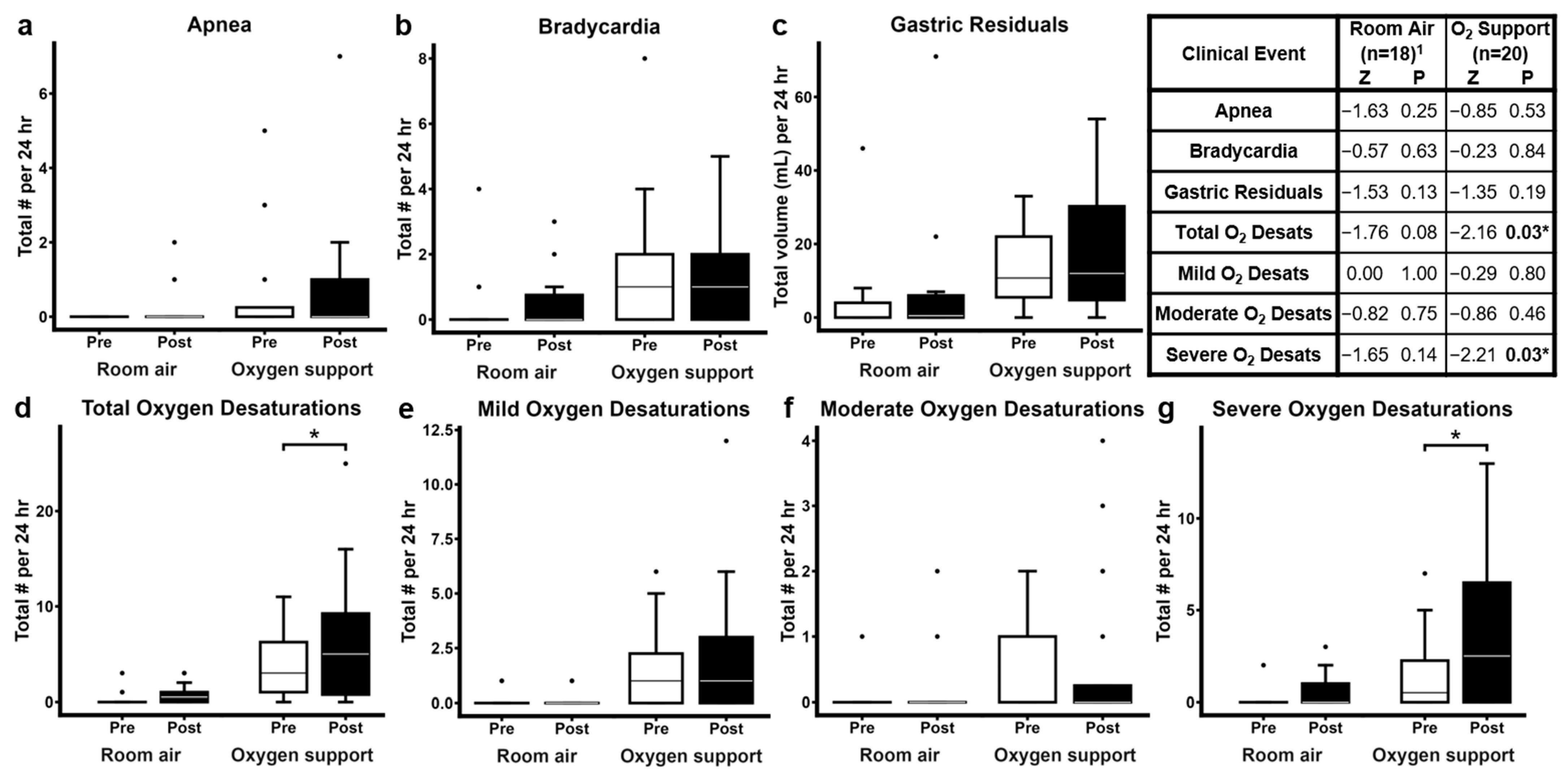
3.3. Clinically Significant Events Before and After ROP Examination
3.4. ROP Diagnosis at Discharge
4. Discussion
4.1. Relationship Between ROP Examination and Urinary Uric Acid-to-Creatinine Concentration Ratios
4.2. ROP Examination and Clinically Significant Oxygen Desaturations
4.3. Conceptualizing the Relationships Between ROP Examination, Increased Urinary Uric Acid-to-Creatinine Concentration Ratios, and Increased Oxygen Desaturations
4.4. Clinical Implications
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-Ap | 2-Aminopurine |
| CGA | Corrected gestational age |
| EGA | Estimated gestational age |
| HPLC | High-performance liquid chromatography |
| NICU | Neonatal intensive care unit |
| NIDCAP | Newborn Individualized Developmental Care and Assessment Program |
| ROP | Retinopathy of prematurity |
| SNAPPE-II | Score for Neonatal Acute Physiology with Perinatal Extension II |
| SpO2 | Systemic peripheral oxygen saturation |
| mStO2 | Mesenteric regional tissue oxygen saturation |
| [UA]/[Cr] | Uric acid-to-creatinine ratio |
References
- Fierson, W.M. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics 2018, 142, e20183061. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.G.; Repka, M.X. Diode laser photocoagulation for threshold retinopathy of prematurity. A randomized study. Ophthalmology 1993, 100, 238–244. [Google Scholar] [CrossRef]
- Iverson, D.A.; Trese, M.T.; Orgel, I.K.; Williams, G.A. Laser photocoagulation for threshold retinopathy of prematurity. Arch. Ophthalmol. 1991, 109, 1342–1343. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.A.; Tasman, W.; Brown, G.C.; Federman, J.L. Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology 1991, 98, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Laser ROP Study Group. Laser therapy for retinopathy of prematurity. Arch. Ophthalmol. 1994, 112, 154–156. [Google Scholar] [CrossRef]
- Behura, S.S.; Dhanawat, A.; Nayak, B.; Panda, S.K. Comparison between oral melatonin and 24% sucrose for pain management during retinopathy of prematurity screening: A randomized controlled trial. Turk. J. Pediatr. 2022, 64, 1013–1020. [Google Scholar] [CrossRef]
- Fajolu, I.B.; Dedeke, I.O.F.; Ezenwa, B.N.; Ezeaka, V.C. Non-pharmacological pain relief interventions in preterm neonates undergoing screening for retinopathy of prematurity: A systematic review. BMJ Open Ophthalmol. 2023, 8, e001271. [Google Scholar] [CrossRef]
- Onuagu, V.; Gardner, F.; Soni, A.; Doheny, K.K. Autonomic measures identify stress, pain, and instability associated with retinopathy of prematurity ophthalmologic examinations. Front. Pain Res. 2022, 3, 1032513. [Google Scholar] [CrossRef]
- Ozgun, U.; Demet, T.; Ozge, K.A.; Zafer, D.; Murat, S.; Mehmet, Y.; Nilgun, K. Fatal necrotising enterocolitis due to mydriatic eye drops. J. Coll. Physicians Surg. Pak. 2014, 24 (Suppl. S2), S147–S149. [Google Scholar]
- Tan, J.B.C.; Dunbar, J.; Hopper, A.; Wilson, C.G.; Angeles, D.M. Differential effects of the retinopathy of prematurity exam on the physiology of premature infants. J. Perinatol. 2019, 39, 708–716. [Google Scholar] [CrossRef]
- Plank, M.S.; Boskovic, D.S.; Tagge, E.; Chrisler, J.; Slater, L.; Angeles, K.R.; Angeles, D.M. An animal model for measuring the effect of common NICU procedures on ATP metabolism. Biol. Res. Nurs. 2011, 13, 283–288. [Google Scholar] [CrossRef]
- Slater, L.; Asmerom, Y.; Boskovic, D.S.; Bahjri, K.; Plank, M.S.; Angeles, K.R.; Phillips, R.; Deming, D.; Ashwal, S.; Hougland, K.; et al. Procedural pain and oxidative stress in premature neonates. J. Pain 2012, 13, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Angeles, D.M.; Boskovic, D.S.; Deming, D.; Hopper, A.; Peverini, R.; Czynski, A.; Hoch, E.; Phillips, R.; Tan, J.B.; Camberos, V.; et al. A pilot study on the biochemical effects of repeated administration of 24% oral sucrose vs. 30% oral dextrose on urinary markers of adenosine triphosphate degradation. J. Perinatol. 2021, 41, 2761–2765. [Google Scholar] [CrossRef]
- Bellos, I.; Fitrou, G.; Pergialiotis, V.; Perrea, D.N.; Papantoniou, N.; Daskalakis, G. Random urine uric acid to creatinine and prediction of perinatal asphyxia: A meta-analysis. J. Matern.-Fetal Neonatal Med. 2019, 32, 3864–3870. [Google Scholar] [CrossRef] [PubMed]
- Bhongir, A.V.; Yakama, A.V.; Saha, S.; Radia, S.B.; Pabbati, J. The urinary uric acid/creatinine ratio is an adjuvant marker for perinatal asphyxia. Eur. J. Pharm. Med. Res. 2015, 2, 520–528. [Google Scholar] [PubMed]
- Patel, K.P.; Makadia, M.G.; Patel, V.I.; Nilayangode, H.N.; Nimbalkar, S.M. Urinary uric acid/creatinine ratio—A marker for perinatal asphyxia. J. Clin. Diagn. Res. 2017, 11, SC08–SC10. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef]
- Gibbins, S.; Stevens, B. Mechanisms of sucrose and non-nutritive sucking in procedural pain management in infants. Pain Res. Manag. 2001, 6, 21–28. [Google Scholar] [CrossRef]
- Moresco, L.; Sjögren, A.; Marques, K.A.; Soll, R.; Bruschettini, M. Caffeine versus other methylxanthines for the prevention and treatment of apnea in preterm infants. Cochrane Database Syst. Rev. 2023, 10, Cd015462. [Google Scholar]
- Eichenwald, E.C.; Hansen, A.R.; Martin, C.R.; Stark, A.R. (Eds.) Cloherty and Stark’s Manual of Neonatal Care, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2017. [Google Scholar]
- Thoyre, S.M.; Carlson, J. Occurrence of oxygen desaturation events during preterm infant bottle feeding near discharge. Early Hum. Dev. 2003, 72, 25–36. [Google Scholar] [CrossRef]
- Holden, M.S.; Hopper, A.; Slater, L.; Asmerom, Y.; Esiaba, I.; Boskovic, D.S.; Angeles, D.M. Urinary hypoxanthine as a measure of increased ATP utilization in late preterm infants. Infant. Child. Adolesc. Nutr. 2014, 6, 240–249. [Google Scholar] [CrossRef] [PubMed]
- George, S.K.; Dipu, M.T.; Mehra, U.R.; Singh, P.; Verma, A.K.; Ramgaokar, J.S. Improved HPLC method for the simultaneous determination of allantoin, uric acid and creatinine in cattle urine. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2006, 832, 134–137. [Google Scholar] [CrossRef]
- Plank, M.S.; Calderon, T.C.; Asmerom, Y.; Boskovic, D.S.; Angeles, D.M. Biochemical measurement of neonatal hypoxia. J. Vis. Exp. 2011, 54, 2948. [Google Scholar]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Richardson, D.K.; Corcoran, J.D.; Escobar, G.J.; Lee, S.K. SNAP-II and SNAPPE-II: Simplified newborn illness severity and mortality risk scores. J. Pediatr. 2001, 138, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Rachuri, S.; Paul, S.; Jaidev, M.D. SNAPPE II score: Predictor of mortality in NICU. Int. J. Contemp. Pediatr. 2019, 6, 422–426. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Green, A.; Jeffs, D.A.; Roberson, P.K. Physiologic effects of retinopathy of prematurity screening examinations. Adv. Neonatal Care 2011, 11, 291–297. [Google Scholar] [CrossRef]
- Lee, J.M.; Kodsi, S.R.; Gaffar, M.A.; Rubin, S.E. Cardiopulmonary arrest following administration of Cyclomydril eyedrops for outpatient retinopathy of prematurity screening. J. AAPOS 2014, 18, 183–184. [Google Scholar] [CrossRef]
- Fairchild, K.D.; Nagraj, V.P.; Sullivan, B.A.; Moorman, J.R.; Lake, D.E. Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr. Res. 2019, 85, 987–993. [Google Scholar] [CrossRef]
- Zarif, N.O.; Arayici, S.; Celik, K.; Kihtir, Z.; Ongun, H. Intranasal dexmedetomidine reduces pain scores in preterm infants during retinopathy of prematurity screening. Front. Pediatr. 2024, 12, 1441324. [Google Scholar]
- Mataftsi, A.; Lithoxopoulou, M.; Seliniotaki, A.K.; Talimtzi, P.; Oustoglou, E.; Diamanti, E.; Soubasi, V.; Ziakas, N.; Haidich, A.B. Avoiding use of lid speculum and indentation reduced infantile stress during retinopathy of prematurity examinations. Acta Ophthalmol. 2022, 100, e128–e134. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Wheaton, W.W.; Chandel, N.S. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am. J. Physiol. Cell Physiol. 2011, 300, C385–C393. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 7th ed.; WH Freeman: New York, NY, USA, 2017. [Google Scholar]
- Bobulescu, I.A.; Moe, O.W. Renal transport of uric acid: Evolving concepts and uncertainties. Adv. Chronic Kidney Dis. 2012, 19, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Mount, D.B. The molecular physiology of uric acid homeostasis. Annu. Rev. Physiol. 2015, 77, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Asmerom, Y.; Slater, L.; Boskovic, D.S.; Bahjri, K.; Holden, M.S.; Phillips, R.; Deming, D.; Ashwal, S.; Fayard, E.; Angeles, D.M. Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. J. Pediatr. 2013, 163, 29–35.e1. [Google Scholar] [CrossRef]
- Johnson, M.A.; Tekkanat, K.; Schmaltz, S.P.; Fox, I.H. Adenosine triphosphate turnover in humans. Decreased degradation during relative hyperphosphatemia. J. Clin. Investig. 1989, 84, 990–995. [Google Scholar] [CrossRef]
- Raivio, K.O.; Kekomäki, M.P.; Mäenpää, P.H. Depletion of liver adenine nucleotides induced by D-fructose. Dose-dependence and specificity of the fructose effect. Biochem. Pharmacol. 1969, 18, 2615–2624. [Google Scholar] [CrossRef]
- Gal, P.; Kissling, G.E.; Young, W.O.; Dunaway, K.K.; Marsh, V.A.; Jones, S.M.; Shockley, D.H.; Weaver, N.L.; Carlos, R.Q.; Ransom, J.L. Efficacy of sucrose to reduce pain in premature infants during eye examinations for retinopathy of prematurity. Ann. Pharmacother. 2005, 39, 1029–1033. [Google Scholar] [CrossRef]
- Sun, X.; Lemyre, B.; Barrowman, N.; O’Connor, M. Pain management during eye examinations for retinopathy of prematurity in preterm infants: A systematic review. Acta Paediatr. 2010, 99, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.N.; Lavery, K.; Dalgleish, S.; Howlett, A.; Hill, V.E.; Dotchin, S.A. Reducing discomfort of eye drops prior to retinal examination in the neonatal intensive care unit. J. Perinatol. 2020, 40, 1857–1862. [Google Scholar] [CrossRef]
- Hatfield, L.A.; Murphy, N.; Karp, K.; Polomano, R.C. A systematic review of behavioral and environmental interventions for procedural pain management in preterm infants. J. Pediatr. Nurs. 2019, 44, 22–30. [Google Scholar] [CrossRef]
- Slevin, M.; Murphy, J.F.; Daly, L.; O’Keefe, M. Retinopathy of prematurity screening, stress related responses, the role of nesting. Br. J. Ophthalmol. 1997, 81, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Rush, R.; Rush, S.; Ighani, F.; Anderson, B.; Irwin, M.; Naqvi, M. The effects of comfort care on the pain response in preterm infants undergoing screening for retinopathy of prematurity. Retina 2005, 25, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Sizun, J.; Westrup, B. Early developmental care for preterm neonates: A call for more research. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F384–F388. [Google Scholar] [CrossRef] [PubMed]
- Catelin, C.; Tordjman, S.; Morin, V.; Oger, E.; Sizun, J. Clinical, physiologic, and biologic impact of environmental and behavioral interventions in neonates during a routine nursing procedure. J. Pain 2005, 6, 791–797. [Google Scholar] [CrossRef]
- Kleberg, A.; Warren, I.; Norman, E.; Morelius, E.; Berg, A.C.; Mat-Ali, E.; Holm, K.; Fielder, A.; Nelson, N.; Hellstrom-Westas, L. Lower stress responses after Newborn Individualized Developmental Care and Assessment Program care during eye screening examinations for retinopathy of prematurity: A randomized study. Pediatrics 2008, 121, e1267–e1278. [Google Scholar] [CrossRef]


| Room Air (n = 18) | Oxygen Support (n = 20) | Test Statistic 1 | p 1 | |
|---|---|---|---|---|
| Birthweight (g) | 1232 ± 438 | 859 ± 255 | 74.50 | 0.002 * |
| EGA at Birth (wk) | 29.4 ± 2.2 | 27.0 ± 2.2 | 75.50 | 0.002 * |
| APGAR, 1 min | 5.1 ± 1.9 | 3.9 ± 2.6 | 131.00 | 0.15 |
| APGAR, 5 min | 7.0 ± 1.2 | 6.5 ± 2.2 | 177.50 | 0.95 |
| SNAPPE-II | 15.7 ± 11.0 | 22.8 ± 14.5 | 139.50 | 0.23 |
| Gender | Female: 8 (44%) Male: 10 (56%) | Female: 8 (40%) Male: 12 (60%) | 0.08 2 (df = 1) | 1.00 2 |
| Race/Ethnicity | Asian/Hispanic: 2 (11%) Black/Hispanic: 1 (6%) Black/Not Hispanic: 4 (22%) Other/Hispanic: 0 (0%) Other White/Hispanic: 1 (6%) Other White/Not Hispanic: 1 (6%) White/Hispanic: 4 (22%) White/Not Hispanic: 3 (17%) >1 Race/Hispanic: 2 (11%) | Asian/Hispanic: 1 (5%) Black/Hispanic: 0 (0%) Black/Not Hispanic: 0 (0%) Other/Hispanic: 2 (10%) Other White/Hispanic: 2 (10%) Other White/Not Hispanic: 1 (5%) White/Hispanic: 12 (60%) White/Not Hispanic: 2 (10%) >1 Race/Hispanic: 0 (%) | 13.80 2 (df = 8) | 0.049 *2 |
| Weight at Exam (g) | 2388 ± 729 | 1721 ± 553 | 79.00 | 0.003 * |
| CGA at Exam (wk) | 36.4 ± 2.7 | 34.1 ± 2.3 | 89.50 | 0.007 * |
| O2 Delivery Mode at Exam | Room air | CPAP: 5 (25%) HFNC: 10 (50%) NAVA: 1 (5%) NC: 2 (10%) NIMV: 2 (10%) | 38.00 2 (df = 5) | <0.001 *2 |
| Max FiO2 24 h Pre-exam (%) | 21.0 ± 0.0 | 29.5 ± 8.0 | 54.00 | <0.001 * |
| Max FiO2 24 h Post-exam (%) | 21.0 ± 0.0 | 33.4 ± 11.9 | 45.00 | <0.001 * |
| Room Air (n = 18) | Oxygen Support (n = 20) | Χ2 | p | |
|---|---|---|---|---|
| ROP Location at Discharge | Zone 1: 0 (0%) Zone 2: 9 (50%) Zone 3: 7 (39%) Mature: 2 (11%) | Zone 1: 0 (0%) Zone 2: 12 (60%) Zone 3: 6 (30%) Mature: 2 (10%) | 0.401 (df = 2) | 0.818 |
| ROP Severity at Discharge | Mature: 2 (11%) Stage 0: 13 (72%) Stage 1: 2 (11%) Stage 2: 1 (6%) Stage 3: 0 (0%) | Mature: 2 (10%) Stage 0: 9 (45%) Stage 1: 2 (10%) Stage 2: 6 (30%) Stage 3: 1 * (5%) | 5.208 (df = 4) | 0.267 |
| Last Documented ROP Diagnosis | ROP resolved: 18 (100%) | ROP resolved: 20 (100%) | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pentecost, A.M.; Boskovic, D.S.; Antimo, A.; Oyoyo, U.; Perry, C.C.; Dunbar, J.; Hopper, A.; Angeles, D.M. Energy Utilization in Premature Neonates Undergoing Screening for Retinopathy of Prematurity. Pediatr. Rep. 2025, 17, 29. https://doi.org/10.3390/pediatric17020029
Pentecost AM, Boskovic DS, Antimo A, Oyoyo U, Perry CC, Dunbar J, Hopper A, Angeles DM. Energy Utilization in Premature Neonates Undergoing Screening for Retinopathy of Prematurity. Pediatric Reports. 2025; 17(2):29. https://doi.org/10.3390/pediatric17020029
Chicago/Turabian StylePentecost, Alena M., Danilo S. Boskovic, Alexis Antimo, Udochukwu Oyoyo, Christopher C. Perry, Jennifer Dunbar, Andrew Hopper, and Danilyn M. Angeles. 2025. "Energy Utilization in Premature Neonates Undergoing Screening for Retinopathy of Prematurity" Pediatric Reports 17, no. 2: 29. https://doi.org/10.3390/pediatric17020029
APA StylePentecost, A. M., Boskovic, D. S., Antimo, A., Oyoyo, U., Perry, C. C., Dunbar, J., Hopper, A., & Angeles, D. M. (2025). Energy Utilization in Premature Neonates Undergoing Screening for Retinopathy of Prematurity. Pediatric Reports, 17(2), 29. https://doi.org/10.3390/pediatric17020029






