Preservation of Pancreatic Function Should Not Be Disregarded When Performing Pancreatectomies for Pancreatoblastoma in Children
Abstract
1. Introduction
2. Morbidity of Standard and Organ-Sparing Pancreatectomies in Children with Pancreatic Tumors Other than PB
3. The Burden of Long-Term Pancreatic Function Deficiencies after Pancreatectomies in Children
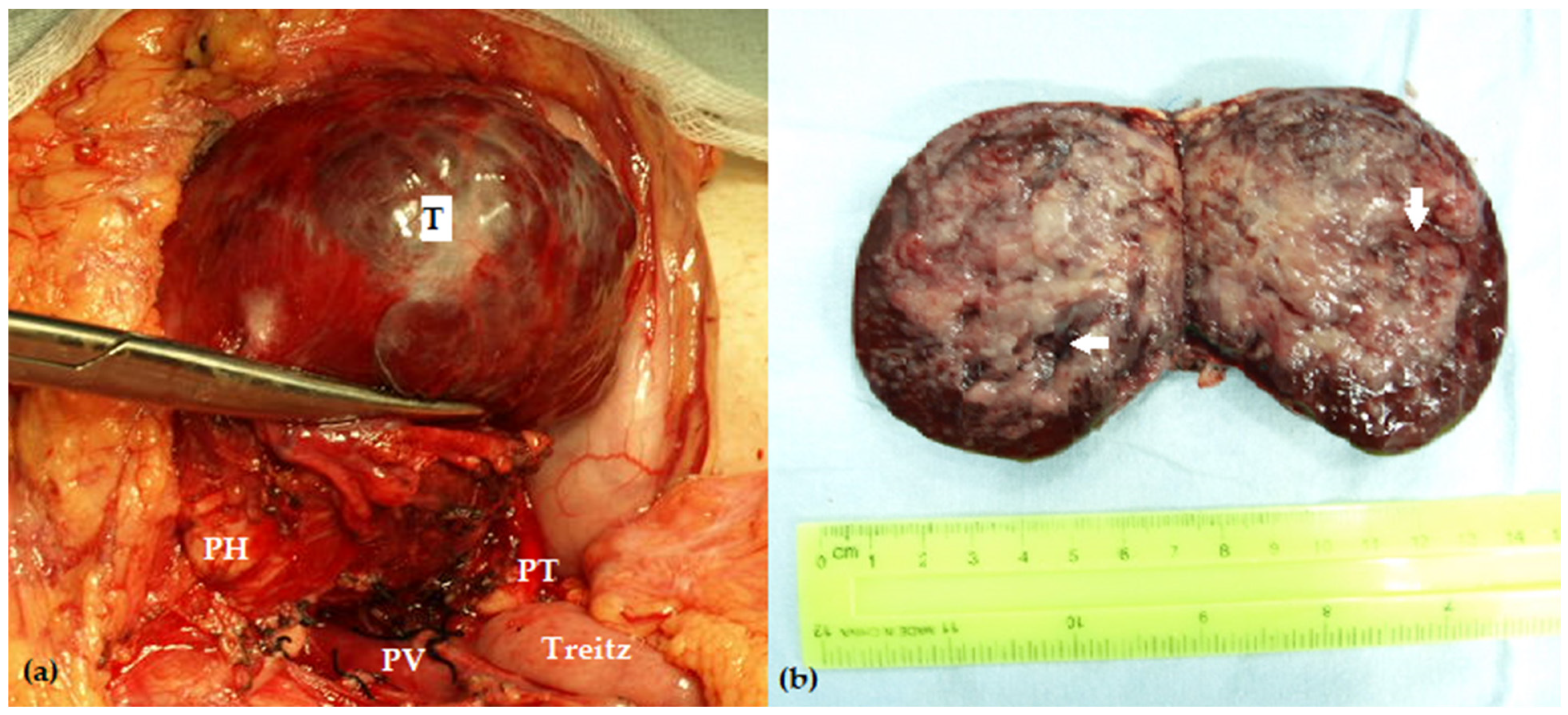
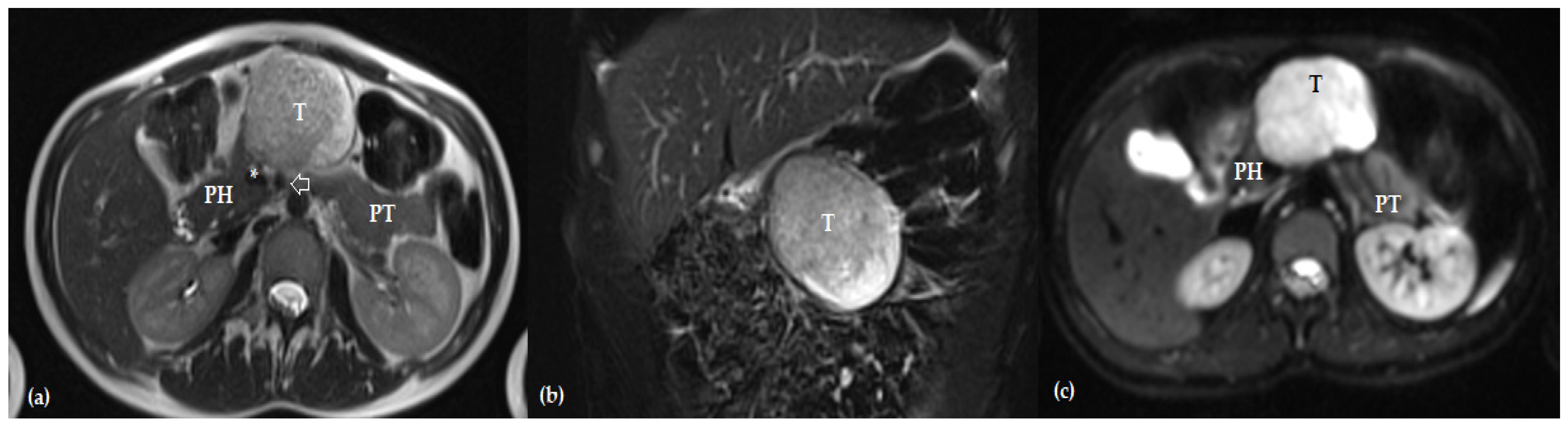
4. Current Surgical Approach and Outcomes of PB in Children
5. The Potential Role of Organ-Sparing Pancreatectomies in PB in Children
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Défachelles, A.S.; Martin De Lassalle, E.; Boutard, P.; Nelken, B.; Schneider, P.; Patte, C. Pancreatoblastoma in Childhood: Clinical Course and Therapeutic Management of Seven Patients. Med. Pediatr. Oncol. 2001, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shorter, N.A.; Glick, R.D.; Klimstra, D.S.; Brennan, M.F.; LaQuaglia, M.P. Malignant Pancreatic Tumors in Childhood and Adolescence: The Memorial Sloan-Kettering Experience, 1967 to Present. J. Pediatr. Surg. 2002, 37, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.A.; Gutierrez, J.C.; Koniaris, L.G.; Neville, H.L.; Thompson, W.R.; Sola, J.E. Malignant Pancreatic Tumors: Incidence and Outcome in 58 Pediatric Patients. J. Pediatr. Surg. 2009, 44, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Dall’Igna, P.; Cecchetto, G.; Bisogno, G.; Conte, M.; Chiesa, P.L.; D’Angelo, P.; De Leonardis, F.; De Salvo, G.; Favini, F.; Ferrari, A.; et al. Pancreatic Tumors in Children and Adolescents: The Italian TREP Project Experience. Pediatr. Blood Cancer 2010, 54, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Ellerkamp, V.; Warmann, S.W.; Vorwerk, P.; Leuschner, I.; Fuchs, J. Exocrine Pancreatic Tumors in Childhood in Germany. Pediatr. Blood Cancer 2012, 58, 366–371. [Google Scholar] [CrossRef]
- Bien, E.; Godzinski, J.; Dall’Igna, P.; Defachelles, A.-S.; Stachowicz-Stencel, T.; Orbach, D.; Bisogno, G.; Cecchetto, G.; Warmann, S.; Ellerkamp, V.; et al. Pancreatoblastoma: A Report from the European Cooperative Study Group for Paediatric Rare Tumours (EXPeRT). Eur. J. Cancer 2011, 47, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Brecht, I.; Schneider, D.; Klöppel, G.; Von Schweinitz, D.; Barthlen, W.; Hamre, M. Malignant Pancreatic Tumors in Children and Young Adults: Evaluation of 228 Patients Identified through the Surveillance, Epidemiology, and End Result (SEER) Database. Klin. Padiatr. 2011, 223, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, K.S.; Doulamis, I.P.; Tsilimigras, D.I.; Nasioudis, D.; Schizas, D.; Masiakos, P.T.; Kelleher, C.M. Solid Pseudopapillary and Malignant Pancreatic Tumors in Childhood: A Systematic Review and Evidence Quality Assessment. Pediatr. Blood Cancer 2018, 65, e27114. [Google Scholar] [CrossRef] [PubMed]
- Picado, O.; Ferrantella, A.; Zabalo, C.; Rao, K.; Thorson, C.M.; Sola, J.E.; Perez, E.A. Treatment Patterns and Outcomes for Pancreatic Tumors in Children: An Analysis of the National Cancer Database. Pediatr. Surg. Int. 2020, 36, 357–363. [Google Scholar] [CrossRef]
- Bien, E.; Roganovic, J.; Krawczyk, M.A.; Godzinski, J.; Orbach, D.; Cecchetto, G.; Barthlen, W.; Defachelles, A.; Ferrari, A.; Weldon, C.B.; et al. Pancreatoblastoma in Children: EXPeRT/PARTNER Diagnostic and Therapeutic Recommendations. Pediatr. Blood Cancer 2021, 68, e29112. [Google Scholar] [CrossRef]
- Li, P.; Kong, Y.; Wan, L.; Guo, J.; Li, W.; Zhang, H.; Yang, G.; Zhang, B. Overall Survival, Late Mortality, and Cancer-Directed Surgery among Children and Adolescents with Ultra-Rare Pediatric Pancreatoblastoma in the United States, 1975–2018. J. Pancreatol. 2023, 6, 61–66. [Google Scholar] [CrossRef]
- Dhebri, A.R.; Connor, S.; Campbell, F.; Ghaneh, P.; Sutton, R.; Neoptolemos, J.P.; Klöppel, G.; Kosmahl, M.; Jänig, U.; Lüttges, J. Diagnosis, Treatment and Outcome of Pancreatoblastoma. Pancreatology 2004, 4, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.N.; Trout, A.T.; Shenoy, A.; Abu-El-Haija, M.; Nathan, J.D. Solid Pancreatic Masses in Children: A Review of Current Evidence and Clinical Challenges. Front. Pediatr. 2022, 10, 966943. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhao, T.; Shi, C.; Chen, L. Pancreatoblastoma in Children: Clinical Management and Literature Review. Transl. Oncol. 2022, 18, 101359. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Trout, A.T.; Ayyala, R.S.; Szabo, S.; Nathan, J.D.; Geller, J.I.; Dillman, J.R. Pancreatic Masses in Children and Young Adults: Multimodality Review with Pathologic Correlation. RadioGraphics 2021, 41, 1766–1784. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gong, Y.; Ji, M.; Yang, B.; Qiao, Z. Differential Diagnosis of Pancreatoblastoma (PB) and Solid Pseudopapillary Neoplasms (SPNs) in Children by CT and MR Imaging. Eur. Radiol. 2021, 31, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.A.; Ha, T.N.; Zhu, H.; Heaton, T.E.; LaQuaglia, M.P.; Murphy, J.T.; Barry, W.E.; Goodhue, C.; Kim, E.S.; Aldrink, J.H.; et al. Pancreaticoduodenectomy for the Treatment of Pancreatic Neoplasms in Children: A Pediatric Surgical Oncology Research Collaborative Study. Pediatr. Blood Cancer 2020, 67, e28425. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.C.; Kuenzler, K.A.; Bodenstein, L.; Chabot, J.A. Central Pancreatectomy with Pancreaticogastrostomy in Children. J. Pediatr. Surg. 2007, 42, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Nachulewicz, P.; Rogowski, B.; Obel, M.; Woźniak, J. Central Pancreatectomy as a Good Solution in Frantz Tumor Resection: A Case Report. Medicine 2015, 94, e1165. [Google Scholar] [CrossRef]
- Leraas, H.J.; Kim, J.; Sun, Z.; Ezekian, B.; Gulack, B.C.; Reed, C.R.; Tracy, E.T. Solid Pseudopapillary Neoplasm of the Pancreas in Children and Adults: A National Study of 369 Patients. J. Pediatr. Hematol./Oncol. 2018, 40, e233–e236. [Google Scholar] [CrossRef]
- Crocoli, A.; Grimaldi, C.; Virgone, C.; De Pasquale, M.D.; Cecchetto, G.; Cesaro, S.; Bisogno, G.; Cecinati, V.; Narciso, A.; Alberti, D.; et al. Outcome after Surgery for Solid Pseudopapillary Pancreatic Tumors in Children: Report from the TREP Project—Italian Rare Tumors Study Group. Pediatr. Blood Cancer 2019, 66, e27519. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, S.; Ren, Q.; Yang, W.; Han, W.; Chang, X.; Zhu, Z.; Qin, H.; Wang, H. Pancreatectomies for Pediatric Pancreatic Tumors: A Single Institute Experience from 2007 to 2018. J. Pediatr. Surg. 2020, 55, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Namgoong, J.-M.; Kim, D.Y.; Kim, S.C.; Kwon, H.H. Suggested Indications for Enucleation of Solid Pseudopapillary Neoplasms in Pediatric Patients. Front. Pediatr. 2019, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Kim, W.; Seo, J.-M.; Lee, S. Prediction of Recurrence of Completely Resected Pancreatic Solid Pseudopapillary Neoplasms in Pediatric Patients: A Single Center Analysis. Children 2021, 8, 632. [Google Scholar] [CrossRef] [PubMed]
- Paredes, O.; Kawaguchi, Y.; Ruiz, E.; Payet, E.; Berrospi, F. Surgery of Pancreas Tumors in Pediatric and Adolescent Patients: A Single Institution Experience in South America. Pediatr. Surg. Int. 2021, 37, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Kim, D.Y.; Kim, S.C.; Kim, S.C.; Kwon, H.; Choi, J.M.; Namgoong, J.-M. Comparison of Splenic Vessel Preserving Distal Pancreatectomy and the Warshaw Technique for Solid Pseudopapillary Neoplasm in Children. Pediatr. Surg. Int. 2022, 38, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Loos, M.; Kinny-Köster, B.; Hackert, T.; Schneider, M.; Mehrabi, A.; Berchtold, C.; Al-Saeedi, M.; Müller, B.P.; Strobel, O.; et al. Pancreatic Surgery in Children: Complex, Safe and Effective. Ann. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.O.; Guérin, F.; Goldzmidt, D.; Fouquet, V.; Franchi-Abella, S.; Fabre, M.; Branchereau, S.; Martelli, H.; Gauthier, F. Pancreatic Resections for Solid or Cystic Pancreatic Masses in Children. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Sugito, K.; Furuya, T.; Kaneda, H.; Masuko, T.; Ohashi, K.; Inoue, M.; Ikeda, T.; Koshinaga, T.; Tomita, R.; Maebayashi, T. Long-Term Follow-Up of Nutritional Status, Pancreatic Function, and Morphological Changes of the Pancreatic Remnant After Pancreatic Tumor Resection in Children. Pancreas 2012, 41, 554–559. [Google Scholar] [CrossRef]
- Sacco Casamassima, M.G.; Gause, C.D.; Goldstein, S.D.; Abdullah, F.; Meoded, A.; Lukish, J.R.; Wolfgang, C.L.; Cameron, J.; Hackam, D.J.; Hruban, R.H.; et al. Pancreatic Surgery for Tumors in Children and Adolescents. Pediatr. Surg. Int. 2016, 32, 779–788. [Google Scholar] [CrossRef]
- Scandavini, C.; Valente, R.; Rangelova, E.; Segersvärd, R.; Arnelo, U.; Permert, J.; Svensson, P.-J.; Stenman, J.; Del Chiaro, M. Pancreatectomies for Pancreatic Neoplasms in Pediatric and Adolescent Age: A Single Institution Experience. Pancreatology 2018, 18, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Pranger, B.K.; Van Dam, J.L.; Groen, J.V.; Van Eijck, C.H.; Koerkamp, B.G.; Bonsing, B.A.; Mieog, J.S.D.; Besselink, M.G.; Busch, O.R.; Kazemier, G.; et al. Pancreatic Resection in the Pediatric, Adolescent and Young Adult Population: Nationwide Analysis on Complications. HPB 2021, 23, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.E.; Zagory, J.A.; Tatum, M.; Tsui, W.S.; Murphy, J. A Retrospective Analysis of Pancreas Operations in Children. Transl. Gastroenterol. Hepatol. 2021, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Bolasco, G.; Capriati, T.; Grimaldi, C.; Monti, L.; De Pasquale, M.D.; Patera, I.P.; Spada, M.; Maggiore, G.; Diamanti, A. Long-Term Outcome of Pancreatic Function Following Oncological Surgery in Children: Institutional Experience and Review of the Literature. WJCC 2021, 9, 7340–7349. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, R.; Kim, P.C.W. Relationship between Surgical Volume and Clinical Outcome: Should Pediatric Surgeons Be Doing Pancreaticoduodenectomies? J. Pediatr. Surg. 2005, 40, 793–796. [Google Scholar] [CrossRef]
- d’Ambrosio, G.; Del Prete, L.; Grimaldi, C.; Bertocchini, A.; Lo Zupone, C.; Monti, L.; De Ville De Goyet, J. Pancreaticoduodenectomy for Malignancies in Children. J. Pediatr. Surg. 2014, 49, 534–538. [Google Scholar] [CrossRef]
- Lindholm, E.B.; Alkattan, A.K.; Abramson, S.J.; Price, A.P.; Heaton, T.E.; Balachandran, V.P.; La Quaglia, M.P. Pancreaticoduodenectomy for Pediatric and Adolescent Pancreatic Malignancy: A Single-Center Retrospective Analysis. J. Pediatr. Surg. 2017, 52, 299–303. [Google Scholar] [CrossRef]
- Van Den Akker, M.; Angelini, P.; Taylor, G.; Chami, R.; Gerstle, J.T.; Gupta, A. Malignant Pancreatic Tumors in Children: A Single-Institution Series. J. Pediatr. Surg. 2012, 47, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Beger, H.G.; Mayer, B.; Poch, B. Long-Term Oncologic Outcome Following Duodenum-Preserving Pancreatic Head Resection for Benign Tumors, Cystic Neoplasms, and Neuroendocrine Tumors: Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2024. [Google Scholar] [CrossRef]
- Beger, H.G.; Mayer, B.; Vasilescu, C.; Poch, B. Long-Term Metabolic Morbidity and Steatohepatosis Following Standard Pancreatic Resections and Parenchyma-Sparing, Local Extirpations for Benign Tumor: A Systematic Review and Meta-Analysis. Ann. Surg. 2022, 275, 54–66. [Google Scholar] [CrossRef]
- Zampieri, N.; Schiavo, N.; Capelli, P.; Scarpa, A.; Bassi, C.; Camoglio, F.S. Pseudopapillary Tumor in Pediatric Age: Clinical and Surgical Management. Pediatr. Surg. Int. 2011, 27, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Jentzsch, C.; Fuchs, J.; Agaimy, A.; Vokuhl, C.; Escherich, G.; Blattmann, C.; Warmann, S.W.; Schmidt, A.; Schäfer, J.; Brecht, I.B.; et al. Solid Pseudopapillary Neoplasms of the Pancreas in Childhood and Adolescence—An Analysis of the German Registry for Rare Pediatric Tumors (STEP). Eur. J. Pediatr. 2023, 182, 5341–5352. [Google Scholar] [CrossRef]
- Snajdauf, J.; Rygl, M.; Petru, O.; Nahlovsky, J.; Frybova, B.; Durilova, M.; Mixa, V.; Keil, R.; Kyncl, M.; Kodet, R.; et al. Indications and Outcomes of Duodenum-Preserving Resection of the Pancreatic Head in Children. Pediatr. Surg. Int. 2019, 35, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Yang, S.; Yang, W.; Han, W.; Cheng, H.; Chang, X.; Zhu, Z.; Ren, Q.; Wang, H. Duodenum-Preserving Pancreas Head Resection in the Treatment of Pediatric Benign and Low-Grade Malignant Pancreatic Tumors. HPB 2020, 22, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; De Lagausie, P.; Escolino, M.; Saxena, A.; Holcomb, G.W.; Settimi, A.; Becmeur, F.; Van Der Zee, D. Laparoscopic Resection of Pancreatic Tumors in Children: Results of a Multicentric Survey. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 533–538. [Google Scholar] [CrossRef]
- Larghi Laureiro, Z.; Angelico, R.; Rigamonti, A.; Saffioti, M.C.; Maritato, S.; Grimaldi, C.; Spada, M. Minimally Invasive Hepatopancreatic and Biliary Surgery in Children: A Large Centre Experience and Review of the Literature. HPB 2022, 24, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Namgoong, J.-M.; Kim, D.-Y.; Kim, S.-C.; Kim, S.-C.; Hwang, J.-H.; Song, K.-B. Laparoscopic Distal Pancreatectomy to Treat Solid Pseudopapillary Tumors in Children: Transition from Open to Laparoscopic Approaches in Suitable Cases. Pediatr. Surg. Int. 2014, 30, 259–266. [Google Scholar] [CrossRef]
- Niec, J.A.; Ghani, M.O.A.; Hilmes, M.A.; McKay, K.G.; Correa, H.; Zamora, I.J.; Lovvorn, H.N. Laparoscopic Resection of Pediatric Solid Pseudopapillary Tumors of the Pancreas. Am. Surg. 2023, 89, 1449–1456. [Google Scholar] [CrossRef]
- Shoup, M. The Value of Splenic Preservation With Distal Pancreatectomy. Arch. Surg. 2002, 137, 164. [Google Scholar] [CrossRef]
- Dumitrascu, T.; Dima, S.; Stroescu, C.; Scarlat, A.; Ionescu, M.; Popescu, I. Clinical Value of Spleen-preserving Distal Pancreatectomy: A Case-matched Analysis with a Special Emphasis on the Postoperative Systemic Inflammatory Response. J. Hepato-Biliary-Pancreat. Sci. 2014, 21, 654–662. [Google Scholar] [CrossRef]
- Dragomir, M.; Petrescu, G.E.D.; Manga, G.; Călin, G.A.; Vasilescu, C. Patients After Splenectomy: Old Risks and New Perspectives. Chirurgia 2016, 111, 393. [Google Scholar] [CrossRef]
- Madenci, A.L.; Armstrong, L.B.; Kwon, N.K.; Jiang, W.; Wolf, L.L.; Koehlmoos, T.P.; Ricca, R.L.; Weldon, C.B.; Haider, A.H.; Weil, B.R. Incidence and Risk Factors for Sepsis after Childhood Splenectomy. J. Pediatr. Surg. 2019, 54, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Luoto, T.T.; Pakarinen, M.P.; Koivusalo, A. Long-Term Outcomes after Pediatric Splenectomy. Surgery 2016, 159, 1583–1590. [Google Scholar] [CrossRef]
- Lee, G.M. Preventing Infections in Children and Adults with Asplenia. Hematology 2020, 2020, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Bram, R.; Bram, J.; Beaman, A.; Lee, A.; Lu, M.; Yheulon, C.; Tabak, B.; Woo, R. High Rates of Pediatric Venous Thromboembolism After Elective Laparoscopic Splenectomy Suggest Need for Perioperative Prophylaxis. J. Surg. Res. 2023, 289, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Dragomir, M.P.; Sabo, A.A.; Petrescu, G.E.D.; Li, Y.; Dumitrascu, T. Central Pancreatectomy: A Comprehensive, up-to-Date Meta-Analysis. Langenbecks Arch. Surg. 2019, 404, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, Y.Y.; Stonogin, S.V.; Donskoy, D.V.; Povarnin, O.Y.; Vilesov, A.V. Laparoscopic Pancreatic Resections for Solid Pseudopapillary Tumor in Children. Eur. J. Pediatr. Surg. 2009, 19, 399–401. [Google Scholar] [CrossRef]
- Dumitrascu, T.; Stanciulea, O.; Herlea, V.; Tomulescu, V.; Ionescu, M. Central Pancreatectomy for Pancreatoblastoma in a 16-Year-Old Girl. J. Pediatr. Surg. 2011, 46, e17–e21. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, W.; Hu, J.; Zhu, Z.; Qin, H.; Han, W.; Wang, H. Diagnosis and Treatment of Pancreatoblastoma in Children: A Retrospective Study in a Single Pediatric Center. Pediatr. Surg. Int. 2019, 35, 1231–1238. [Google Scholar] [CrossRef]
- Van Ramshorst, T.M.E.; Zwart, M.J.W.; Voermans, R.P.; Festen, S.; Daams, F.; Busch, O.R.; Oomen, M.W.N.; Besselink, M.G. Robotic Central Pancreatectomy with Roux-En-Y Pancreaticojejunostomy. J. Vis. Exp. 2021, 62862. [Google Scholar] [CrossRef]
- Sheng, M.; Zhang, R.; Ma, X.; Zhou, H. CT Manifestations of Childhood Pancreatoblastoma. World J. Pediatr. Surg. 2022, 5, e000398. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, T.; Scarlat, A.; Ionescu, M.; Popescu, I. Central Pancreatectomy versus Spleen-Preserving Distal Pancreatectomy: A Comparative Analysis of Early and Late Postoperative Outcomes. Dig. Surg. 2012, 29, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Nahm, C.B.; Jamieson, N.B.; Samra, J.; Clifton-Bligh, R.; Mittal, A.; Tsang, V. Risk Factors for Development of Diabetes Mellitus (Type 3c) after Partial Pancreatectomy: A Systematic Review. Clin. Endocrinol. 2020, 92, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Van Bodegraven, E.A.; Lof, S.; Jones, L.; Aussilhou, B.; Yong, G.; Jishu, W.; Klotz, R.; Rocha-Castellanos, D.M.; Matsumato, I.; De Ponthaud, C.; et al. Tailoring the Use of Central Pancreatectomy Through Prediction Models for Major Morbidity and Postoperative Diabetes: International Retrospective Multicenter Study. Ann. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, T.; De Pastena, M.; Paiella, S.; Marchegiani, G.; Landoni, L.; Festini, M.; Ramera, M.; Marinelli, V.; Casetti, L.; Esposito, A.; et al. Pancreatic Enucleation Patients Share the Same Quality of Life as the General Population at Long-Term Follow-Up: A Propensity Score-Matched Analysis. Ann. Surg. 2023, 277, e609–e616. [Google Scholar] [CrossRef] [PubMed]
- Brozzetti, S.; Carati, M.; Sterpetti, A.V. Systematic Review and Meta-Analysis of Clinical Outcomes after Enucleation of Pancreatic Metastases from Renal Cell Carcinoma. Dig. Surg. 2023, 40, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Kim, H.-Y.; Jung, S.-E.; Lee, S.-C.; Park, K.-W. Long-Term Functional Outcomes of PPPD in Children—Nutritional Status, Pancreatic Function, GI Function and QOL. J. Pediatr. Surg. 2016, 51, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Shamberger, R.C.; Hendren, W.H.; Leichtner, A.M. Long-Term Nutritional and Metabolic Consequences of Pancreaticoduodenectomy in Children. Surgery 1994, 115, 382–388. [Google Scholar]
- Beger, H.G.; Mayer, B. Early Postoperative and Late Metabolic Morbidity after Pancreatic Resections: An Old and New Challenge for Surgeons—A Review. Am. J. Surg. 2018, 216, 131–134. [Google Scholar] [CrossRef]
- Okano, K.; Murakami, Y.; Nakagawa, N.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Takahashi, S.; Sueda, T. Remnant Pancreatic Parenchymal Volume Predicts Postoperative Pancreatic Exocrine Insufficiency after Pancreatectomy. Surgery 2016, 159, 885–892. [Google Scholar] [CrossRef]
- Lim, P.-W.; Dinh, K.H.; Sullivan, M.; Wassef, W.Y.; Zivny, J.; Whalen, G.F.; LaFemina, J. Thirty-Day Outcomes Underestimate Endocrine and Exocrine Insufficiency after Pancreatic Resection. HPB 2016, 18, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, T.; Luque, A.Á.; Pinilla, I.; Lassaletta, L. Pancreatic Regeneration after Near-Total Pancreatectomy in Children with Nesidioblastosis. Pediatr. Radiol. 2005, 35, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Arya, V.B.; Senniappan, S.; Demirbilek, H.; Alam, S.; Flanagan, S.E.; Ellard, S.; Hussain, K. Pancreatic Endocrine and Exocrine Function in Children Following Near-Total Pancreatectomy for Diffuse Congenital Hyperinsulinism. PLoS ONE 2014, 9, e98054. [Google Scholar] [CrossRef] [PubMed]
- Budipramana, V.S.; Witarto, A.P.; Witarto, B.S.; Pramudito, S.L.; Ratri, L.C.; Wairooy, N.A.P.; Er Putra, A.J. Risk Factors for Exocrine Pancreatic Insufficiency after Pancreatic Surgery: A Systematic Review and Meta-Analysis. CJS 2022, 65, E770–E781. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Sun, R.; Han, X.; Liu, Z. New-Onset Diabetes Mellitus After Distal Pancreatectomy: A Systematic Review and Meta-Analysis. J. Laparoendosc. Adv. Surg. Tech. 2020, 30, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, D. Diagnosis and Treatment of Pancreatoblastoma in China. Pancreas 2007, 34, 92–95. [Google Scholar] [CrossRef]
- Klimstra, D.S.; Wenig, B.M.; Adair, C.F.; Heffess, C.S. Pancreatoblastoma A Clinicopathologic Study and Review of the Literature. Am. J. Surg. Pathol. 1995, 19, 1371–1389. [Google Scholar] [CrossRef]
- Murakami, T.; Ueki, K.; Kawakami, H.; Gondo, T.; Kuga, T.; Esato, K.; Furukawa, S. Pancreatoblastoma: Case Report and Review of Treatment in the Literature. Med. Pediatr. Oncol. 1996, 27, 193–197. [Google Scholar] [CrossRef]
- Imamura, A.; Nakagawa, A.; Okuno, M.; Takai, S.; Komada, H.; Kwon, A.-H.; Uetsuji, S.; Kamiyama, Y.; Sakaida, N.; Okamura, A. Pancreatoblastoma in an Adolescent Girl: Case Report and Review of 26 Japanese Cases. Eur. J. Surg. 2003, 164, 309–312. [Google Scholar] [CrossRef]
- Ohata, R.; Okazaki, T.; Ishizaki, Y.; Fujimura, J.; Shimizu, T.; Lane, G.J.; Yamataka, A.; Kawasaki, S. Pancreaticoduodenectomy for Pancreatoblastoma: A Case Report and Literature Review. Pediatr. Surg. Int. 2010, 26, 447–450. [Google Scholar] [CrossRef]
- Pennella, C.; Bosaleh, A.; Rose, A.; Zubizarreta, P.; Cacciavillano, W. Pancreatoblastoma. Case Report and Review of Literature. ACTA Gastroenterol. Latinoam. 2020, 50, 163–168. [Google Scholar] [CrossRef]
- Mylonas, K.S.; Nasioudis, D.; Tsilimigras, D.I.; Doulamis, I.P.; Masiakos, P.T.; Kelleher, C.M. A Population-Based Analysis of a Rare Oncologic Entity: Malignant Pancreatic Tumors in Children. J. Pediatr. Surg. 2018, 53, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Defachelles, A.-S.; Rocourt, N.; Branchereau, S.; Peuchmaur, M. Le pancréatoblastome chez l’enfant: Du diagnostic à la prise en charge thérapeutique. Bull. Cancer 2012, 99, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.F. Pancreatoduodenectomy for Carcinoma of the Pancreas in an Infant: Report of a Case. Ann. Surg. 1957, 145, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, T.; Scarlat, A.; Ionescu, M.; Popescu, I. Central Pancreatectomy: An Oncologically Safe Option to Treat Metastases of Other Neoplasms of the Mid-Portion of the Pancreas? Ann. Hepato-Biliary-Pancreat. Surg. 2017, 21, 76. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-N.; Chowdhury, T.A. Diabetes: An Overview for Clinical Oncologists. Clin. Oncol. 2020, 32, 579–590. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Cohen, L.E.; Mostoufi-Moab, S.; Patterson, B.C.; Simmons, J.H.; Meacham, L.R.; Van Santen, H.M.; Sklar, C.A. Endocrine Late Effects in Childhood Cancer Survivors. JCO 2018, 36, 2153–2159. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.N.; Tonorezos, E.S.; Cohen, P. Diabetes and Metabolic Syndrome in Survivors of Childhood Cancer. Horm. Res. Paediatr. 2019, 91, 118–127. [Google Scholar] [CrossRef]
- Amutha, A.; Mohan, V. Diabetes Complications in Childhood and Adolescent Onset Type 2 Diabetes—A Review. J. Diabetes Its Complicat. 2016, 30, 951–957. [Google Scholar] [CrossRef]
- TODAY Study Group. Long-Term Complications in Youth-Onset Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 416–426. [Google Scholar] [CrossRef]
- Soheilipour, F.; Abbasi Kasbi, N.; Imankhan, M.; Eskandari, D. Complications and Treatment of Early-Onset Type 2 Diabetes. Int. J. Endocrinol. Metab. 2023, 21, e135004. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | No of Patients | Overall Morbidity | POPF | Impaired Pancreatic Function | Follow-Up Time (Months) |
|---|---|---|---|---|---|
| SPT | |||||
| Fisher, 2007 [18] | 1 | 0% | 0% | 0% | 10 |
| Sokolov, 2009 [57] | 1 | 0% | 0% | NR | 24 |
| Muller, 2012 [28] | 2 | 50% | 50% | E—0%, Ex—50% | 50 |
| Nachulewicz, 2015 [19] | 1 | 100% | 100% | NR | 36 |
| Crocoli, 2018 [21] | 4 | 0% | 0% | 0% | NR |
| Cho, 2019 [23] | 51 | NR | 63% | E—3.9% | 442 |
| van Ramshorst, 2021 [60] | 1 | 100% | 100% | 0% | NR |
| Jones, 2021 [33] | 1 | 100% | 100% | NR | 13 |
| Jentzsch, 2023 [42] | 1 | 100% | 0% | 0% | 5 |
| PB | |||||
| Dumitrascu, 2011 [58] | 1 | 0% | 0% | 0% | 180 |
| Author, Year | No of pts | Overall Morbidity | POPF | Endocrine Insufficiency | Exocrine Insufficiency | Median Follow-Up Time (Months) |
|---|---|---|---|---|---|---|
| Pancreaticoduodenectomies | ||||||
| Dasgupta, 2005 [35] | 5 | 40% | NR | NR | NR | NR |
| Muller, 2012 [28] | 11 | 54.5% | 9.1% | NR | NR | NR |
| D’Ambrosio, 2014 [36] | 5 | 0% | 0% | 20% | 0% | 24 |
| Park, 2016 [67] | 10 | NR | NR | 10% | 30% | 126 |
| Lindholm, 2017 [37] | 12 | 50%/16.7% a | 8.3% | 0% | 83.3% | NR |
| Scandavini, 2017 [31] | 5 | 40% | 20% | 0% | 0% | 80 |
| Huang, 2019 [59] | 6 | NR | NR | NR | NR | NR |
| Qin, 2019 [44] | 6 | 16.7% | 0% | 0% | 0% | 31 |
| Vasudevan, 2020 [17] | 65 | 16.9%/12.3% a | 13.8% | 13.8% | 29.2% | 45.6 |
| Jones, 2021 [33] | 9 | NR | 11% | NR | NR | NR |
| Bolasco, 2021 [34] | 8 | NR | NR | 25% | 50% | 83.4 |
| Fuchs, 2023 [27] | 19 | 11% a | NR | NR | NR | NR |
| Duodenum-preserving pancreatic head resections | ||||||
| Snajdauf, 2019 [43] | 21 | 9.5%/4.8% a | NR | 0% | 36% | 115.2 |
| Qin, 2019 [44] | 22 | 63.6% | 50%/31.8% b | 0% | 4.5% | 31 |
| Fuchs, 2023 [27] | 10 | 0% | NR | NR | NR | NR |
| Distal spleno-pancreatectomies | ||||||
| Huang, 2019 [59] | 3 | NR | NR | NR | NR | NR |
| Bolasco, 2021 [34] | 3 | NR | NR | 0% | 0% | 30 |
| Spleen-preserving distal pancreatectomies | ||||||
| Huang, 2019 [59] | 5 | NR | NR | NR | NR | NR |
| Bolasco, 2021 [34] | 4 | NR | NR | 0% | 0% | 61.2 |
| Kwon, 2022 [26] | 28 | 57.1%/10.7% a | 89.3%/10.7% b | NR | NR | NR |
| Enucleations | ||||||
| Scandavini, 2017 [31] | 3 | 33.3% | 33.3% | 0% | 0% | 80 |
| Cho, 2019 [23] | 15 | NR | 66.7% | 0% | 0% | 746.8 |
| Qin, 2019 [44] | 7 | 57.1% | 28.6% b | 0% | 0% | 31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrascu, T. Preservation of Pancreatic Function Should Not Be Disregarded When Performing Pancreatectomies for Pancreatoblastoma in Children. Pediatr. Rep. 2024, 16, 385-398. https://doi.org/10.3390/pediatric16020033
Dumitrascu T. Preservation of Pancreatic Function Should Not Be Disregarded When Performing Pancreatectomies for Pancreatoblastoma in Children. Pediatric Reports. 2024; 16(2):385-398. https://doi.org/10.3390/pediatric16020033
Chicago/Turabian StyleDumitrascu, Traian. 2024. "Preservation of Pancreatic Function Should Not Be Disregarded When Performing Pancreatectomies for Pancreatoblastoma in Children" Pediatric Reports 16, no. 2: 385-398. https://doi.org/10.3390/pediatric16020033
APA StyleDumitrascu, T. (2024). Preservation of Pancreatic Function Should Not Be Disregarded When Performing Pancreatectomies for Pancreatoblastoma in Children. Pediatric Reports, 16(2), 385-398. https://doi.org/10.3390/pediatric16020033






