Association between Paediatric Complementary and Alternative Medicine Use and Parental Health Literacy, Child Health, and Socio-Economic Variables: A Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Recruitment
2.2. Study Population
2.3. Outcomes Measures
2.3.1. Demographic Information
- Child age and sex;
- Parent age and sex;
- Parental education level;
- Family ethnicity;
- Residential address;
- Annual household income;
- Family structure.
2.3.2. Child Health Information
- Prescription medications;
- Chronic health conditions;
- Reason for hospital attendance;
- Overall health in the last week (0–10 scale).
2.3.3. Healthcare Visits
- Primary care physician visits within the previous 12 months;
- Emergency department visits within the previous 12 months;
- Outpatient visits within the previous 12 months.
2.3.4. CAM Use
- Child CAM use;
- If yes, type, frequency and duration;
- Reason for CAM use;
- CAM efficacy and side effects;
- Disclosure of CAM to medical team;
- Parental CAM use;
- Sibling CAM use.
2.3.5. Parental Opinion Survey
2.3.6. Health Literacy Assessment
2.3.7. Child Well-Being
2.3.8. Socio-Economic Deprivation
2.4. Ethics and Consent
2.5. Statistical Analysis
2.6. Sample Size Calculation
3. Results
3.1. Patient Demographics
3.2. CAM Type, Duration, Frequency, and Side Effects
3.3. Factors Associated with CAM Use by Children
3.3.1. Socio-Demographic Variables
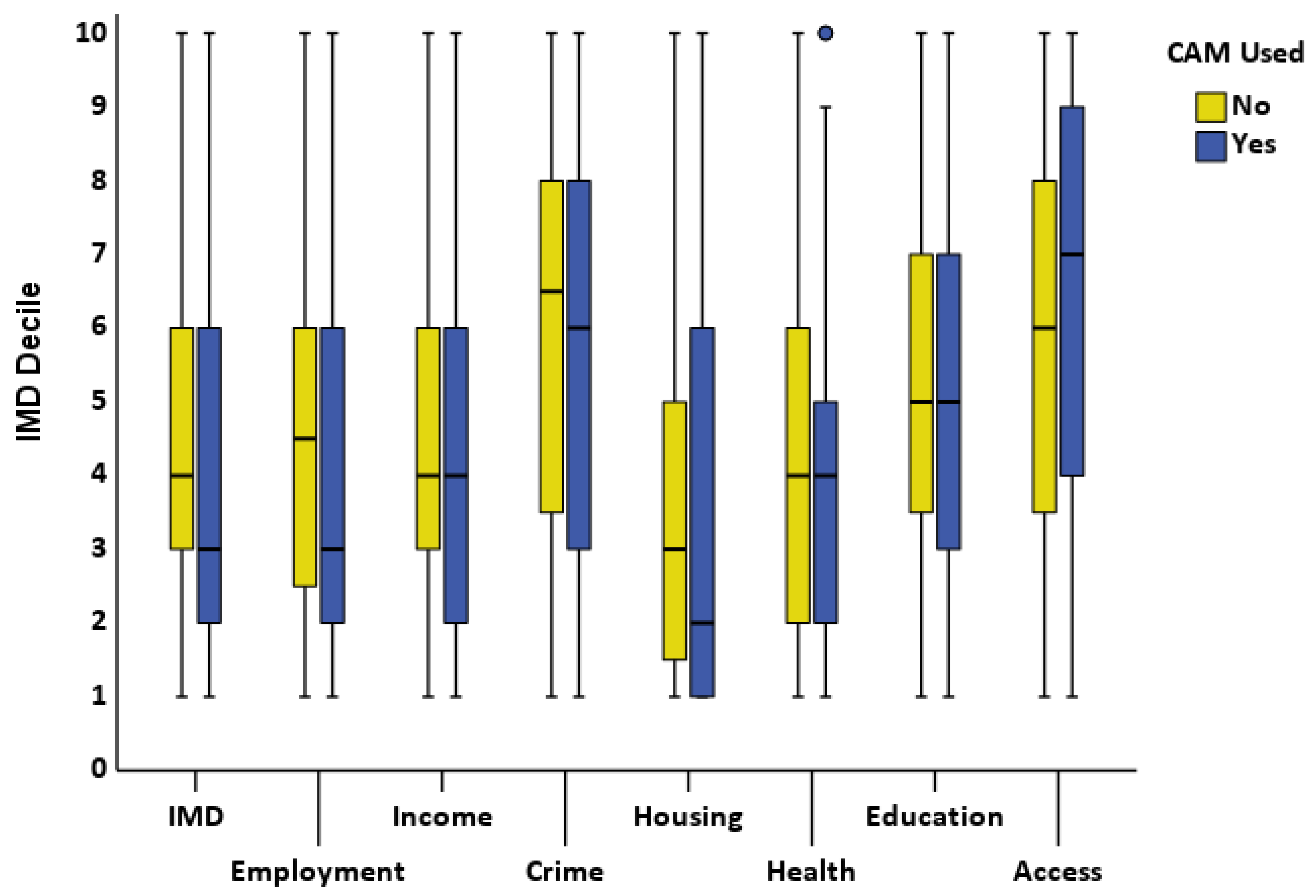
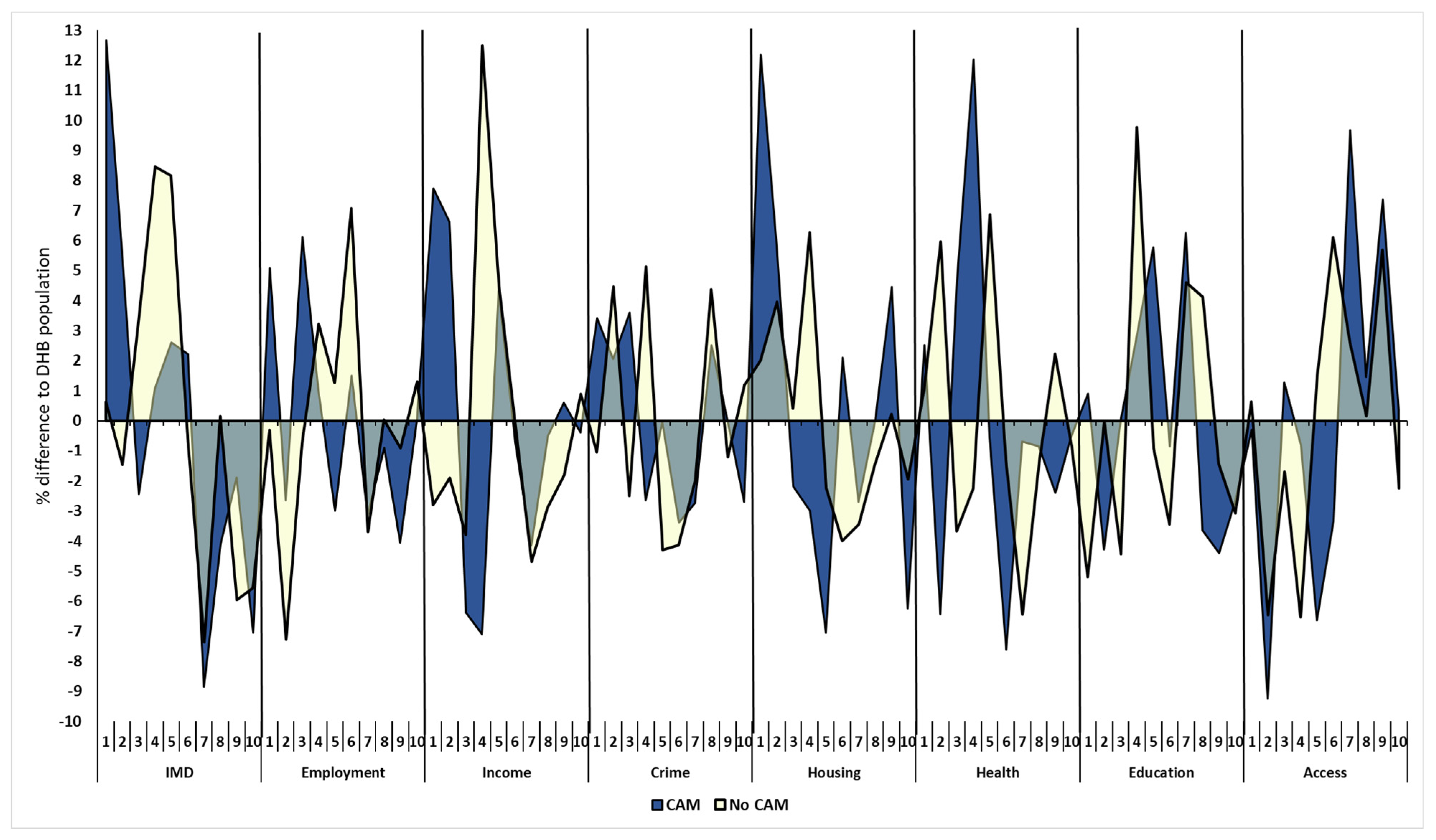
3.3.2. Socio-Economic Deprivation Deciles
3.3.3. Parental Opinion
3.3.4. Parental Health Literacy
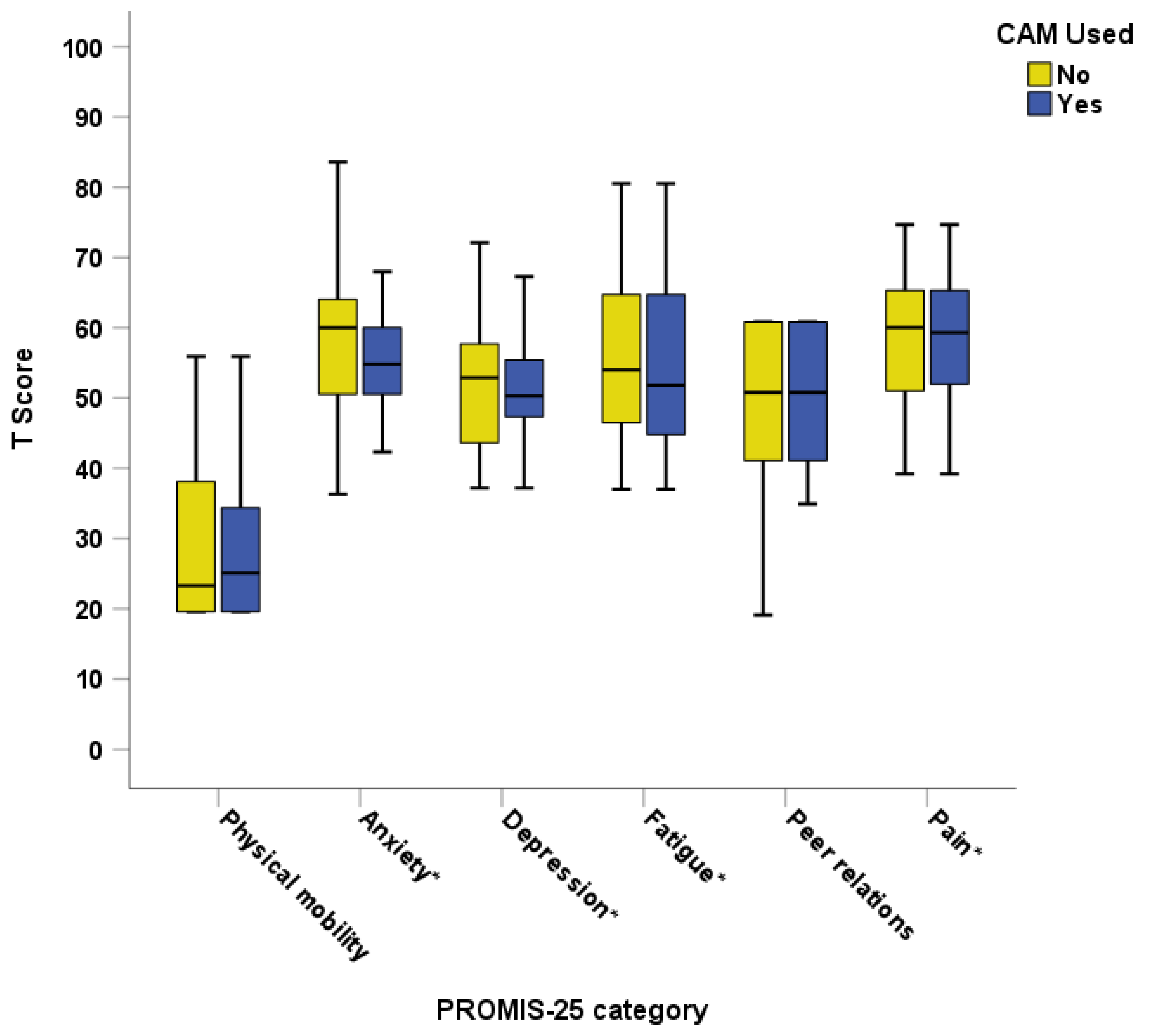
3.3.5. Child Well-Being
3.4. CAM Disclosure Rates
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ng, J.Y.; Dhawan, T.; Dogadova, E.; Taghi-Zada, Z.; Vacca, A.; Wieland, L.S.; Moher, D. Operational definition of complementary, alternative, and integrative medicine derived from a systematic search. BMC Complement. Med. Ther. 2022, 22, 104. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, T.; Şen, V.; Kelekçi, S.; Karabel, M.; Şahin, C.; Uluca, Ü.; Karabel, D.; Haspolat, Y.K. Use of complementary and alternative medicine in children who have no chronic disease. Turk. Pediatr. Ars. 2014, 49, 148–153. [Google Scholar] [CrossRef]
- Italia, S.; Wolfenstetter, S.; Teuner, C. Patterns of Complementary and Alternative Medicine (CAM) use in children: A systematic review. Eur. J. Pediatr. 2014, 173, 1413–1428. [Google Scholar] [CrossRef] [PubMed]
- Lüthi, E.; Diezi, M.; Danon, N.; Dubois, J.; Pasquier, J.; Burnand, B. Complementary and alternative medicine use by pediatric oncology patients before, during, and after treatment. BMC Complement. Altern. Med. 2021, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Gottschling, S.; Gronwald, B.; Schmitt, S.; Schmitt, C.; Längler, A.; Leidig, E.; Meyer, S.; Baan, A.; Shamdeen, M.G.; Berrang, J.; et al. Use of complementary and alternative medicine in healthy children and children with chronic medical conditions in Germany. Complement. Ther. Med. 2013, 21, S61–S69. [Google Scholar] [CrossRef] [PubMed]
- Samdup, D.Z.; Smith, R.G.; Il Song, S. The use of complementary and alternative medicine in children with chronic medical conditions. Am. J. Phys. Med. Rehabil. 2006, 85, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Dagenais, S.; Clifford, T.; Baydala, L.; King, W.J.; Hervas-Malo, M.; Moher, D.; Vohra, S. Complementary and alternative medicine use by pediatric specialty outpatients. Pediatrics 2013, 131, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sathiyan, J.; Faeyza, N.; Ramasamy, K.; Ng, W.S.; Ganapathy, S. Complementary and Alternative Medicine Use Among Pediatric Emergency Department Patients in Singapore. Pediatr. Emerg. Care 2021, 37, e1566–e1570. [Google Scholar] [CrossRef] [PubMed]
- Oren-Amit, A.; Berkovitch, M.; Bahat, H.; Goldman, M.; Kozer, E.; Ziv-Baran, T.; Abu-Kishk, I. Complementary and alternative medicine among hospitalized pediatric patients. Complement. Ther. Med. 2017, 31, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Vernon-Roberts, A.; Denny, A.; Day, A.S. Point Prevalence of Complementary or Alternative Medicine Use among Children Attending a Tertiary Care Hospital. Children 2023, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Cranswick, N.; South, M. Adverse events associated with the use of complementary and alternative medicine in children. Arch. Dis. Child. 2011, 96, 297. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Schroeder, N.; Gottschling, S. Complementary and alternative medicine in children. Eur. J. Pediatr. 2013, 172, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.G.; Croitoru, S.K.; Silverberg, H.M.; Steinhart, V.A.; Weizman, V.A. Use of Complementary and Alternative Medicine for Inflammatory Bowel Disease Is Associated with Worse Adherence to Conventional Therapy: The COMPLIANT Study. Inflamm. Bowel Dis. 2016, 22, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Birdee, G.S.; Phillips, R.S.; Davis, R.B.; Gardiner, P. Factors Associated With Pediatric Use of Complementary and Alternative Medicine. Pediatrics 2010, 125, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.E.; Duffy, C.; De Civita, M.; Malleson, P.; Philibert, L.; Gibbon, M.; Ortiz-Alvarez, O.; Dobkin, P.L. Factors associated with the use of complementary and alternative medicine in juvenile idiopathic arthritis. Arthritis Care Res. 2004, 51, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, P.R.; Feinberg, I.; Spratling, R. The Relationship of Parental Health Literacy to Health Outcomes of Children with Medical Complexity. J. Pediatr. Nurs. 2021, 60, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, L.; Huang, Z.; Li, D.; Tao, Q.; Zhang, F. The effects of parent’s health literacy and health beliefs on vaccine hesitancy. Vaccine 2023, 41, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E.; Halpern, D.J.; Crotty, K. Low Health Literacy and Health Outcomes: An Updated Systematic Review. Ann. Intern. Med. 2011, 155, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.K.; Glick, A.; Yin, H.S. Health Literacy: Implications for Child Health. Pediatr. Rev. 2019, 40, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Tek Ayaz, S. The roles of health literacy in parents’ honey use and the use of complementary alternative medicine in a Turkish population. BMC Complement. Med. Ther. 2023, 23, 376. [Google Scholar] [CrossRef] [PubMed]
- Fleary, S.; Heffer, R.W.; McKyer, E.L.; Taylor, A. A Parent-Focused Pilot Intervention to Increase Parent Health Literacy and Healthy Lifestyle Choices for Young Children and Families. ISRN Fam. Med. 2013, 2013, 619389. [Google Scholar] [CrossRef] [PubMed]
- Johri, M.; Subramanian, S.V.; Sylvestre, M.-P.; Dudeja, S.; Chandra, D.; Koné, G.K.; Sharma, J.K.; Pahwa, S. Association between maternal health literacy and child vaccination in India: A cross-sectional study. J. Epidemiol. Community Health 2015, 69, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Dowson, C.; Mangin, D. Prevalence of complementary and alternative medicine use in Christchurch, New Zealand: Children attending general practice versus paediatric outpatients. N. Z. Med. J. 2007, 120, U2464. [Google Scholar]
- Barnes, J.; Butler, R. Community pharmacists’ views on the regulation of complementary medicines and complementary-medicines practitioners: A qualitative study in New Zealand. Int. J. Pharm. Pract. 2018, 26, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Butler, R. Community pharmacists’ professional practices for complementary medicines: A qualitative study in New Zealand. Int. J. Clin. Pharm. 2020, 42, 1109–1117. [Google Scholar] [CrossRef]
- Medical Council of New Zealand. Doctors and Complementary and Alternative Medicine (CAM). 2022. Available online: https://www.mcnz.org.nz/assets/standards/Complementary-and-alternative-medicine.pdf (accessed on 29 April 2024).
- Pharmacy Council New Zealand. Pharmacy Council Complementary and Alternative Medicines—Statement and Protocol for Pharmacists. 2021. Available online: https://pharmacycouncil.org.nz/wp-content/uploads/2021/03/Complementary-and-Alternative-Medicines-CAM-Statement-1.pdf (accessed on 29 April 2024).
- Ministerial Advisory Committee on Complementary and Alternative Health. Ministerial Advisory Committee on Complementary and Alternative Health. 2004. Available online: https://www.beehive.govt.nz/sites/default/files/MACCAH%20Advice%20to%20Minister.pdf (accessed on 29 April 2024).
- The College of Nurses ANI. Complementary and Alternative Therapies Policy. 2019. Available online: https://www.nurse.org.nz/user/file/2118/Complimetnary%20Therapies%202019.pdf (accessed on 29 April 2024).
- Veziari, Y.; Kumar, S.; Leach, M.J. An exploration of barriers and enablers to the conduct and application of research among complementary and alternative medicine stakeholders in Australia and New Zealand: A qualitative descriptive study. PLoS ONE 2022, 17, 0264221. [Google Scholar] [CrossRef]
- Lee, E.L.; Harrison, J.; Barnes, J. Mapping prevalence and patterns of use of, and expenditure on, traditional, complementary and alternative medicine in New Zealand: A scoping review of qualitative and quantitative studies. N. Z. Med. J. 2021, 134, 57–74. [Google Scholar]
- Posadzki, P.; Watson, L.; Alotaibi, A.; Ernst, E. Prevalence of complementary and alternative medicine (CAM)-use in UK paediatric patients: A systematic review of surveys. Complement. Ther. Med. 2013, 21, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.E.; Cooper, K.L.; Relton, C.; Thomas, K.J. Prevalence of complementary and alternative medicine (CAM) use by the general population: A systematic review and update. Int. J. Clin. Pract. 2012, 66, 924–939. [Google Scholar] [CrossRef] [PubMed]
- James, P.B.; Wardle, J.; Steel, A.; Adams, J. Traditional, complementary and alternative medicine use in Sub-Saharan Africa: A systematic review. BMJ Glob. Health 2018, 3, e000895. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B.D.; Mays, M.Z.; Martz, W.; Castro, K.M.; DeWalt, D.A.; Pignone, M.P.; Mockbee, J.; Hale, F.A. Quick assessment of literacy in primary care: The newest vital sign. Ann. Fam. Med. 2005, 3, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.K.; Schapira, M.M.; Hoffmann, R.G.; Brousseau, D.C. Measuring Health Literacy in Caregivers of Children: A Comparison of the Newest Vital Sign and S-TOFHLA. Clin. Pediatr. 2014, 53, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.E.; Gross, H.E.; Stucky, B.D.; Thissen, D.; DeWitt, E.M.; Lai, J.S.; Amtmann, D.; Khastou, L.; Varni, J.W.; A DeWalt, D. Development of six PROMIS pediatrics proxy-report item banks. Health Qual. Life Outcomes 2012, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Exeter, D.J. The New Zealand Indices of Multiple Deprivation (IMD): A new suite of indicators for social and health research in Aotearoa, New Zealand. PLoS ONE 2017, 12, e0181260. [Google Scholar] [CrossRef] [PubMed]
- University of Auckland. Deprivation and Health Geography within NZ. Deprivation Maps of 20 DHB Areas Using the IMD18 New Zealand: University of Auckland. 2023. Available online: https://imdmap.auckland.ac.nz/download/ (accessed on 16 May 2023).
- Jeon, S.-R.; Kang, J.W.; Ang, L.; Lee, H.W.; Lee, M.S.; Kim, T.-H. Complementary and alternative medicine (CAM) interventions for COVID-19: An overview of systematic reviews. Integr. Med. Res. 2022, 11, 100842. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, V.; Sun, S.; Hussain, R.; Abutaleb, M.H.; Hedima, E.W. Complementary and alternative medicines use in COVID-19: A global perspective on practice, policy and research. Res. Soc. Adm. Pharm. 2022, 18, 2524–2528. [Google Scholar] [CrossRef] [PubMed]
- Pitetti, R.; Singh, S.; Hornyak, D.; Garcia, S.E.; Herr, S. Complementary and alternative medicine use in children. Pediatr. Emerg. Care 2001, 17, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Bains, S.S.; Egede, L.E. Association of health literacy with complementary and alternative medicine use: A cross-sectional study in adult primary care patients. BMC Complement. Altern. Med. 2011, 11, 138. [Google Scholar] [CrossRef]
- Gardiner, P.; Mitchell, S.; Filippelli, A.C.; Sadikova, E.; White, L.F.; Paasche-Orlow, M.K.; Jack, B.W. Health literacy and complementary and alternative medicine use among underserved inpatients in a safety net hospital. J. Health Commun. 2013, 18 (Suppl. 1), 290–297. [Google Scholar] [CrossRef] [PubMed]
- Zaidman, E.A.; Scott, K.M.; Hahn, D.; Bennett, P.; Caldwell, P.H.Y. Impact of parental health literacy on the health outcomes of children with chronic disease globally: A systematic review. J. Paediatr. Child. Health 2023, 59, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.M.; Dreyer, B.P.; Mendelsohn, A.L.; Bailey, S.C.; Sanders, L.M.; Wolf, M.S.; Parker, R.M.; Patel, D.A.; Kim, K.Y.A.; Jimenez, J.J.; et al. Liquid Medication Dosing Errors by Hispanic Parents: Role of Health Literacy and English Proficiency. Acad. Pediatr. 2017, 17, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Tek, S.; Topuz, A. The Effect of the Health Literacy Levels of Parents on Medication Errors. Int. J. Caring Sci. 2021, 14, 1388–1395. [Google Scholar]
- De Buhr, E.; Tannen, A. Parental health literacy and health knowledge, behaviours and outcomes in children: A cross-sectional survey. BMC Public Health 2020, 20, 1096. [Google Scholar] [CrossRef] [PubMed]
- DeWalt, D.A.; Hink, A. Health literacy and child health outcomes: A systematic review of the literature. Pediatrics 2009, 124 (Suppl. 3), S265–S274. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Weller, J.; Rahiri, J.-L.; Harwood, M.; Pitama, S. Māori experiences of hospital care: A qualitative systematic review. AlterNative Int. J. Indig. Peoples 2022, 18, 455–464. [Google Scholar] [CrossRef]
- Wilson, D.; Moloney, E.; Parr, J.M.; Aspinall, C.; Slark, J. Creating an Indigenous Māori-centred model of relational health: A literature review of Māori models of health. J. Clin. Nurs. 2021, 30, 3539–3555. [Google Scholar] [CrossRef] [PubMed]
- Rolleston, A.; Miskelly, P.; McDonald, M.; Wiles, J.; Poppe, K.; Doughty, R. Cultural context in New Zealand: Incorporating kaupapa Māori values in clinical research and practice. Health Promot. Int. 2022, 37, daac065. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, J.; Lamont, A.E.; Ohm, J.; Alcantara, J. The Quality of Life of Children Under Chiropractic Care Using PROMIS-25: Results from a Practice-Based Research Network. J. Altern. Complement. Med. 2017, 24, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Hoogeboom, T.J.; Nijhuis-van der Sanden, M.W.G.; de Bie, R.A.; Bart Staal, J. Spinal manual therapy in infants, children and adolescents: A systematic review and meta-analysis on treatment indication, technique and outcomes. PLoS ONE 2019, 14, 0218940. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, G.V.; Salum, G.A.; Sugaya, L.S.; Caye, A.; Rohde, L.A. Annual Research Review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J. Child. Psychol. Psychiatry 2015, 56, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Simkin, D.R.; Swick, S.; Taneja, K.S.; Ranjbar, N. Complementary and Integrative Medicine for Anxiety in Children, Adolescents, and Young Adults. Child. Adolesc. Psychiatr. Clin. N. Am. 2023, 32, 193–216. [Google Scholar] [CrossRef]
- Ng, J.Y.; Jain, A. Complementary and alternative medicine mention and recommendations in guidelines for anxiety: A systematic review and quality assessment. Psychiatry Res. 2022, 309, 114388. [Google Scholar] [CrossRef] [PubMed]
- Akhgarjand, C.; Asoudeh, F.; Bagheri, A.; Kalantar, Z.; Vahabi, Z.; Shab-bidar, S.; Rezvani, H.; Djafarian, K. Does Ashwagandha supplementation have a beneficial effect on the management of anxiety and stress? A systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2022, 36, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Dobson, E.T.; Mills, J.A.; Cornwall, G.J.; Sakolsky, D.; Birmaher, B.; Compton, S.N.; Piacentini, J.; McCracken, J.T.; Ginsburg, G.S.; et al. Placebo Response in Pediatric Anxiety Disorders: Results from the Child/Adolescent Anxiety Multimodal Study. J. Child. Adolesc. Psychopharmacol. 2017, 27, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Zuzak, T.J.; Zuzak-Siegrist, I.; Simões-Wüst, A.P.; Rist, L.; Staubli, G. Use of complementary and alternative medicine by patients presenting to a paediatric Emergency Department. Eur. J. Pediatr. 2009, 168, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sawni, A.; Ragothaman, R.; Thomas, R.L.; Mahajan, P. The Use of Complementary/Alternative Therapies Among Children Attending an Urban Pediatric Emergency Department. Clin. Pediatr. 2007, 46, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.M.; Dhir, R.; Craig, S.S.; Lammers, T.; Gardiner, K.; Hunter, K.; Joffe, P.; Krieser, D.; E Babl, F. Complementary and alternative medicine use among paediatric emergency department patients. J. Paediatr. Child. Health 2015, 51, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.-L.; Taylor, D.M.; Lee, M.; Johnson, O.G.; Ashok, A.; Griffiths, M.; Simma, L.; Craig, S.S.; Cheek, J.A.; Babl, F.E. Observational study of alternative therapies among paediatric emergency department patients. Emerg. Med. Australas. 2017, 29, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Zuzak, T.; Rauber-Lüthy, C.; Simões-Wüst, A. Accidental intakes of remedies from complementary and alternative medicine in children—Analysis of data from the Swiss Toxicological Information Centre. Eur. J. Pediatr. 2010, 169, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, B.; George, M.; Thompson, L.; Vangala, S.V.; Chang, T.; Okelo, S. Inhaled corticosteroid beliefs, complementary and alternative medicine in children presenting to the emergency department for asthma. J. Asthma 2021, 58, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.K.; Murdock, K.K.; McQuaid, E.L. Complementary and Alternative Medication (CAM) Use and Asthma Outcomes in Children: An Urban Perspective. J. Asthma 2007, 44, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Karine, T.-A.; Debbie Ehrmann, F.; Maria Victoria, Z.; Martin, D.; Peter, M.; Ciarán, M.D. Is Complementary and Alternative Healthcare Use Associated with Better Outcomes in Children with Juvenile Idiopathic Arthritis? J. Rheumatol. 2009, 36, 2302. [Google Scholar]
- Sato, M.; Yamamoto-Hanada, K.; Yang, L.; Irahara, M.; Ishikawa, F.; Iwama-Mitsui, M.; Saito-Abe, M.; Miyaji, Y.; Inagaki, S.; Fukuie, T.; et al. Complementary and alternative medicine and atopic dermatitis in children. J. Dermatol. Sci. 2020, 97, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Mittal, R.; Walser, S.A.; Lehman, E.; Kumar, A.; Paudel, S.; Mainali, G. Complementary and Alternative Medicine (CAM) use in Children with Epilepsy. J. Child. Neurol. 2022, 37, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Serpico, M.R.; Boyle, B.M.; Kemper, K.J.; Kim, S.C. Complementary and Alternative Medicine Use in Children With Inflammatory Bowel Diseases: A Single-Center Survey. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Phatak, U.P.; Alper, A.; Pashankar, D.S. Complementary and Alternative Medicine Use in Children With Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 157–160. [Google Scholar] [CrossRef] [PubMed]
- KidsHealth. Advice For Parents about Complementary & Alternative Medicine. 2021. Available online: https://www.kidshealth.org.nz/advice-parents-about-complementary-alternative-medicine (accessed on 29 April 2024).

| Variable | Category | Mean (SD) or N (%) |
|---|---|---|
| Child age | Years | 6.7 (4.9) |
| Child sex | Male Female | 66 (51) 64 (49) |
| Family ethnicity * | NZ European Māori Pacific MELAA Asian | 105 (81) 21 (16) 4 (3) 5 (4) 15 (12) |
| Number of adults in family home | 1 2 3 4–5 | 15 (12) 99 (76) 8 (6) 8 (6) |
| Number of children in family home | 1 2 3 4–6 | 37 (29) 54 (42) 29 (22) 10 (7) |
| Child chronic health condition | No Yes Under investigation | 87 (67) 29 (22) 14 (11) |
| Child on prescription medications | No Yes | 83 (64) 47 (36) |
| Reason for attending hospital | Accident/injury New illness/condition Pre-existing condition Investigations | 26 (20) 58 (45) 21 (16) 25 (19) |
| Parent age | Years | 38.0 (7.7) |
| Parent sex | Male Female | 19 (14) 111 (86) |
| Parent education level | High School College/vocational training University Post-graduate | 41 (31.5) 20 (15.4) 46 (35.4) 23 (17.7) |
| Household income (NZD) | Up to 50,000 50,000–100,000 100,000–150,000 150,000–200,000 200,000+ Not stated | 13 (10) 36 (28) 29 (22) 24 (19) 17 (13) 11 (8) |
| CAM used by parents CAM used by siblings | Yes Yes | 81 (62.3) 62 (47.7) |
| Child health in last week | 1–10 scale | 6.3 (2.7) |
| ED visits in last 12 months PCP visits in last 12 months OPA visits in last 12 months | Yes Yes Yes | 84 (64.6) 109 (83.8) 68 (52.3) |
| Variable | Category | Frequency N (%) |
|---|---|---|
| Used for a chronic condition | Yes No | 25 (30) 58 (70) |
| Side effects | None | 80 (92) |
| Mild | 7 (8) | |
| Moderate | 0 | |
| Severe | 0 | |
| Benefits seen | None | 10 (12) |
| Improved slightly | 44 (52) | |
| Improved lots | 31 (36) | |
| Reason for using * | Treatment of symptoms | 56 (39) |
| Prevention of symptoms | 37 (26) | |
| To complement conventional treatment | 21 (14) | |
| Knowledge of it working for other people | 15 (10) | |
| Lack of conventional treatment | 5 (3) | |
| Worry about side effects from conventional treatment | 4 (3) | |
| Lack of confidence in conventional treatment | 4 (3) | |
| More effective than conventional treatment | 3 (2) | |
| Duration | More than 12 months | 46 (53) |
| 6–12 months | 12 (14) | |
| 1–6 months | 19 (22) | |
| Less than 1 month | 10 (11) | |
| Frequency | When needed | 21 (24) |
| Yearly | 0 | |
| Every 6 months | 8 (9) | |
| Monthly | 1 (1) | |
| Weekly | 20 (23) | |
| Daily | 37 (43) |
| Variable | Mean Difference or χ2 (Phi) | p-Value | 95% CI |
|---|---|---|---|
| Child age | −1.0 | 0.25 | −2.7, 0.7 |
| Parent age | −1.3 | 0.33 | −4.0, 1.4 |
| Overall health rating | 0.0 | 0.99 | −0.9, 0.9 |
| Child gender | 0.1 (−0.0) | 0.72 | −0.1, 0.2 |
| Ethnicity NZ European | 1.1 (0.1) | 0.30 | −0.2, 0.1 |
| Ethnicity Māori | 1.4 (0.1) | 0.24 | −0.2, 0.1 |
| Ethnicity Pacific | 1.5 (0.1) | 0.23 | −0.1, 0.0 |
| Ethnicity MELAA | 0.4 (0.1) | 0.51 | −0.1, 0.0 |
| Ethnicity Asian | 2.4 (−0.1) | 0.12 | −0.0, 0.2 |
| Number of adults in family home | 1.2 (0.1) | 0.76 | 0.8, 0.8 |
| Number of children in family home | 0.2 (0.0) | 0.97 | 0.9, 0.9 |
| Child chronic health condition | 0.9 (0.1) | 0.42 | −0.3, 0.1 |
| Child on prescription medications | 1.0 (0.1) | 0.33 | −0.3, 0.1 |
| Reason for attending hospital | 1.9 (0.1) | 0.72 | −0.5, 0.3 |
| Parent gender | 3.3 (0.2) | 0.07 | −0.2, 0.1 |
| Parent education level | 7.3 (0.2) | 0.77 | −0.4, 0.3 |
| Household income (NZD) | 0.9 (0.1) | 0.75 | −0.4, 0.5 |
| CAM used by parents | 26.8 (0.5) | <0.001 | −1.3, −0.7 |
| CAM used by siblings | 59.4 (0.7) | <0.001 | −1.6, −0.9 |
| ED visits in last 12 months | 4.7 (0.2) | 0.03 | −0.3, 0.0 |
| PCP visits in last 12 months | 0.1 (−0.0) | 0.82 | −0.1, 0.1 |
| OPA visits in last 12 months | 0.1 (−0.0) | 0.76 | −0.1, 0.2 |
| Opinion Statement | CAM Use | Disagree N (%) | Neutral N (%) | Agree N (%) | χ2 (Phi) | p Value |
|---|---|---|---|---|---|---|
| Doctors should be supportive of people using CAM | No CAM CAM | 0 (0) 0 (0) | 18 (24) 7 (14) | 55 (76) 50 (86) | 2.2 (0.1) | 0.14 |
| Doctors should ask patients if they are using CAM | No CAM CAM | 0 (0) 0 (0) | 16 (23) 11 (19) | 57 (77) 46 (81) | 0.3 (0.0) | 0.59 |
| Doctors should know about CAM and be able to give advice | No CAM CAM | 2 (3) 1 (2) | 15 (21) 12 (20) | 56 (76) 44 (78) | 0.2 (0.0) | 0.91 |
| I would only use CAM for my child if a doctor recommended it | No CAM CAM | 24 (34) 33 (56) | 20 (27) 15 (27) | 29 (39) 9 (17) | 9.2 (0.3) | 0.01 |
| CAMs do not interfere with prescribed drugs | No CAM CAM | 13 (17) 15 (27) | 41 (56) 24 (42) | 19 (27) 18 (31) | 0.2 (0.2) | 0.23 |
| Enough is known about the effectiveness of CAM | No CAM CAM | 26 (34) 21 (39) | 34 (48) 19 (32) | 13 (18) 17 (29) | 3.7 (0.2) | 0.16 |
| Enough is known about the safety of CAM | No CAM CAM | 15 (20) 14 (26) | 37 (51) 18 (32) | 21 (29) 25 (42) | 5.6 (0.2) | 0.10 |
| Enough is known about the side effects of CAM | No CAM CAM | 17 (21) 14 (27) | 38 (54) 19 (32) | 18 (25) 24 (41) | 6.2 (0.2) | 0.05 |
| There is sufficient information available about CAM | No CAM CAM | 28 (38) 22 (39) | 29 (39) 16 (29) | 16 (23) 19 (32) | 2.2 (0.1) | 0.34 |
| CAMs have fewer side effects than prescribed or conventional treatment. | No CAM CAM | 4 (6) 6 (10) | 47 (63) 24 (44) | 22 (31) 27 (46) | 4.9 (0.2) | 0.09 |
| CAM is more effective than prescribed or conventional treatment | No CAM CAM | 26 (37) 12 (20) | 42 (56) 38 (68) | 5 (7) 7 (12) | 4.4 (0.2) | 0.11 |
| CAM therapists/practitioners should be qualified and registered. | No CAM CAM | 1 (2) 1 (2) | 13 (18) 13 (22) | 59 (80) 43 (76) | 0.3 (0.0) | 0.86 |
| CAM is used by people due to a lack of conventional treatment for an illness or condition. | No CAM CAM | 38 (52) 31 (54) | 27 (38) 18 (31) | 8 (10) 8 (15) | 1.3 (0.1) | 0.52 |
| CAM is used by people due to a lack of conventional treatment for an illness or condition | No CAM CAM | 26 (35) 22 (39) | 33 (47) 23 (39) | 14 (18) 12 (22) | 0.8 (0.1) | 0.68 |
| CAM can be used to replace conventional treatment | No CAM CAM | 26 (35) 24 (43) | 32 (44) 21 (37) | 15 (21) 12 (20) | 0.8 (0.1) | 0.68 |
| The cost of CAM puts people off using it | No CAM CAM | 11 (15) 11 (18) | 24 (34) 24 (41) | 38 (51) 22 (41) | 1.3 (0.1) | 0.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denny, A.; Day, A.S.; Vernon-Roberts, A. Association between Paediatric Complementary and Alternative Medicine Use and Parental Health Literacy, Child Health, and Socio-Economic Variables: A Prospective Study. Pediatr. Rep. 2024, 16, 368-384. https://doi.org/10.3390/pediatric16020032
Denny A, Day AS, Vernon-Roberts A. Association between Paediatric Complementary and Alternative Medicine Use and Parental Health Literacy, Child Health, and Socio-Economic Variables: A Prospective Study. Pediatric Reports. 2024; 16(2):368-384. https://doi.org/10.3390/pediatric16020032
Chicago/Turabian StyleDenny, Abida, Andrew S. Day, and Angharad Vernon-Roberts. 2024. "Association between Paediatric Complementary and Alternative Medicine Use and Parental Health Literacy, Child Health, and Socio-Economic Variables: A Prospective Study" Pediatric Reports 16, no. 2: 368-384. https://doi.org/10.3390/pediatric16020032
APA StyleDenny, A., Day, A. S., & Vernon-Roberts, A. (2024). Association between Paediatric Complementary and Alternative Medicine Use and Parental Health Literacy, Child Health, and Socio-Economic Variables: A Prospective Study. Pediatric Reports, 16(2), 368-384. https://doi.org/10.3390/pediatric16020032






.png)

