Predictive Value of Neutrophil-to-Monocyte Ratio, Lymphocyte-to-Monocyte Ratio, C-Reactive Protein, Procalcitonin, and Tumor Necrosis Factor Alpha for Neurological Complications in Mechanically Ventilated Neonates Born after 35 Weeks of Gestation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Inclusion and Exclusion Criteria
2.3. Laboratory Investigations
2.4. Respiratory Support and Mechanical Ventilation Description
2.5. Statistical Analysis
3. Results
Patient Demographics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaltsogianni, O.; Dassios, T.; Greenough, A. Neonatal respiratory support strategies—Short and long-term respiratory outcomes. Front. Pediatr. 2023, 11, 1212074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakkarapani, A.A.; Adappa, R.; Ali, S.K.M.; Gupta, S.; Soni, N.B.; Chicoine, L.; Hummler, H.D. “Current concepts in assisted mechanical ventilation in the neonate”–Part 2: Understanding various modes of mechanical ventilation and recommendations for individualized disease-based approach in neonates. Int. J. Pediatr. Adolesc. Med. 2020, 7, 201–208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rocha, G.; Soares, P.; Gonçalves, A.; Silva, A.I.; Almeida, D.; Figueiredo, S.; Pissarra, S.; Costa, S.; Soares, H.; Flôr-De-Lima, F.; et al. Respiratory Care for the Ventilated Neonate. Can. Respir. J. 2018, 2018, 7472964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haribhai, S.; Mahboobi, S.K. Ventilator Complications. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560535/ (accessed on 24 January 2024).
- Sood, S.; Ganatra, H.A.; Marques, F.P.; Langner, T.R. Complications during mechanical ventilation—A pediatric intensive care perspective. Front. Med. 2023, 10, 1016316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bano, S.; Chaudhary, V.; Garga, U.C. Neonatal Hypoxic-Ischemic Encephalopathy: A Radiological Review. J. Pediatr. Neurosci. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamity, R.; Kapavarapu, P.K.; Chandel, A. Feeding Problems and Long-Term Outcomes in Preterm Infants—A Systematic Approach to Evaluation and Management. Children 2021, 8, 1158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patra, A.; Huang, H.; Bauer, J.; Giannone, P.J. Neurological consequences of systemic inflammation in the premature neonate. Neural Regen. Res. 2017, 12, 890–896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dankhara, N.; Holla, I.; Ramarao, S.; Thekkeveedu, R.K. Bronchopulmonary Dysplasia: Pathogenesis and Pathophysiology. J. Clin. Med. 2023, 12, 4207. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Nayak, M.K.; Rath, S.; Das, P. The Utility of the Neutrophil-Lymphocyte Ratio as an Early Diagnostic Marker in Neonatal Sepsis. Cureus 2021, 13, e12891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, X.; Wang, Y.; Xie, M.; Qiu, S.; Zhou, J. Elevated neutrophil–to-monocyte ratio as a prognostic marker for poor outcomes in neonatal sepsis. Heliyon 2022, 8, e11181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ucar, B.; Yildiz, B.; Aksit, M.A.; Yarar, C.; Colak, O.; Akbay, Y.; Colak, E. Serum amyloid A, procalcitonin, tumor necrosis factor-α, and interleukin-1β levels in neonatal late-onset sepsis. Mediat. Inflamm. 2008, 2008, 737141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Machado, J.R.; Soave, D.F.; da Silva, M.V.; de Menezes, L.B.; Etchebehere, R.M.; Monteiro, M.L.; dos Reis, M.A.; Corrêa, R.R.M.; Celes, M.R.N. Neonatal sepsis and inflammatory mediators. Mediat. Inflamm. 2014, 2014, 269681. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eichberger, J.; Resch, E.; Resch, B. Diagnosis of Neonatal Sepsis: The Role of Inflammatory Markers. Front. Pediatr. 2022, 10, 840288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shang, Q.-X.; Yang, Y.-S.; Hu, W.-P.; Yuan, Y.; He, Y.; Zhao, J.-Y.; Ji, A.-F.; Chen, L.-Q. Clinical and prognostic significance of preoperative lymphocyte-monocyte ratio, neutrophil-lymphocyte ratio and neutrophil-monocyte ratio on esophageal squamous cell carcinoma patients. Transl. Cancer Res. 2020, 9, 3903–3914. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Pogorelić, Z.; Agrawal, A.; Muñoz, C.M.L.; Kainth, D.; Verma, A.; Jindal, B.; Agarwala, S.; Anand, S. Utility of Ischemia-Modified Albumin as a Biomarker for Acute Appendicitis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 5486. [Google Scholar] [CrossRef] [PubMed]
- Rhally, A.; Griffa, A.; Kremer, S.; Uginet, M.; Breville, G.; Stancu, P.; Assal, F.; Lalive, P.H.; Lövblad, K.-O.; Allali, G. C-reactive protein and white matter microstructural changes in COVID-19 patients with encephalopathy. J. Neural Transm. 2021, 128, 1899–1906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bian, J.; Guo, S.; Huang, T.; Li, X.; Zhao, S.; Chu, Z.; Li, Z. CRP as a potential predictor of outcome in acute ischemic stroke. Biomed. Rep. 2023, 18, 17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Q.-T.; Chen, C.; Yu, Q.-Y.; Chen, S.-Y.; Huang, X.; Zhong, Y.-L.; Luo, S.-P.; Gao, J. The benefits of higher LMR for early threatened abortion: A retrospective cohort study. PLoS ONE 2020, 15, e0231642. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumitro, K.R.; Utomo, M.T.; Widodo, A.D.W. Neutrophil-to-Lymphocyte Ratio as an Alternative Marker of Neonatal Sepsis in Developing Countries. Oman Med. J. 2021, 36, e214. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, B.; Huang, J.; Yuan, H.; Yan, W.; Hu, G.; Wang, J. Tumor necrosis factor-α as a diagnostic marker for neonatal sepsis: A meta-analysis. Sci. World J. 2014, 2014, 471463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, M.; Jung, K.; Ko, Y.; Kim, I.-S.; Hwang, K.; Jang, J.-H.; Shin, J.E.; Park, K.I. TNF-α Pretreatment Improves the Survival and Function of Transplanted Human Neural Progenitor Cells Following Hypoxic-Ischemic Brain Injury. Cells 2020, 9, 1195. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, T.; Dong, G.; Zhang, M.; Xu, Z.; Hu, Y.; Xie, B.; Wang, Y.; Xu, B. Association of Neutrophil–Lymphocyte Ratio and the Presence of Neonatal Sepsis. J. Immunol. Res. 2020, 2020, 7650713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bohrer, B.; Silveira, R.C.; Neto, E.C.; Procianoy, R.S. Mechanical ventilation of newborns infant changes in plasma pro- and anti-inflammatory cytokines. J. Pediatr. 2010, 156, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Numis, A.L.; Foster-Barber, A.; Deng, X.; Rogers, E.E.; Barkovich, A.J.; Ferriero, D.M.; Glass, H.C. Early changes in pro-inflammatory cytokine levels in neonates with encephalopathy are associated with remote epilepsy. Pediatr. Res. 2019, 86, 616–621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, X.-B.; Huang, L.-X.; Huang, Z.-R.; Lu, L.-M.; Luo, B.; Cai, W.-Q.; Liu, A.-M.; Wang, S.-W. The lymphocyte-to-monocyte ratio predicts intracranial atherosclerotic stenosis plaque instability. Front. Immunol. 2022, 13, 915126. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.H.; Zhou, L.; Li, Y.; McIntire, L.B.; Nordvig, A.; Butler, T.; de Leon, M.; Chiang, G.C. Peripheral immune cell imbalance is associated with cortical beta-amyloid deposition and longitudinal cognitive decline. Sci. Rep. 2023, 13, 8847. [Google Scholar] [CrossRef] [PubMed]
- Kaminiów, K.; Kozak, S.; Paprocka, J. Neonatal Seizures Revisited. Children 2021, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Massaro, A.N.; Chang, T.; Kadom, N.; Tsuchida, T.; Scafidi, J.; Glass, P.; McCarter, R.; Baumgart, S.; Vezina, G.; Nelson, K.B. Biomarkers of brain injury in neonatal encephalopathy treated with hypothermia. J. Pediatr. 2012, 161, 434–440. [Google Scholar] [CrossRef] [PubMed]

| Variables | Neurological Complications (n = 26) | No Neurological Complications (n = 59) | p-Value |
|---|---|---|---|
| Gender, n (%) | 0.895 | ||
| Male | 18 (69.23%) | 40 (67.80%) | |
| Female | 8 (30.77%) | 19 (32.20%) | |
| Gestational age, weeks (mean ± SD) | 36.69 ± 1.59 | 36.29 ± 1.82 | 0.335 |
| Birth weight, grams (mean ± SD) | 2941 ± 966 | 2626 ± 614 | 0.073 |
| APGAR score 1 min, (mean ± SD) | 5 ± 2 | 7 ± 1 | <0.001 |
| APGAR score 5 min, (mean ± SD) | 6 ± 1 | 7 ± 1 | <0.001 |
| Monitored pregnancy, n (%) | 15 (57.69%) | 36 (61.02%) | 0.773 |
| Cesarean birth, n (%) | 17 (65.38%) | 41 (69.49%) | 0.707 |
| Meconium aspiration, n (%) | 3 (11.54%) | 5 (8.47%) | 0.655 |
| Transient tachypnea, n (%) | 8 (30.77%) | 23 (38.89%) | 0.468 |
| Variables | Neurological Complications (n = 26) | No Neurological Complications (n = 59) | p-Value |
|---|---|---|---|
| Neonatal sepsis, n (%) | 5 (19.23%) | 11 (18.64%) | 0.949 |
| Respiratory complications | |||
| ARDS | 3 (11.54%) | 11 (18.64%) | 0.415 |
| Congenital pneumonia | 5 (19.23%) | 13 (22.03%) | 0.770 |
| Neonatal asphyxia | 10 (38.46%) | 5 (8.47%) | <0.001 |
| Pneumothorax | 5 (19.23%) | 9 (15.25%) | 0.648 |
| Pulmonary hemorrhage | 3 (11.54%) | 7 (11.86%) | 0.965 |
| Recurrent pneumonia | 8 (30.77%) | 20 (33.90%) | 0.777 |
| Neurological complications | |||
| HIE | 19 (73.80%) | – | |
| Intracranial hemorrhage | 6 (23.08%) | – | |
| Seizures | 16 (61.54%) | – | |
| Interventions | |||
| nIPPV | 13 (50.00%) | 15 (25.42%) | 0.026 |
| nCPAP/nIPPV | 11 (42.31%) | 33 (55.93%) | 0.246 |
| SIMV/IPPV | 9 (34.62%) | 19 (32.20%) | 0.827 |
| Days on ventilation (>7) | 15 (57.69%) | 23 (38.89%) | 0.109 |
| Variables (Mean ± SD) * | Neurological Complications (n = 26) | No Neurological Complications (n = 59) | p-Value ** |
|---|---|---|---|
| CRP (0–5 mg/L) | 11.39 ± 10.51 | 8.29 ± 11.58 | 0.245 |
| Procalcitonin (0–0.5 ng/mL) | 16.16 ± 13.27 | 9.44 ± 15.64 | 0.059 |
| WBC (9–30 × 103/μL) | 20.70 ± 16.79 | 15.39 ± 5.67 | 0.032 |
| Lymphocyte count (2–17 × 103/μL) | 4.61 ± 2.02 | 2.41 ± 3.55 | 0.004 |
| Neutrophil count (1.5–22 × 103/μL) | 9.63 ± 6.99 | 7.20 ± 6.68 | 0.131 |
| Monocyte count (0.1–1.36 × 103/μL) | 2.03 ± 1.65 | 2.17 ± 4.15 | 0.868 |
| NMR | 7.59 ± 4.09 | 5.44 ± 4.34 | 0.035 |
| LMR | 6.18 ± 12.06 | 2.63 ± 1.31 | 0.027 |
| TNF-alpha (pg/mL) | 17.33 ± 15.76 | 11.06 ± 7.21 | 0.013 |
| Variables (Mean ± SD) * | Neurological Complications (n = 26) | No Neurological Complications (n = 59) | p-Value ** |
|---|---|---|---|
| CRP (0–5 mg/L) | 12.64 ± 12.91 | 6.89 ± 10.85 | 0.036 |
| Procalcitonin (0–0.5 ng/mL) | 8.56 ± 7.44 | 4.43 ± 8.94 | 0.042 |
| WBC (9–30 × 103/μL) | 17.35 ± 10.47 | 13.51 ± 4.52 | 0.020 |
| Lymphocyte count (2–17 × 103/μL) | 4.59 ± 2.06 | 3.66 ± 3.24 | 0.181 |
| Neutrophil count (1.5–22 × 103/μL) | 7.58 ± 4.53 | 3.41 ± 2.91 | <0.001 |
| Monocyte count (0.1–1.36 × 103/μL) | 2.50 ± 3.04 | 1.87 ± 0.88 | 0.145 |
| NMR | 5.42 ± 3.47 | 3.83 ± 3.35 | 0.049 |
| LMR | 4.22 ± 6.09 | 2.71 ± 2.00 | 0.005 |
| TNF-alpha (pg/mL) | 19.59 ± 9.57 | 13.65 ± 14.35 | 0.022 |
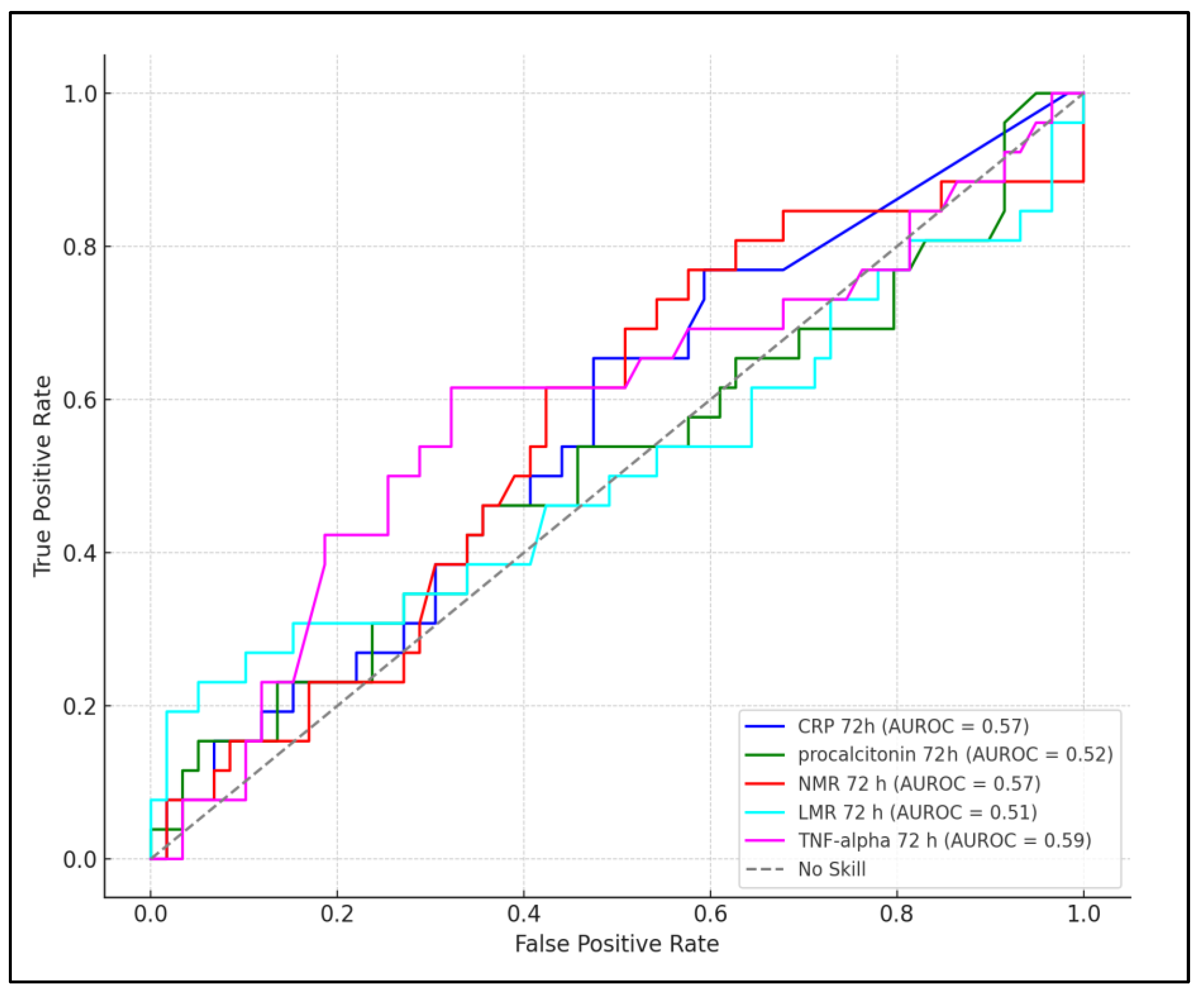
| Laboratory Parameter | Timeframe | Best Cutoff Value | Sensitivity | Specificity | AUC | p-Value |
|---|---|---|---|---|---|---|
| CRP | 24 h | >10.7 mg/L | 68% | 62% | 0.431 | 0.168 |
| Procalcitonin | 24 h | >12.2 ng/mL | 66% | 59% | 0.429 | 0.183 |
| NMR | 24 h | >5.3 | 78% | 67% | 0.562 | 0.029 |
| LMR | 24 h | >4.2 | 71% | 64% | 0.558 | 0.038 |
| TNF-alpha | 24 h | >12.8 pg/mL | 82% | 69% | 0.574 | 0.005 |
| CRP | 72 h | >15.4 mg/L | 74% | 66% | 0.570 | 0.032 |
| Procalcitonin | 72 h | >0.32 ng/mL | 70% | 61% | 0.520 | 0.059 |
| NMR | 72 h | >6.1 | 76% | 68% | 0.567 | 0.025 |
| LMR | 72 h | >3.7 | 68% | 63% | 0.510 | 0.076 |
| TNF-alpha | 72 h | >14.3 pg/mL | 87% | 72% | 0.593 | <0.001 |
| Factors above the Best Cutoff | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| CRP | 1.41 | 1.06–4.81 | 0.030 |
| Procalcitonin | 1.30 | 0.94–3.17 | 0.093 |
| NMR | 2.16 | 1.18–4.09 | 0.022 |
| LMR | 1.94 | 1.32–4.26 | 0.008 |
| TNF-alpha | 3.32 | 2.06–6.39 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioboata, D.M.; Boia, M.; Manea, A.M.; Costescu, O.C.; Costescu, S.; Doandes, F.M.; Popa, Z.L.; Sandesc, D. Predictive Value of Neutrophil-to-Monocyte Ratio, Lymphocyte-to-Monocyte Ratio, C-Reactive Protein, Procalcitonin, and Tumor Necrosis Factor Alpha for Neurological Complications in Mechanically Ventilated Neonates Born after 35 Weeks of Gestation. Pediatr. Rep. 2024, 16, 313-326. https://doi.org/10.3390/pediatric16020027
Cioboata DM, Boia M, Manea AM, Costescu OC, Costescu S, Doandes FM, Popa ZL, Sandesc D. Predictive Value of Neutrophil-to-Monocyte Ratio, Lymphocyte-to-Monocyte Ratio, C-Reactive Protein, Procalcitonin, and Tumor Necrosis Factor Alpha for Neurological Complications in Mechanically Ventilated Neonates Born after 35 Weeks of Gestation. Pediatric Reports. 2024; 16(2):313-326. https://doi.org/10.3390/pediatric16020027
Chicago/Turabian StyleCioboata, Daniela Mariana, Marioara Boia, Aniko Maria Manea, Oana Cristina Costescu, Sergiu Costescu, Florina Marinela Doandes, Zoran Laurentiu Popa, and Dorel Sandesc. 2024. "Predictive Value of Neutrophil-to-Monocyte Ratio, Lymphocyte-to-Monocyte Ratio, C-Reactive Protein, Procalcitonin, and Tumor Necrosis Factor Alpha for Neurological Complications in Mechanically Ventilated Neonates Born after 35 Weeks of Gestation" Pediatric Reports 16, no. 2: 313-326. https://doi.org/10.3390/pediatric16020027
APA StyleCioboata, D. M., Boia, M., Manea, A. M., Costescu, O. C., Costescu, S., Doandes, F. M., Popa, Z. L., & Sandesc, D. (2024). Predictive Value of Neutrophil-to-Monocyte Ratio, Lymphocyte-to-Monocyte Ratio, C-Reactive Protein, Procalcitonin, and Tumor Necrosis Factor Alpha for Neurological Complications in Mechanically Ventilated Neonates Born after 35 Weeks of Gestation. Pediatric Reports, 16(2), 313-326. https://doi.org/10.3390/pediatric16020027






