High-Altitude Pulmonary Edema in Two Pediatric Patients with Pre-Existing Lung Disease
Abstract
1. Introduction
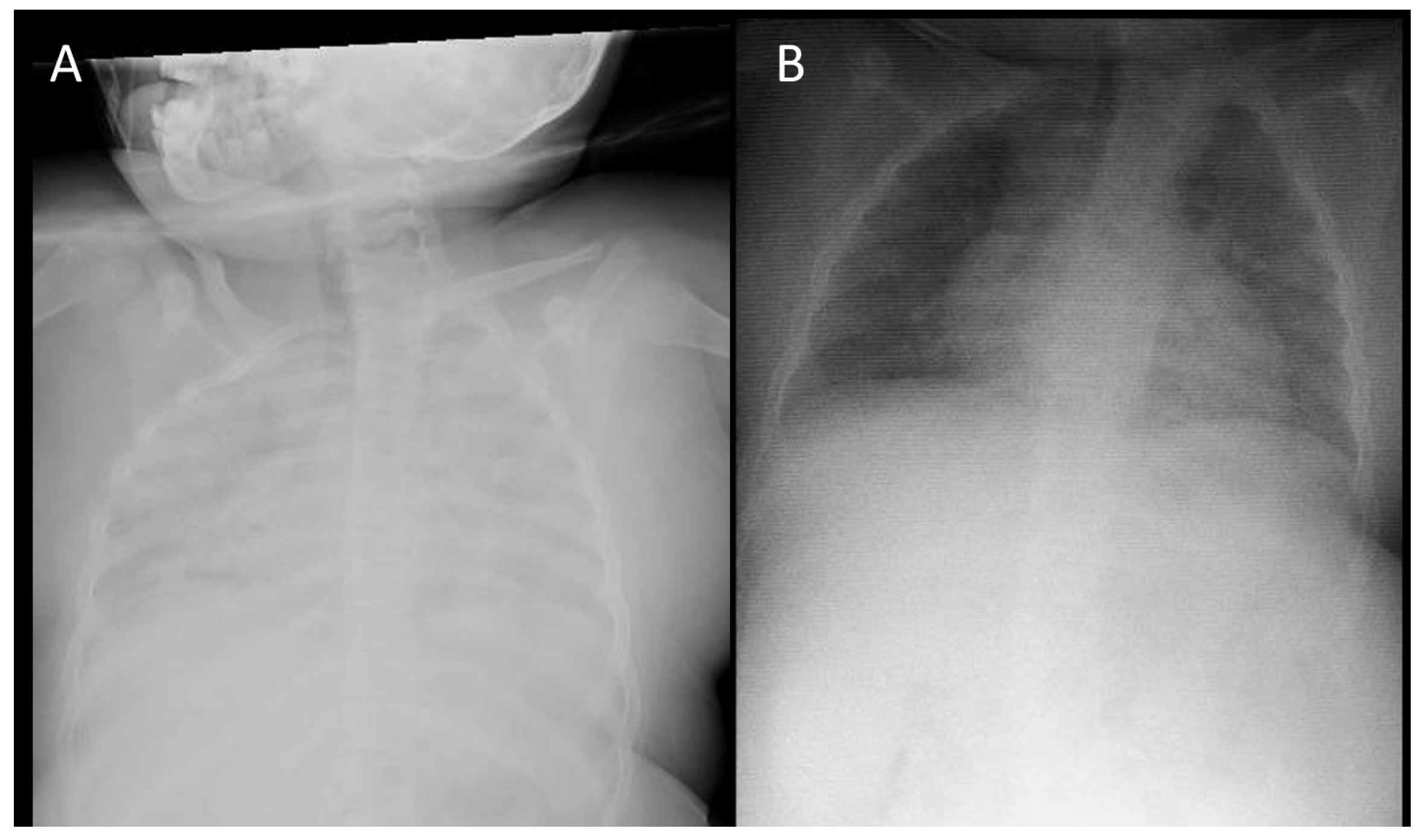
2. Cases
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paralikar, S.J. High altitude pulmonary edema-clinical features, pathophysiology, prevention and treatment. Indian J. Occup. Environ. Med. 2012, 16, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Garlick, V.; O’Connor, A.; Shubkin, C.D. High-altitude illness in the pediatric population: A review of the literature on prevention and treatment. Curr. Opin. Pediatr. 2017, 29, 503–509. [Google Scholar] [CrossRef] [PubMed]
- West, J.B. High-altitude medicine. Am. J. Respir. Crit. Care Med. 2012, 186, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Das, B.B.; Wolfe, R.R.; Chan, K.C.; Larsen, G.L.; Reeves, J.T.; Ivy, D. High-altitude pulmonary edema in children with underlying cardiopulmonary disorders and pulmonary hypertension living at altitude. Arch. Pediatr. Adolesc. Med. 2004, 158, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Bärtsch, P.; Swenson, E.R. Acute High-Altitude Illnesses. N. Engl. J. Med. 2013, 369, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Luks, A.M.; Swenson, E.R. Travel to high altitude with pre-existing lung disease. Eur. Respir. J. 2007, 29, 770–792. [Google Scholar] [CrossRef] [PubMed]
- Liptzin, D.R.; Abman, S.H.; Giesenhagen, A.; Ivy, D.D. An approach to children with pulmonary edema at high altitude. High Alt. Med. Biol. 2018, 19, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Adamo, M.; Prokopakis, K.E.; Bolotin, T. High Altitude Pulmonary Edema in a Healthy Pediatric Patient Traveling from Denver to Breckenridge. Open Access Emerg. Med. 2022, 14, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Asseri, A.A.; Asiri, İ.A.; Alwabel, H.H.; Asiri, A.M.; Asiri, W.I. Severe acute reentry high altitude pulmonary edema in pediatric patients: Report of three cases and literature review. Turk. J. Pediatr. 2022, 64, 400–407. [Google Scholar] [CrossRef]
- Hultgren, H.N. High Altitude Pulmonary Edema: Hemodynamic Aspects. Int. J. Sport Med. 1997, 18, 20–25. [Google Scholar] [CrossRef]
- Bhagi, S.; Srivastava, S.; Singh, S.B. High-altitude Pulmonary Edema: Review. J. Occup. Health 2014, 56, 235–243. [Google Scholar] [CrossRef]
- Zhou, Q. Standardization of methods for early diagnosis and on-site treatment of high-altitude pulmonary edema. Pulm. Med. 2011, 2011, 190648. [Google Scholar] [CrossRef]
- Baniya, S.; Holden, C.; Basnyat, B. Reentry High Altitude Pulmonary Edema in the Himalayas. High Alt. Med. Biol. 2017, 18, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Cubides Diaz, D.A.; Muñoz Angulo, N.; Herrera Alzate, L.A.; Martin Arsanios, D.; Ovalle Monroy, A.L.; Velandia, O.; Calderón Vargas, C.M. High altitude pulmonary edema at 2640 m altitude associated with an acute Rhinovirus infection. First case in the literature. Respir. Med. Case Rep. 2023, 41, 101791. [Google Scholar] [CrossRef] [PubMed]
- Busch, T.; Bärtsch, P.; Pappert, D.; Grünig, E.; Hildebrandt, W.; Elser, H.; Falke, K.J.; Swenson, E.R. Hypoxia Decreases Exhaled Nitric Oxide in Mountaineers Susceptible to High-Altitude Pulmonary Edema. Am. J. Respir. Crit. Care Med. 2001, 163, 368–373. [Google Scholar] [CrossRef]
- Berger, M.M.; Macholz, F.; Mairbäurl, H.; Bärtsch, P. Remote ischemic preconditioning for prevention of high-altitude diseases: Fact or fiction? J. Appl. Physiol. 2015, 119, 1143–1151. [Google Scholar] [CrossRef]
- Sartori, C.; Vollenweider, L.; Löffler, B.-M.; Delabays, A.; Nicod, P.; Bärtsch, P.; Scherrer, U. Exaggerated Endothelin Release in High-Altitude Pulmonary Edema. Circulation 1999, 99, 2665–2668. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Hesse, C.; Dehnert, C.; Siedler, H.; Kleinbongard, P.; Bardenheuer, H.J.; Kelm, M.; Bärtsch, P.; Haefeli, W.E. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 2005, 172, 763–767. [Google Scholar] [CrossRef] [PubMed]
- O’toole, S.J.; Irish, M.S.; Holm, B.A.; Glick, P.L. Congenital diaphragmatic hernia pulmonary vascular abnormalities in congenital diaphragmatic hernia. Clin. Perinatol. 1996, 23, 781–794. [Google Scholar] [CrossRef]
- Murray, F.; Insel, P.A.; Yuan, J.X.J. Role of O2-sensitive K+ and Ca2+ channels in the regulation of the pulmonary circulation: Potential role of caveolae and implications for high altitude pulmonary edema. Respir. Physiol. Neurobiol. 2006, 151, 192–208. [Google Scholar] [CrossRef]
- Pollard, A.J.; Durmowicz, A.; Durrer, B.; Eldridge, M.; Hackett, P.; Jean, D.; Kriemler, S.; Litch, J.A.; Murdoch, D.; Nickol, A.; et al. Children at high altitude: An international consensus statement by an ad hoc committee of the International Society for Mountain Medicine, March 12, 2001. High Alt. Med. Biol. 2001, 2, 389–401. [Google Scholar] [CrossRef] [PubMed]

| Variables | Reference Range | Patient 1 | Patient 2 |
|---|---|---|---|
| Blood and biochemical tests | |||
| White blood cells (×109/L) | 4500–11,000 | 5.56 | 26.34 |
| Absolute lymphocyte count per mm3 | 1000–4800 | 780 | 1120 |
| Absolute neutrophil count per mm3 | 1800–7700 | 4040 | 24,920 |
| Hemoglobin (g/dL) | 12.0–16.0 | 10.8 | 11.4 |
| Hematocrit | 36–46% | 38.0 | 36.3 |
| Platelets (×109/L) | 150,000–450,000 | 189 | 358 |
| ESR (mm/h) | 0–13 | 4 | 80 |
| BUN (mg/dL) | 8.0–25 | 14 | 10 |
| Creatinine (mg/dL) | 0.30–1.00 | 0.36 | 0.39 |
| Sodium (mEq/L) | 135–145 | 137 | 136 |
| ALT (IU/L) | 10–55 | 12 | 9 |
| AST (IU/L) | 9.0–32 | 36 | 22 |
| Albumin (g/dL) | 3.4–5.4 | 3.49 | 3.6 |
| pH | 7.35–7.45 | 7.37 | 7.41 |
| PCO2 (mmHg) | 35–45 | 36.3 | 45 |
| HCO3 (mEq/L) | 22–26 | 21.1 | 27.4 |
| Base excess (mEq/L) | −2–+2 | −3.9 | 2.1 |
| Oxygen saturation (%) | >95% | 40 | 71 |
| FiO2 (%) | - | 100% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asseri, A.A.; Assiri, M.; Alshehri, N.; Alyazidi, N.S.; Alasmari, A.; Alshabab, S.Q.; Asiri, N.A. High-Altitude Pulmonary Edema in Two Pediatric Patients with Pre-Existing Lung Disease. Pediatr. Rep. 2024, 16, 271-277. https://doi.org/10.3390/pediatric16020023
Asseri AA, Assiri M, Alshehri N, Alyazidi NS, Alasmari A, Alshabab SQ, Asiri NA. High-Altitude Pulmonary Edema in Two Pediatric Patients with Pre-Existing Lung Disease. Pediatric Reports. 2024; 16(2):271-277. https://doi.org/10.3390/pediatric16020023
Chicago/Turabian StyleAsseri, Ali Alsuheel, Marei Assiri, Norah Alshehri, Noha Saad Alyazidi, Ahmed Alasmari, Saud Q. Alshabab, and Nada Abdullah Asiri. 2024. "High-Altitude Pulmonary Edema in Two Pediatric Patients with Pre-Existing Lung Disease" Pediatric Reports 16, no. 2: 271-277. https://doi.org/10.3390/pediatric16020023
APA StyleAsseri, A. A., Assiri, M., Alshehri, N., Alyazidi, N. S., Alasmari, A., Alshabab, S. Q., & Asiri, N. A. (2024). High-Altitude Pulmonary Edema in Two Pediatric Patients with Pre-Existing Lung Disease. Pediatric Reports, 16(2), 271-277. https://doi.org/10.3390/pediatric16020023






