Pediatric Lemierre’s Syndrome: A Comprehensive Literature Review
Abstract
1. Introduction
2. Materials and Methods
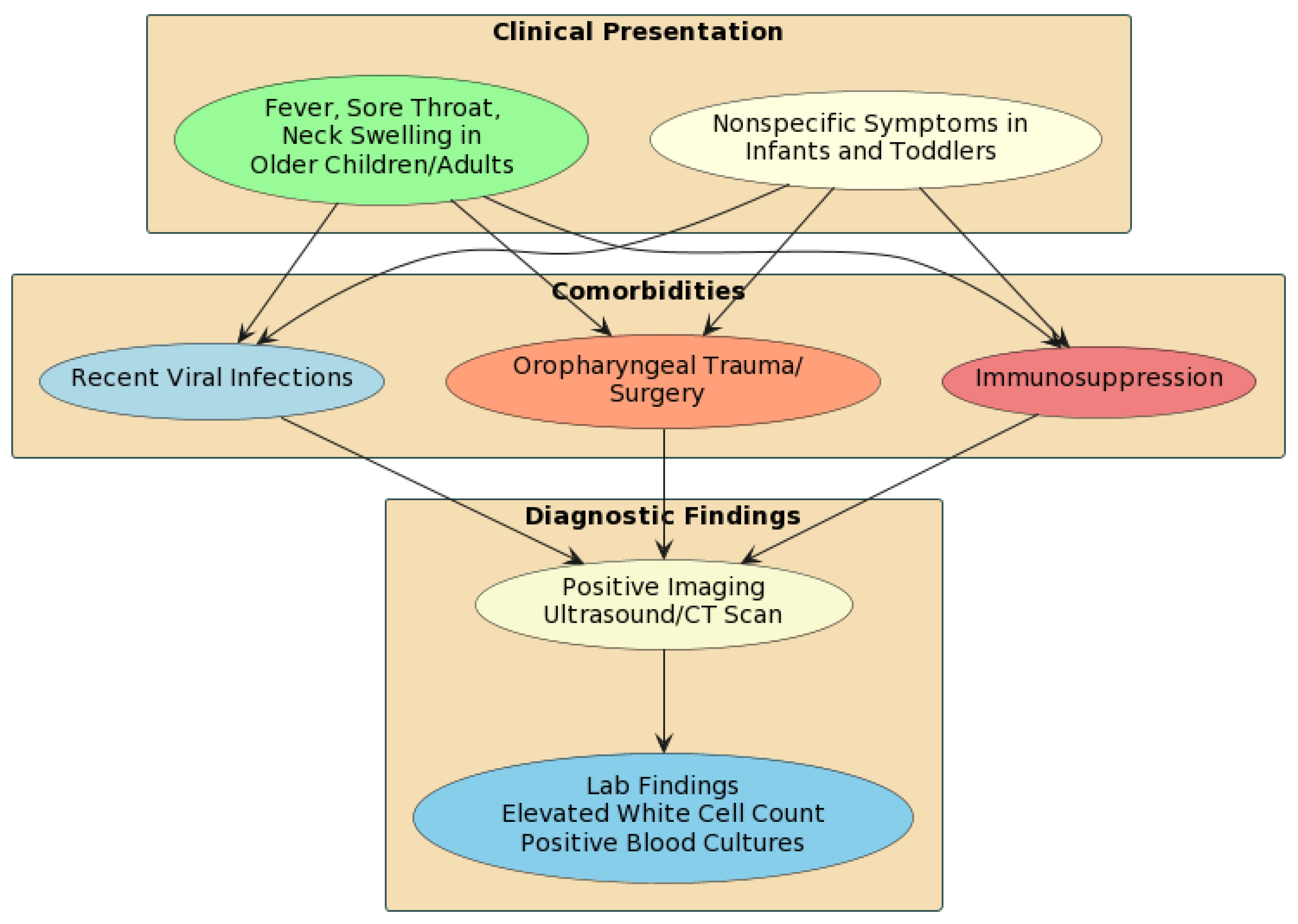
2.1. What Is the Typical Presentation of Lemierre’s Syndrome in Children?
2.2. What Organisms Commonly Cause Lemierre’s Syndrome?
2.3. What Imaging Modalities Are Recommended for Diagnosis?
2.4. What Is the Diagnostic Accuracy of CT vs. MRI?
2.5. What Treatment Regimens Are Most Used?
2.6. What Complications and Metastatic Foci Occur in Pediatric Lemierre’s Syndrome?
2.7. What Are the Common Morbidity and Mortality Rates with Appropriate Treatment?
2.8. What Risk Factors Predispose Children to Developing Lemierre’s Syndrome?
2.9. How Do the Pathogenesis, Presentation, and Management Differ between Children and Adults?
2.10. What Are the Long-Term Outcomes for Pediatric Patients?
3. Discussion
4. Conclusions/Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weesner, C.L.; Cisek, J.E. Lemierre syndrome: The forgotten disease. Ann. Emerg. Med. 1993, 22, 256–258. [Google Scholar] [CrossRef] [PubMed]
- Lemierre, A. On certain septicaemias due to anaerobic organisms. Lancet 1927, 227, 701–703. [Google Scholar] [CrossRef]
- Valerio, L.; Zane, F.; Sacco, C.; Granziera, S.; Nicoletti, T.; Russo, M.; Corsi, G.; Holm, K.; Hotz, M.A.; Righini, C.; et al. Patients with Lemierre syndrome have a high risk of new thromboembolic complications, clinical sequelae and death: An analysis of 712 cases. J. Intern. Med. 2021, 289, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.I.; Baring, D.; Addidle, M.; Murray, C.; Adams, C. Lemierre Syndrome: Two Cases and a Review. Laryngoscope 2007, 117, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Venditto, L.; Giampietro, C.; Sica, F.; Biondi, A. Lung abscess as a complication of Lemierre Syndrome in adolescents: A single center case reports and review of the literature. Ital. J. Pediatr. 2023, 49, 96. [Google Scholar] [CrossRef]
- Riordan, T. Human Infection with Fusobacterium necrophorum (Necrobacillosis), with a Focus on Lemierre’s Syndrome. Clin. Microbiol. Rev. 2007, 20, 622–659. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Chao, F.Y.; Ding, Y.E.; Huang, W.H.; Lee, C.Y. Case report of atypical Lemierre’s Syndrome associated with Fusobacterium nucleatum infection without internal or external jugular venous thrombophlebitis. Respir. Med. Case Rep. 2022, 37, 101651. [Google Scholar] [CrossRef]
- Holm, K.; Lilje, B.; Jönsson-Mejda, K.; Johansson, E. The role of Fusobacterium necrophorum in pharyngotonsillitis—A review. Anaerobe 2016, 42, 89–97. [Google Scholar] [CrossRef]
- Kuppalli, K.; Livorsi, D.; Talati, N.J.; Osborn, M. Lemierre’s syndrome due to Fusobacterium necrophorum. Lancet Infect. Dis. 2012, 12, 808–815. [Google Scholar] [CrossRef]
- Noguchi, S.; Yoshino, M.; Kudo, T.; Takasaki, J.; Takano, T. The clinical features of respiratory infections caused by the Streptococcus anginosus group. BMC Pulm. Med. 2015, 15, 133. [Google Scholar] [CrossRef]
- Camacho-Cruz, J.; Preciado, H.; Beltrán, N.; Fierro, L.; Carrillo, J. Lemierre’s Syndrome Caused by Streptococcus anginosus Presenting as Postseptal Cellulitis in a Pediatric Patient. ORL 2019, 81, 234–239. [Google Scholar] [CrossRef]
- Nadir, N.; Stone, M.B.; Chao, J. Diagnosis of Lemierre’s Syndrome by Bedside Sonography. Acad. Emerg. Med. 2010, 17, 548–551. [Google Scholar] [CrossRef]
- Eilbert, W.; Singla, N. Lemierre’s syndrome. Int. J. Emerg. Med. 2013, 6, 40. [Google Scholar] [CrossRef]
- Walkty, A.; Embil, J. Lemierre’s Syndrome. N. Engl. J. Med. 2019, 380, e16. [Google Scholar] [CrossRef]
- Simka, M.; Czaja, J.; Kowalczyk, D. Collapsibility of the internal jugular veins in the lateral decubitus body position: A potential protective role of the cerebral venous outflow against neurodegeneration. Med. Hypotheses 2019, 133, 109397. [Google Scholar] [CrossRef]
- Hong, P.; MacCormick, J.; Lamothe, A.; Corsten, M. Lemierre Syndrome: Presentation of Three Cases. J. Otolaryngol. 2005, 34, 352. [Google Scholar] [PubMed]
- Wright, W.F.; Shiner, C.N.; Ribes, J.A. Lemierre Syndrome. South. Med. J. 2012, 105, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Cloutet, A.; Botta, R.K.; Kulkarni, S.R.; Ponna, P. A Complex Case of Lemierre’s Syndrome with Facial Vein Involvement. Cureus 2022, 14, e23420. [Google Scholar] [CrossRef] [PubMed]
- Karnov, K.K.S.; Lilja-Fischer, J.; Randrup, T.S. Isolated facial vein thrombophlebitis: A variant of lemierre syn-drome. Open Forum Infect. Dis. 2014, 1, ofu053. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Kawabata-Naya, C.; Yamashita, M.; Yoshikawa, M.; Ito, A. Atypical Lemierre syndrome, thrombophlebitis of the facial vein. Am. J. Emerg. Med. 2013, 31, 460.e1–460.e3. [Google Scholar] [CrossRef] [PubMed]
- Righini, C.A.; Reynaud, Q.; Assouline, B.; Abid, S.; Jegoux, F. Lemierre syndrome: Study of 11 cases and literature review. Head Neck. 2014, 36, 1044–1051. [Google Scholar] [CrossRef]
- Morizono, S.; Masuzaki, T.; Inoue, A.; Yanagawa, R. Lemierre’s Syndrome: Porphyromonas asaccharolytica as a Putative Pathogen. Intern. Med. 2005, 44, 350–353. [Google Scholar] [CrossRef][Green Version]
- Johannesen, K.; Bodtger, U. Lemierre’s syndrome: Current perspectives on diagnosis and management. Infect. Drug Resist. 2016, 9, 221–227. [Google Scholar] [CrossRef]
- Gore, M.R. Lemierre Syndrome: A Meta-analysis. Int. Arch. Otorhinolaryngol. 2020, 24, e379–e385. [Google Scholar] [CrossRef]
- Kristensen, L.H.; Prag, J. Lemierre’s syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: A prospective epidemiological and clinical survey. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 779–789. [Google Scholar] [CrossRef]
- Goldenberg, N.A.; Knapp-Clevenger, R.; Hays, T.; Manco-Johnson, M.J. Lemierre’s and Lemierre’s-like syndromes in children: Survival and thromboembolic outcomes. Pediatrics 2005, 116, e543–e548. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.N.; Levi, J.R.; Cohen, M.B. Lemierre’s syndrome in the pediatric population: Trends in disease presentation and management in literature. Int. J. Pediatr. Otorhinolaryngol. 2020, 136, 110213. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, J.; Nogueira-Silva, L.; Ferreira, T.; Mendes, F.; Canhão, H. To anticoagulate? Controversy in the management of thrombotic complications of head & neck infections. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 129–135. [Google Scholar]
- Brook, I. Fusobacterial head and neck infections in children. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Leugers, C.M.; Clover, R. Lemierre syndrome: Postanginal sepsis. J. Am. Board Fam. Pract. 1995, 8, 384–391. [Google Scholar] [PubMed]
- de Benedictis, F.M.; Ronchi, A.; Bragazzi, N.L.; Larici, A.R.; Delledonne, V.; Caminiti, C.; Montella, S.; Plebani, M. Complicated pneumonia in children. Lancet 2020, 396, 786–798. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Lichtstein, D.M.; Garcia, J.; Tamariz, L.J. The evolution of Lemierre’s syndrome: Report of 2 cases and review of the literature. Medicine 2002, 81, 458–465. [Google Scholar] [CrossRef]
- Bouziri, A.; Douira, W.; Khaldi, A.; Mrad, S.; Bouyahia, O.; Smaoui, H.; Sammoud, A.; Menif, K.; Jaballah, N.B. Neurological variant of Lemierre’s Syndrome with purulent meningitis: A case report and literature review. Fetal Pediatr Pathol. 2013, 31, 1–6. [Google Scholar] [CrossRef]
- Bentham, J.R.; Pollard, A.J.; Milford, C.A.; Anslow, P.; Pike, M.G. Cerebral infarct and meningitis secondary to Lemierre’s syndrome. Pediatr. Neurol. 2004, 30, 281–283. [Google Scholar] [CrossRef]
- Man, M.Y.; Shum, H.P.; Yan, W.W.; Lau, S.K.P. A Case of Lemierre’s Syndrome in Intensive Care Unit. Indian J. Crit. Care Med. 2018, 22, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Jean, S.S.; Chen, F.L.; Hsieh, S.M.; Hsueh, P.R. Lemierre’s Syndrome: A Forgotten and Re-Emerging Infection. J. Microbiol. Immunol. Infect. 2020, 53, 513–517. [Google Scholar] [CrossRef]
- Hochmair, M.; Valipour, A.; Oschatz, E.; Hollaus, P.; Huber, M.; Burghuber, C. From a Sore Throat to the Intensive Care Unit: The Lemierre Syndrome. Wien. Klin. Wochenschr. 2006, 118, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Papaconstantinou, H.; Assimakopoulos, D.A. Lemierre’s syndrome: A systematic review. Laryngoscope 2009, 119, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Frederick, D.; Urwiler, L. Lemierre’s Syndrome. J. Neurosci. Nurs. 2015, 47, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Bourgeaud, J.; Delabays, B.; Van den Bogaart, L.; Ratano, D. Complex Lemierre Syndrome with Multisystemic Abscesses. BMJ Case Rep. 2023, 16, e254638. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Liao, K.H.; Jean, S.S.; Ou, T.Y.; Chen, F.L.; Lee, W.S. Lemierre Syndrome with Cervical Spondylodiscitis and Epidural Abscess Associated with Direct Injection of Heroin into the Jugular Vein. J. Microbiol. Immunol. Infect. 2015, 48, 238–239. [Google Scholar] [CrossRef]
- Lee, W.S.; Wang, F.D.; Shieh, Y.H.; Teng, S.O.; Ou, T.Y. Lemierre Syndrome Complicating Multiple Brain Abscesses Caused by Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae Cured by Fosfomycin and Meropenem Combination Therapy. J. Microbiol. Immunol. Infect. 2012, 45, 72–74. [Google Scholar] [CrossRef]
- Briggs, S.; Pappachan, J.; Argent, J.; McGill, N.; Marsh, M. Lemierre Disease in the Pediatric Intensive Care Unit, Clinical Course, and the Use of High-Frequency Oscillatory Ventilation. Pediatr. Crit. Care Med. 2003, 4, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Quast, D.R.; Lotz, T.A.; Breuer, T.G.K. Lemierre Syndrome After Tonsillectomy. Dtsch. Arztebl. Int. 2017, 114, 679. [Google Scholar] [CrossRef] [PubMed]
- Constantin, J.M.; Mira, J.P.; Guerin, R.; Cayot-Constantin, S.; Lesens, O.; Gourdon, F.; Romaszko, J.P.; Linval, P.; Laurichesse, H.; Bazin, J.E. Lemierre’s Syndrome and Genetic Polymorphisms: A Case Report. BMC Infect. Dis. 2006, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Frizzola, M.A.; Hertzog, J.H. Lemierre Syndrome in a 22-Month-Old Due to Streptococcus pyogenes: A Case Report. Pediatr. Emerg. Care 2011, 27, 1078–1080. [Google Scholar] [CrossRef]
- Kosoko, A.A.; Clement, O.O. A Previously Healthy Infant with Lemierre Syndrome in the Emergency Department: Case Report. Clin. Pract. Cases Emerg. Med. 2023, 7, 148–152. [Google Scholar] [CrossRef]
- Aspesberro, F.; Siebler, T.; Van Nieuwenhuyse, J.P.; Panosetti, E.; Berthet, F. Lemierre Syndrome in a 5-Month-Old Male Infant: Case Report and Review of the Pediatric Literature. Pediatr. Crit. Care Med. 2008, 9, e35–e37. [Google Scholar] [CrossRef] [PubMed]
- Moretti, M.; De Geyter, D.; Goethal, L.; Allard, S.D. Lemierre’s syndrome in adulthood, a case report and systematic review. Acta Clin. Belg. 2021, 76, 324–334. [Google Scholar] [CrossRef]
- Carius, B.M.; Koyfman, A.; Long, B. High risk and low prevalence diseases: Lemierre’s syndrome. Am. J. Emerg. Med. 2022, 61, 98–104. [Google Scholar] [CrossRef]
- Creemers-Schild, D.; de Boer, M.G.J.; Jansen, N.J.G.; van de Noort, V.; Cate, F.T.E.N.; van der Ende, A.; Arensman, R.M. Fusobacterium necrophorum, an Emerging Pathogen of Otogenic and Paranasal Infections? New Microbes New Infect. 2014, 2, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.; Than, M. Lemierre’s syndrome: Diagnosis in the emergency department. Emerg. Med. Australas. 2012, 24, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Kristensen, L.H.; Prag, J. Detection of Fusobacterium necrophorum subsp. funduliforme in tonsillitis in young adults by real-time PCR. Clin. Microbiol. Infect. 2007, 13, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Marchello, C.; Ebell, M.H. Prevalence of Group C Streptococcus and Fusobacterium necrophorum in Patients with Sore Throat: A Meta-Analysis. Ann. Fam. Med. 2016, 14, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Monagle, P.; Chalmers, E.; Chan, A.; DeVeber, G.; Kirkham, F.; Massicotte, P.; Michelson, A.D. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e737S–e801S. [Google Scholar] [CrossRef] [PubMed]
- Campo, F.; Paíga, P.; Cruz, D.; Matos, A. Antibiotic and Anticoagulation Therapy in Lemierre’s Syndrome: Case Report and Review. J. Chemother. 2019, 31, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bondy, P.; Grant, T. Lemierre’s Syndrome: What are the Roles for Anticoagulation and Long-Term Antibiotic Therapy? Ann. Otol. Rhinol. Laryngol. 2008, 117, 679–683. [Google Scholar] [CrossRef]
- Pólai, Z.; Balla, Z.; Andrási, N.; Kőhalmi, K.V.; Temesszentandrási, G.; Benedek, S.; Varga, L.; Farkas, H. A follow-up survey of patients with acquired angioedema due to C1-inhibitor deficiency. J. Intern. Med. 2021, 289, 547–558. [Google Scholar] [CrossRef]
- Osowicki, J.; Kapur, S.; Phuong, L.K.; Dobson, S. The Long Shadow of Lemierre’s Syndrome. J. Infect. 2017, 74, S47–S53. [Google Scholar] [CrossRef]
- Novotny, S.; Serrano, K.; Bazer, D.; Manganas, L. Multiple Cranial Nerve Palsies in a Pediatric Case of Lemierre’s Syndrome due to Streptococcus viridans. Case Rep. Neurol. Med. 2021, 4455789. [Google Scholar] [CrossRef]
- Blessing, K.; Toepfner, N.; Kinzer, S.; Möllmann, C.; Geiger, J.; Serr, A.; Hufnagel, M.; Müller, C.; Krüger, M.; Ridder, G.J.; et al. Lemierre Syndrome Associated with 12th Cranial Nerve Palsy—A Case Report and Review. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1585–1588. [Google Scholar] [CrossRef]
- Ibrahim, I.G.; Osman, A.A.; Elmi, A.M.; Küsbeci, M.; Ali Jama, S.M.; Ali, A.Y.; Farah, F.A. Lemierre’s Syndrome with Cranial Epidural Abscess Complication: A Case Report. Ann. Med. Surg. 2022, 81, 104478. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.M.; Parikh, D.A.; Wright, R.; Holden, P.; Armstrong, W.; Camilon, F.; Wong, B.J. Lemierre Syndrome: A Pediatric Case Series and Review of Literature. Am. J. Otolaryngol. 2010, 31, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Valerio, L.; Corsi, G.; Sebastian, T.; Barco, S. Lemierre syndrome: Current evidence and rationale of the Bacteria-Associated Thrombosis, Thrombophlebitis and LEmierre syndrome (BATTLE) registry. Thromb. Res. 2020, 196, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, A.N.; Morovic, S.; Menegatti, E.; Viselner, G.; Zamboni, P. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound: Recommendations for a protocol. Funct. Neurol. 2011, 26, 229–248. [Google Scholar] [PubMed]
- Caprioli, S.; Tagliafico, A.; Fiannacca, M.; Borda, F.; Picasso, R.; Conforti, C.; Casaleggio, A.; Cittadini, G. Imaging assessment of deep neck spaces infections: An anatomical approach. Radiol. Med. 2023, 128, 81–92. [Google Scholar] [CrossRef]
- Bista, P.K.; Pillai, D.; Narayanan, S.K. Characterization of Three New Outer Membrane Adhesion Proteins in Fusobacterium necrophorum. Microorganisms 2023, 11, 2968. [Google Scholar] [CrossRef]
- Sacco, C.; Kyrle, P.A.; Bauersachs, R.; Brenner, B.; Pabinger, I. Lemierre Syndrome: Clinical Update and Protocol for a Systematic Review and Individual Patient Data Meta-analysis. Hamostaseologie 2019, 39, 76–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavalle, S.; Masiello, E.; Cocuzza, S.; Pavone, P.; Di Nora, A.; Calvo-Henriquez, C.; Lechien, J.R.; Yanez, M.M.; Praticò, A.; Ceccarelli, M.; et al. Pediatric Lemierre’s Syndrome: A Comprehensive Literature Review. Pediatr. Rep. 2024, 16, 201-213. https://doi.org/10.3390/pediatric16010018
Lavalle S, Masiello E, Cocuzza S, Pavone P, Di Nora A, Calvo-Henriquez C, Lechien JR, Yanez MM, Praticò A, Ceccarelli M, et al. Pediatric Lemierre’s Syndrome: A Comprehensive Literature Review. Pediatric Reports. 2024; 16(1):201-213. https://doi.org/10.3390/pediatric16010018
Chicago/Turabian StyleLavalle, Salvatore, Edoardo Masiello, Salvatore Cocuzza, Piero Pavone, Alessandra Di Nora, Christian Calvo-Henriquez, Jerome Rene Lechien, Miguel Mayo Yanez, Andrea Praticò, Manuela Ceccarelli, and et al. 2024. "Pediatric Lemierre’s Syndrome: A Comprehensive Literature Review" Pediatric Reports 16, no. 1: 201-213. https://doi.org/10.3390/pediatric16010018
APA StyleLavalle, S., Masiello, E., Cocuzza, S., Pavone, P., Di Nora, A., Calvo-Henriquez, C., Lechien, J. R., Yanez, M. M., Praticò, A., Ceccarelli, M., Iannella, G., Pace, A., Parisi, F. M., Magliulo, G., & Maniaci, A. (2024). Pediatric Lemierre’s Syndrome: A Comprehensive Literature Review. Pediatric Reports, 16(1), 201-213. https://doi.org/10.3390/pediatric16010018












