Chronic Nonbacterial Osteomyelitis of the Jaw in a 3-Year-Old Girl
Abstract
1. Introduction
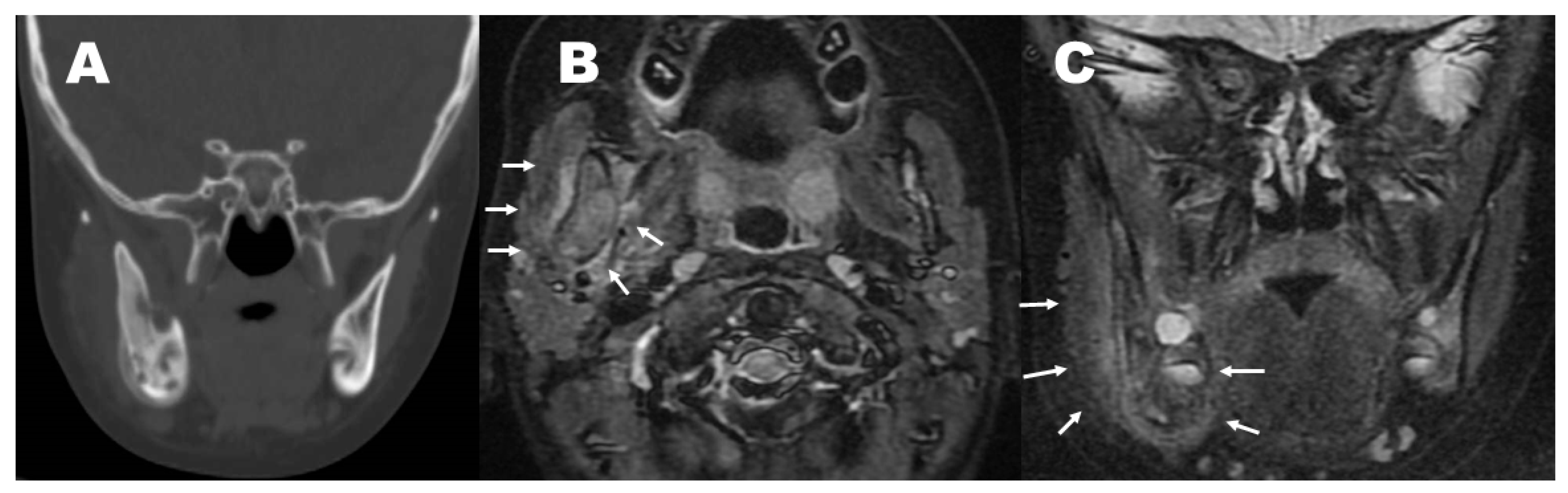
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofmann, S.R.; Kapplusch, F.; Girschick, H.J.; Morbach, H.; Pablik, J.; Ferguson, P.J.; Hedrich, C.M. Chronic Recurrent Multifocal Osteomyelitis (CRMO): Presentation, Pathogenesis, and Treatment. Curr. Osteoporos. Rep. 2017, 15, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Taddio, A.; Ferrara, G.; Insalaco, A.; Pardeo, M.; Gregori, M.; Finetti, M.; Pastore, S.; Tommasini, A.; Ventura, A.; Gattorno, M. Dealing with Chronic Non-Bacterial Osteomyelitis: A practical approach. Pediatr. Rheumatol. Online J. 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed]
- Kanda, R.; Nakano, K.; Miyagawa, I.; Iwata, S.; Nakayamada, S.; Tanaka, Y. A case of bone destruction caused by chronic non-bacterial osteomyelitis (CNO) successfully repaired with a tumour necrosis factor-α (TNF-α) inhibitor, adalimumab. Mod. Rheumatol. Case Rep. 2020, 4, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Beretta-Piccoli, B.C.; Sauvain, M.J.; Gal, I.; Schibler, A.; Saurenmann, T.; Kressebuch, H.; Bianchetti, M.G. Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome in childhood: A report of ten cases and review of the literature. Eur. J. Pediatr. 2000, 159, 594–601. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The role of Cutibacterium acnes in auto-inflammatory bone disorders. Eur. J. Pediatr. 2019, 178, 89–95. [Google Scholar] [CrossRef]
- Jiang, N.; Zhang, P.; Hu, W.-R.; Yao, Z.L.; Yu, B. Similarities and Differences between Clavicular Bacterial Osteomyelitis and Nonbacterial Osteitis: Comparisons of 327 Reported Cases. J. Immunol. Res. 2021, 2021, 4634505. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.K. Chronic non-bacterial osteomyelitis in the jaw. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 68–75. [Google Scholar] [CrossRef]
- Padwa, B.L.; Dentino, K.; Robson, C.D.; Woo, S.B.; Kurek, K.; Resnick, C.M. Pediatric chronic non- bacterial osteomyelitis of the jaw: Clinical, radiographic, and histopathologic features. J. Oral Maxillofac. Surg. 2016, 74, 2393–2402. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, W.; Lu, Y.; Wei, W.; Han, Z.; Chen, M. Monofocal Chronic Nonbacterial Osteomyelitis in the Mandible Accompanied with Mucocutaneous Disease. J. Craniofac. Surg. 2017, 28, e547–e551. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, K.; Shang, W.; Song, K. Oral administration of alendronate and vitamin D3 for the treatment of chronic non-bacterial osteomyelitis of the jaw. Int. J. Oral Maxillofac. Surg. 2020, 49, 1595–1598. [Google Scholar] [CrossRef]
- Gaal, A.; Basiaga, M.L.; Zhao, Y.; Egbert, M. Pediatric chronic nonbacterial osteomyelitis of the mandible: Seattle Children’s hospital 22-patient experience. Pediatr. Rheumatol. Online J. 2020, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Girschick, H.J.; Morbach, H.; Schwarz, T.; Yimam, T.; Frenkel, J.; van Gijn, M.E. Mutation screening of the IL-1 receptor antagonist gene in chronic non-bacterial osteomyelitis of childhood and adolescence. Clin. Exp. Rheumatol. 2011, 29, 1040–1043. [Google Scholar] [PubMed]
- Jansson, A.; Renner, E.D.; Ramser, J.; Mayer, A.; Haban, M.; Meindl, A.; Grote, V.; Diebold, J.; Jansson, V.; Schneider, K.; et al. Classification of non-bacterial osteitis: Retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford) 2007, 46, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.M.; Ferguson, P.J. Autoinflammatory bone diseases. Rheum. Dis. Clin. N. Am. 2013, 39, 735–749. [Google Scholar] [CrossRef]
- Bader-Meunier, B.; Van Nieuwenhove, E.; Breton, S.; Wouters, C. Bone involvement in monogenic autoinflammatory syndromes. Rheumatology (Oxford) 2018, 57, 606–618. [Google Scholar] [CrossRef]
- Tlaskalová-Hogenová, H.; Stepánková, R.; Hudcovic, T.; Tučková, L.; Cukrowska, B.; Lodinová-Žádnıková, R.; Kozáková, H.; Rossmann, P.; Bártová, J.; Sokol, D.; et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol. Lett. 2004, 93, 97–108. [Google Scholar] [CrossRef]
- Schnabel, A.; Range, U.; Hahn, G.; Siepmann, T.; Berner, R.; Hedrich, C.M. Unexpectedly high incidences of chronic non-bacterial as compared to bacterial osteomyelitis in children. Rheumatol. Int. 2016, 36, 1737–1745. [Google Scholar] [CrossRef]
- Skrabl-Baumgartner, A.; Singer, P.; Greimel, T.; Gorkiewicz, G.; Hermann, J. Chronic non-bacterial osteomyelitis: A comparative study between children and adults. Pediatr. Rheumatol. Online J. 2019, 17, 49. [Google Scholar] [CrossRef]
- Zhao, Y.; Ferguson, P.J. Chronic Nonbacterial Osteomyelitis and Chronic Recurrent Multifocal Osteomyelitis in Children. Pediatr. Clin. N. Am. 2018, 65, 783–800. [Google Scholar] [CrossRef]
- Miettunen, P.; Wei, X.; Kaura, D.; Reslan, W.A.; Aguirre, A.N.; Kellner, J.D. Dramatic pain relief and resolution of bone inflammation following pamidronate in 9 pediatric patients with persistent Chronic Recurrent Multifocal Osteomyelitis (CRMO). Pediatr. Rheumatol. Online J. 2009, 7, 2. [Google Scholar] [CrossRef]
- Perkins, A.; Stevens, A.M.; Ferguson, P.J.; Zhao, Y. Urinary N-telopeptide as a Biomarker of Disease Activity in Patients with Chronic Nonbacterial Osteomyelitis Who Have Not Received Bisphosphonates. Rheumatology 2020, 47, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Hirano, D.; Chiba, K.; Yamada, S.; Ida, H. Oral alendronate in pediatric chronic recurrent multifocal osteomyelitis. Pediatr. Int. 2017, 59, 506–508. [Google Scholar] [CrossRef]

| CBC | Inflammatory Markers | ||
|---|---|---|---|
| WBC (3300–8600/µL) | 6300 | CRP (<0.14) mg/dL | 0.91 |
| Hb (11.6–14.8 g/dL) | 10.7 | Procalcitonin (<0.4) ng/mL | NT |
| Platelet counts (158 K–348 K/µL) | 405 K | ESR (1 h; 3–15) mm | 42 |
| Hepatic function | ESR (2 h; NA) mm | 74 | |
| AST (13–30) U/L | 23 | Renal function | |
| ALT (10–42) U/L | 10 | BUN (8.0–20) mg/dL | 13.1 |
| LDH (124–222) U/L | 200 | Creatinine (0.65–1.07) mg/dL | 0.24 |
| ALP (38–113) U/L | 284 | Uric acid (3.7–7.8) mg/dL | 3.0 |
| Total protein (6.6–8.1) g/dL | 7.2 | Other (vitamin C) | NT |
| Albumin (4.1–5.1) g/dL | 3.9 | Bacterial study | |
| Immunological | Blood culture | neg | |
| IgE (RIST) (<30) IU/mL | 223 | Urine culture | NT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makino, S.; Oshige, H.; Shinozuka, J.; Imashuku, S. Chronic Nonbacterial Osteomyelitis of the Jaw in a 3-Year-Old Girl. Pediatr. Rep. 2023, 15, 209-214. https://doi.org/10.3390/pediatric15010016
Makino S, Oshige H, Shinozuka J, Imashuku S. Chronic Nonbacterial Osteomyelitis of the Jaw in a 3-Year-Old Girl. Pediatric Reports. 2023; 15(1):209-214. https://doi.org/10.3390/pediatric15010016
Chicago/Turabian StyleMakino, Shigeru, Hideo Oshige, Jun Shinozuka, and Shinsaku Imashuku. 2023. "Chronic Nonbacterial Osteomyelitis of the Jaw in a 3-Year-Old Girl" Pediatric Reports 15, no. 1: 209-214. https://doi.org/10.3390/pediatric15010016
APA StyleMakino, S., Oshige, H., Shinozuka, J., & Imashuku, S. (2023). Chronic Nonbacterial Osteomyelitis of the Jaw in a 3-Year-Old Girl. Pediatric Reports, 15(1), 209-214. https://doi.org/10.3390/pediatric15010016







