Recognizing the Emergent and Submerged Iceberg of the Celiac Disease: ITAMA Project—Global Strategy Protocol
Abstract
1. Introduction
2. Aims and Hypothesis of the ITAMA Project
- To determine if a rapid and cheap POCT can bridge the diagnostic gap of CD in a large target population in Malta.
- To evaluate the diagnostic accuracy of the POCT utilized in the study.
- To analyze whether serial testing with POCT and conventional celiac serology may decrease the need for intestinal biopsy for diagnosing CD in children as still indicated by ESPGHAN.
- To assess the negative predictive value of POCT.
- To delve deep in the “coeliac iceberg” in order to identify people with biomarkers of potential gluten related disorders, such as TGA-IgA intestinal mucosal deposits (MD), who might benefit from a gluten-free diet.
- To develop and validate an artificial intelligence-based system to support clinical decisions in CD diagnosis.
- To investigate the potential cost savings resulting from the project.
3. Methods
- Fulfilment of aims 1 to 3—In Maltese primary schools and the general hospital, Mater Dei Hospital, all children with a suspicion of CD after a positive POCT result where referred for further secondary confirmatory tests.
- Fulfilment of aims 4 and 5—In Sicily, at the Messina University Hospital Digestive Endoscopy Unit and the Regional Center for CD and at the Buccheri La Ferla Hospital in Palermo, centralized serology testing, both from Maltese and Sicilian patients, was performed.
- Fulfilment of aims 6 and 7—Project coordination, leadership, and cost control, together with the development of the CDSS, were undertaken by the Physics and Chemistry Department “E. Segre” at the University of Palermo and AcrossLimits Ltd. in Malta respectively.
3.1. Study Protocol in Malta—School and Hospital Settings
3.1.1. Target Population
3.1.2. Initial Screening
3.1.3. Follow-Ups
3.1.4. Study Outcomes
3.1.5. Statistical Analysis
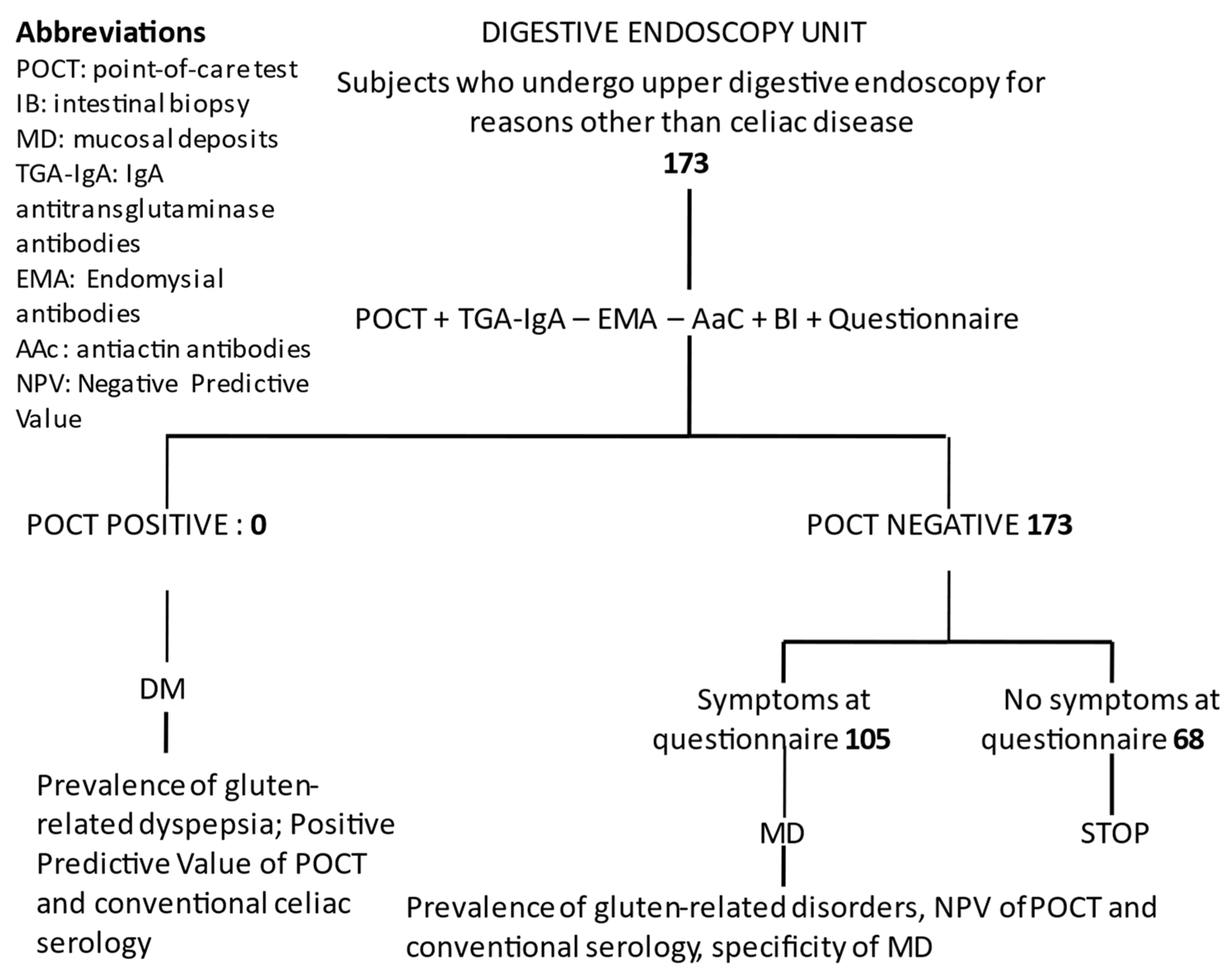
3.2. Study Protocol in Sicily—Digestive Endoscopy Unit Setting
3.2.1. Study Participants
3.2.2. Initial Testing and Procedures
3.2.3. Confirmation Testing
3.2.4. Study Outcomes
3.2.5. Statistical Analysis
3.2.6. Sample Size
3.3. Study Protocol in Sicily—Celiac Disease Center Setting
3.3.1. Study Participants
3.3.2. Initial Testing
3.3.3. Confirmatory Testing and Procedures
3.3.4. Study Outcome
3.3.5. Statistical Analysis
3.3.6. Development and Validation of an AI-Based System to Support Clinical Decisions in CD Diagnosis
3.3.7. Analysis of the Costs Saved as Consequence of the Project
- ○
- Increased avoidance of conventional serology and invasive procedures, such as upper digestive endoscopy with intestinal biopsy and increment of diagnosis.
- ○
- Better reduction of diagnostic timeframes.
- ○
- Reduction of outpatient consultations.
- ○
- Reduction of social costs, such as loss of work and school days (however this is compensated with the increase in voucher volume).
- ○
- whether a screening action is feasible, cost-effective, and well accepted by the population,
- ○
- both possible disinvestments and investments that improve outcomes,
- ○
- costs for undiagnosed children in a case-finding action.
4. Results
Database Storing and CDSS Performances
- 20,454 patient basic information, spread on 4 tables and 103,513 rows:
- ○
- Demographic data (age, sex, ethnicity)
- ○
- Medical history (answers to 29 multiple-choice questions)
- ○
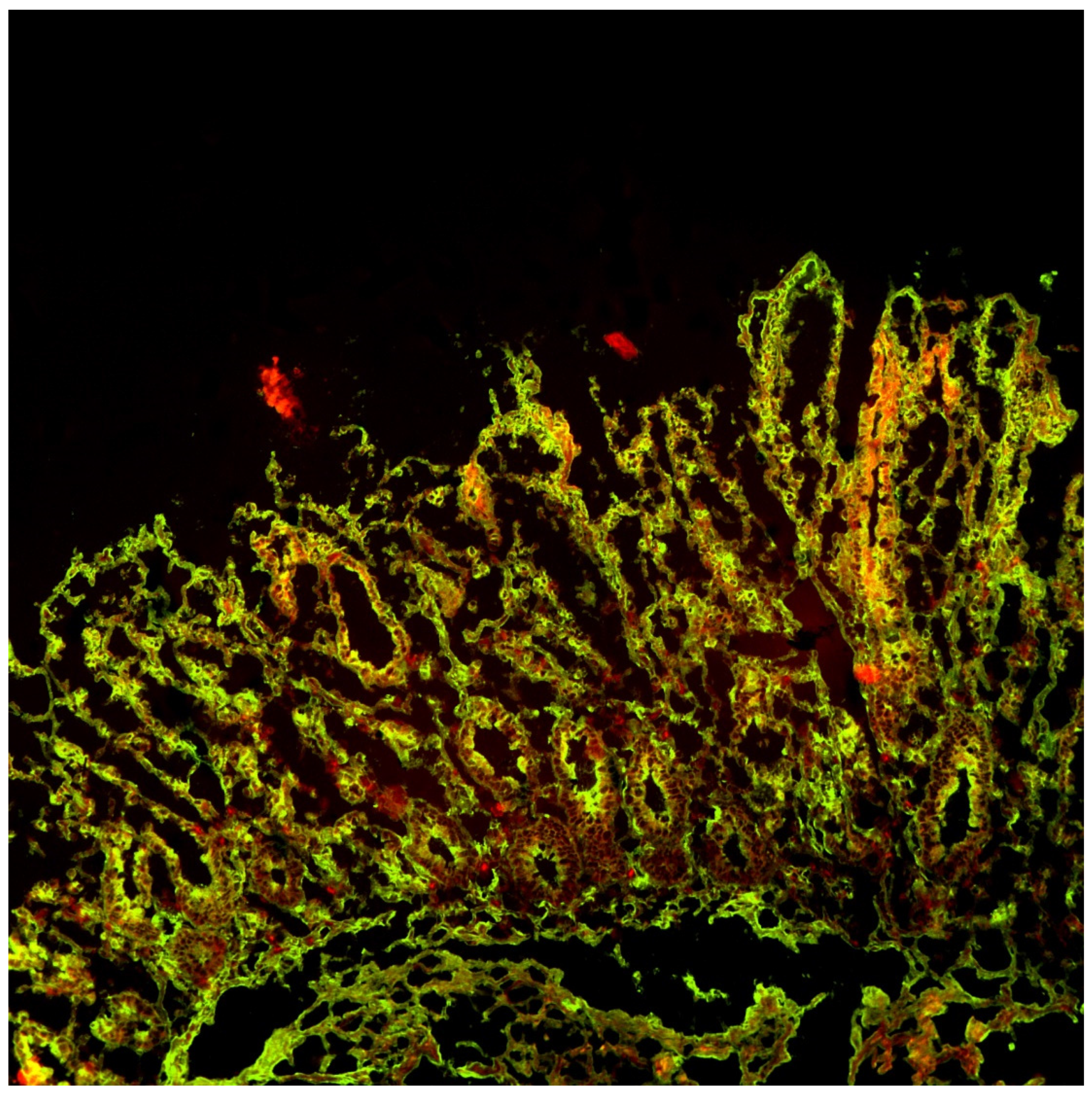
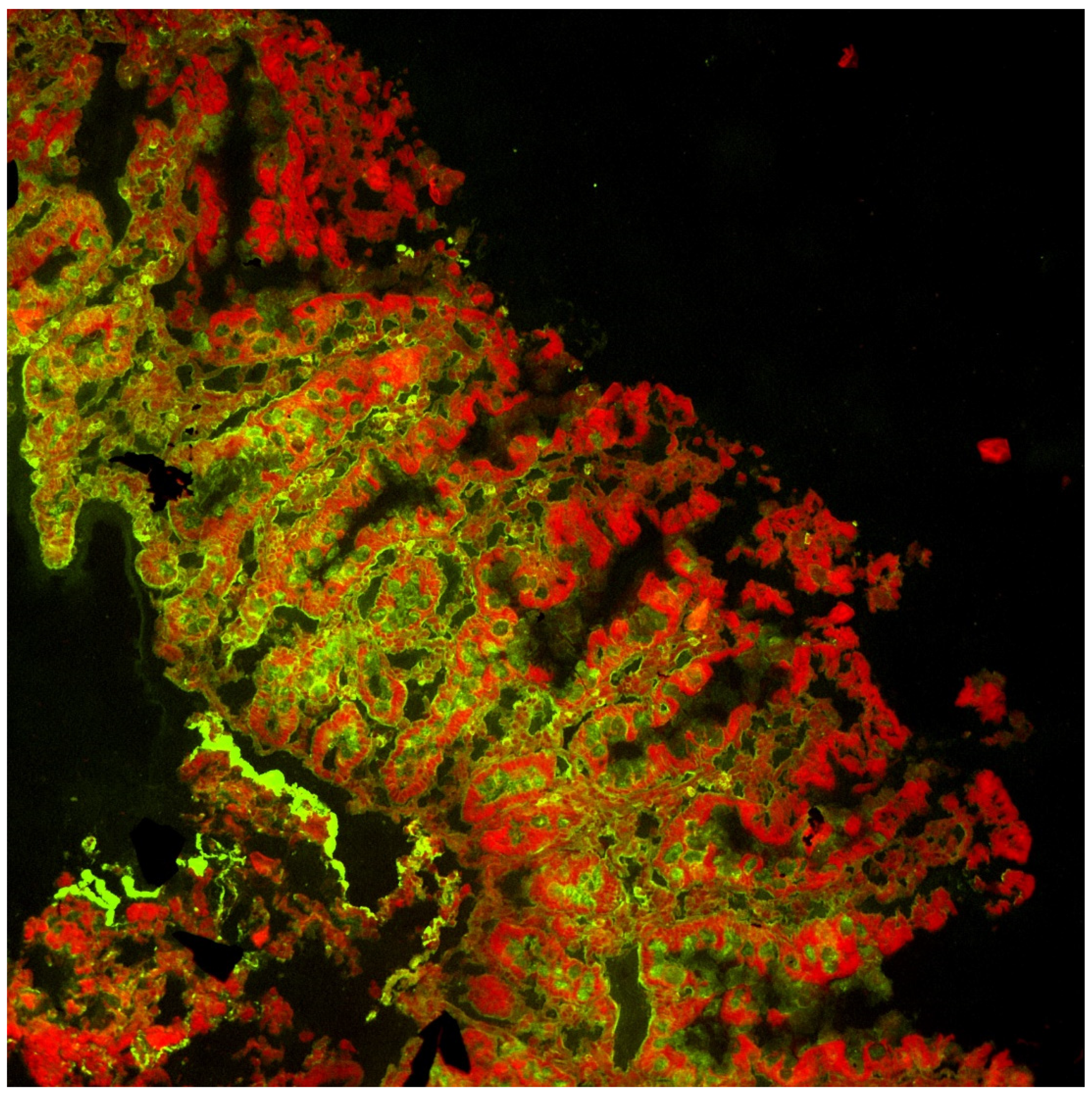
- Point-of-Care (POCT) data (pictures and results)
- 875 patient second-level exams results, spread on 12 tables and 2573 rows:
- ○
- Blood tests (ant-TTG-IgA, anti-TTG-IgG, Total IgA, EMA(pictures), Anti-Actin AAC, anti-Deaminated Gliadin Peptide (DPG)-IgG)
- 165 patient third-level (endoscopy) exam results, spread on 2 tables and 228 rows:
- ○
- Biopsy results (based on Marsh index)
- ○
- Mucosal deposits (pictures and evaluation)
- 19,418 final diagnosis details, spread on 2 tables and 39,807 rows:
- ○
- Diagnostic pathways (doctors’ decision on the diagnostic pathway for each participant)
- ○
- Final diagnosis (Coeliac/Non-coeliac)
5. Discussion
Limitations
6. Conclusions
- They improve the quality of life of the inhabitants of the territory by often facing common challenges in cross-border regions.
- Where possible, they reduce the costs for diagnosis and those due to delayed diagnosis.
- Furthermore, they offer job opportunities to companies in the area that deal with diagnostic tools or diagnostic support.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Prim. 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Greco, L.; Timpone, L.; Abkari, A.; Abu-Zekry, M.; Attard, T.; Bouguerrà, F.; Cullufi, P.; Kansu, A.; Micetic-Turk, D.; Mišak, Z.; et al. Burden of celiac disease in the Mediterranean area. World J. Gastroenterol. 2011, 17, 4971–4978. [Google Scholar] [CrossRef] [PubMed]
- Long, K.H.; Rubio-Tapia, A.; Wagie, A.E.; Iii, L.J.M.; Lahr, B.D.; Van Dyke, C.T.; Murray, J.A. The economics of coeliac disease: A population-based study. Aliment. Pharmacol. Ther. 2010, 32, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatric Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatric Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Rosén, A.; Sandström, O.; Carlsson, A.; Högberg, L.; Olén, O.; Stenlund, H.; Ivarsson, A. Usefulness of symptoms to screen for celiac disease. Pediatrics 2014, 133, 211–218. [Google Scholar] [CrossRef]
- Baker, S.S. Rethinking strategies to screen for celiac disease. Pediatrics 2014, 133, 331–332. [Google Scholar] [CrossRef]
- Gatti, S.; Lionetti, E.; Balanzoni, L.; Verma, A.K.; Galeazzi, T.; Gesuita, R.; Scattolo, N.; Cinquetti, M.; Fasano, A.; Catassi, C.; et al. Increased Prevalence of Celiac Disease in School-age Children in Italy. Clin. Gastroenterol. Hepatol. 2019, 18, 596–603. [Google Scholar] [CrossRef]
- Stahl, M.G.; Rasmussen, C.G.; Dong, F.; Waugh, K.; Norris, J.M.; Baxter, J.; Yu, L.; Steck, A.K.; Frohnert, B.I.; Liu, E.; et al. Mass Screening for Celiac Disease: The Autoimmunity Screening for Kids Study. Am. J. Gastroenterol. 2020, 116, 180–187. [Google Scholar] [CrossRef]
- Korponay-Szabó, I.R.; Szabados, K.; Pusztai, J.; Uhrin, K.; Ludmány, E.; Nemes, E.; Kaukinen, K.; Kapitány, A.; Koskinen, L.; Sipka, S.; et al. Population screening for coeliac disease in primary care by district nurses using a rapid antibody test: Diagnostic accuracy and feasibility study. BMJ 2007, 335, 1244–1247. [Google Scholar] [CrossRef]
- Costa, S.; Astarita, L.; Ben-Hariz, M.; Currò, G.; Dolinsek, J.; Kansu, A.; Magazzu’, G.; Marvaso, S.; Micetic-Turku, D.; Pellegrino, S.; et al. A Point-of-Care test for facing the burden of undiagnosed celiac disease in the Mediterranean area: A pragmatic design study. BMC Gastroenterol. 2014, 14, 219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Mäki, M.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Diagnostic Accuracy of Point of Care Tests for Diagnosing Celiac Disease: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2019, 53, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Meijer-Boekel, C.; Akker, M.v.D.; van Bodegom, L.; Escher, J.; van Geloven, N.; van Overveld, F.; Rings, E.H.H.; Smit, L.; de Vries, M.C.; Mearin, M.L. Early diagnosis of coeliac disease in the Preventive Youth Health Care Centres in the Netherlands: Study protocol of a case finding study (GLUTENSCREEN). BMJ Paediatr. Open 2021, 5, e001152. [Google Scholar] [CrossRef] [PubMed]
- Primavera, G.; Aiello, A.; Grosso, C.; Trifirò, G.; Costa, S.; Grima, A.; Pallio, S.; Toumi, M.; Magazzu, G.; Pellegrino, S.; et al. Point-of-Care test screening versus Case finding for paediatric coeliac disease: A pragmatic study in primary care. Acta Paediatr. 2020, 110, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Scoglio, R.; Trifirò, G.; Sandullo, A.; Marangio, G.; D’Agate, C.; Costa, S.; Pellegrino, S.; Alibrandi, A.; Aiello, A.; Currò, G.; et al. Diagnostic Yield of 2 Strategies for Adult Celiac Disease Identification in Primary Care. J. Clin. Gastroenterol. 2019, 53, 15–22. [Google Scholar] [CrossRef]
- Catassi, C.; Rätsch, I.M.; Fabiani, E.; Rossini, M.; Bordicchia, F.; Candela, F.; Coppa, G.V.; Giorgi, P.L. Coeliac disease in the year 2000: Exploring the iceberg. Lancet 1994, 343, 200–203. [Google Scholar] [CrossRef]
- Korponay-Szabó, I.R.; Halttunen, T.; Szalai, Z.; Laurila, K.; Király, R.; Kovács, J.B.; Fésüs, L.; Mäki, M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut 2004, 53, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Hadjivassiliou, M.; Gibson, A.; Davies-Jones, G.A.; Lobo, A.J.; Stephenson, T.J.; Milford-Ward, A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet 1996, 347, 369–371. [Google Scholar] [CrossRef]
- Maglio, M.; Florian, F.; Vecchiet, M.; Auricchio, R.; Paparo, F.; Spadaro, R.; Zanzi, D.; Rapacciuolo, L.; Franzese, A.; Sblattero, D.; et al. Majority of Children With Type 1 Diabetes Produce and Deposit Anti-Tissue Transglutaminase Antibodies in the Small Intestine. Diabetes 2009, 58, 1578–1584. [Google Scholar] [CrossRef]
- Costa, S.; Currò, G.; Pellegrino, S.; Lucanto, M.C.; Tuccari, G.; Ieni, A.; Visalli, G.; Magazzù, G.; Santoro, D. Case report on pathogenetic link between gluten and IgA nephropathy. BMC Gastroenterol. 2018, 18, 64. [Google Scholar] [CrossRef]
- Hadjivassiliou, M.; Mäki, M.; Sanders, D.S.; Williamson, C.A.; Grünewald, R.A.; Woodroofe, N.M.; Korponay-Szabó, I.R. Autoantibody targeting of brain and intestinal transglutaminase in gluten ataxia. Neurology 2006, 66, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Molder, A.; Balaban, D.V.; Jinga, M.; Molder, C.-C. Current Evidence on Computer-Aided Diagnosis of Celiac Disease: Systematic Review. Front. Pharmacol. 2020, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Pota, M.; Esposito, M.; De Pietro, G. Designing rule-based fuzzy systems for classification in medicine. Knowl.-Based Syst. 2017, 124, 105–132. [Google Scholar] [CrossRef]
- Piccialli, F.; Calabrò, F.; Crisci, D.; Cuomo, S.; Prezioso, E.; Mandile, R.; Troncone, R.; Greco, L.; Auricchio, R. Precision medicine and machine learning towards the prediction of the outcome of potential celiac disease. Sci Rep. 2021, 11, 5683. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Volgelsang, H. The histopathology of celiac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Singh, A.D.; Ellias, S.; Singh, P.; Ahuja, V.; Makharia, G.K. The Prevalence of the Celiac Disease in Patients with Dyspepsia: A Systematic Review and Meta-Analysis. Am. J. Dig. Dis. 2021, 1–13. [Google Scholar] [CrossRef]
- Salmi, T.T.; Collin, P.; Jarvinen, O.; Haimila, K.; Partanen, J.; Laurila, K.; Korponay-Szabo, I.R.; Huhtala, H.; Reunala, T.; Maki, M.; et al. Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming coeliac disease. Aliment. Pharmacol. Ther. 2006, 24, 541–552. [Google Scholar] [CrossRef]
- Lau, M.S.Y.; Sanders, D.S. Point of care testing for paediatric coeliac disease in the new ESPGHAN era. Rev. Española Enferm. Dig. 2017, 109, 741–742. [Google Scholar] [CrossRef]
- Tangermann, P.; Branchi, F.; Itzlinger, A.; Aschenbeck, J.; Schubert, S.; Maul, J.; Liceni, T.; Schröder, A.; Heller, F.; Spitz, W.; et al. Low Sensitivity of Simtomax Point of Care Test in Detection of Celiac Disease in a Prospective Multicenter Study. Clin. Gastroenterol. Hepatol. 2018, 17, 1780–1787.e5. [Google Scholar] [CrossRef]
- Greenhalgh, T. How to read a paper. Papers that report diagnostic or screening tests. BMJ. 1997, 315, 540–543, Erratum in BMJ 1997, 315, 942; Erratum in BMJ 1998, 316, 225. [Google Scholar] [CrossRef]
- Tommasini, A.; Not, T.; Kiren, V.; Baldas, V.; Santon, D.; Trevisiol, C.; Berti, I.; Neri, E.; Gerarduzzi, T.; Bruno, I.; et al. Mass screening for coeliac disease using antihuman transglutaminase antibody assay. Arch. Dis. Child. 2004, 89, 512–515. [Google Scholar] [CrossRef]


| The ICT (Information and Communication Technologies) Tools for the diagnosis of Autoimmune diseases (AD) in the Mediterranean Area (ITAMA) is an INTERREG V-A Italia—Malta Cooperation Project funded by the European Regional Development Fund. The Program Investment Priority axis is to “Promote the sustainable and smart growth through research and innovation” with specific objective to “Increase the innovation and research activities to improve the quality of life and cultural heritage fruition”. |
| The Common Challenge is enhancing health and quality of life by improving the diagnosis of AD, third in the world after cardiovascular and cancer in terms of incidence, with a focus on the study of celiac disease in the Mediterranean. |
| The Overall Objective is to activate a network between research and productive environments in the healthcare sectors to develop innovative ICT Tools for the diagnosis of AD, and related technology transfer tools. Expected change concerns the anticipation of diagnosis time through the optimization of the diagnostic path. |
| The two main outputs produced are: Database and innovative ICT tools to support the diagnosis of celiac disease for Healthcare delivery services; Technology transfer services by modeling production processes based on the project’s results for health companies and specialized enterprises. |
The adopted approach is:
|
Project is innovative in three aspects:
|
| Do You Have Any Family Relatives with Coeliac Disease? | |||
|---|---|---|---|
| Yes|No|Unknown|Father|Mother|Paternal Grandfather|Paternal Grandmother | |||
| Maternal Grandfather|Maternal Grandmother|Sister|Brother|Other: | |||
| 1. Persistently tired/weak/low energy | Yes | No | Unknown |
| 2. Immunodeficiency | Yes | No | Unknown |
| 3. Vomiting (more than 1 episode per month in last 3 months) | Yes | No | Unknown |
| 4. Liver problems | Yes | No | Unknown |
| 5. Diabetes (type 1) | Yes | No | Unknown |
| 6. Anaemia (pallor, low blood level) | Yes | No | Unknown |
| 7. Rheumatoid Arthritis | Yes | No | Unknown |
| 8. Renal problems | Yes | No | Unknown |
| 9. Epilepsy | Yes | No | Unknown |
| 10. Severe dental decay | Yes | No | Unknown |
| 11. Mood changes | Yes | No | Unknown |
| 12. Persistent loose stools | Yes | No | Unknown |
| 13. Repeatedly complains of abdominal pain | Yes | No | Unknown |
| 14. Thyroid problems | Yes | No | Unknown |
| 15. Abdominal distention/bloating, flatulence | Yes | No | Unknown |
| 16. Irregular bowel habits | Yes | No | Unknown |
| 17. Alopecia (hair loss) | Yes | No | Unknown |
| 18. Vitiligo (white skin patches) | Yes | No | Unknown |
| 19. Down’s, Williams or Turner’s syndrome | Yes | No | Unknown |
| 20. Recurrent Mouth Ulcers | Yes | No | Unknown |
| 21. Difficulty with balance/walking | Yes | No | Unknown |
| 22. Poor weight gain, anorexia, weight loss | Yes | No | Unknown |
| 23. Short stature/growth failure | Yes | No | Unknown |
| 24. Weak bones | Yes | No | Unknown |
| 25. Constipation | Yes | No | Unknown |
| 1. Weakness/fatigue | Yes | No | Unknown |
| 2. Total IgA deficiency | Yes | No | Unknown |
| 3. Isolated and persistent hyper-transaminasemia (ALT-AST level two times the normal range for at least 3 months) | Yes | No | Unknown |
| 4. Insulin-dependent type I diabetes | Yes | No | Unknown |
| 5. Anaemia | Yes | No | Unknown |
| 6. Rheumatoid Arthritis | Yes | No | Unknown |
| 7. IgA nephropathy | Yes | No | Unknown |
| 8. Epilepsies resistant to pharmacological treatment or epilepsies with intracranic calcification | Yes | No | Unknown |
| 9. Teeth enamel defects | Yes | No | Unknown |
| 10. Depression (treated with drugs) | Yes | No | Unknown |
| 11. Chronic diarrhoea and/or malabsorption | Yes | No | Unknown |
| 12. Repeatedly complains of abdominal pain (IBS) | Yes | No | Unknown |
| 13. Thyroid disorders with positive antibodies | Yes | No | Unknown |
| 14. Abdominal distention/bloating, flatulence (IBS) | Yes | No | Unknown |
| 15. Irregular bowel habits (IBS) | Yes | No | Unknown |
| 16. Alopecia | Yes | No | Unknown |
| 17. Vitiligo | Yes | No | Unknown |
| 18. Down syndrome and Turner syndrome | Yes | No | Unknown |
| 19. Recurrent aphtous stomatitis (more than four episodes/year) | Yes | No | Unknown |
| 20. Ataxia | Yes | No | Unknown |
| 21. Weight loss | Yes | No | Unknown |
| 22. Short stature | Yes | No | Unknown |
| 23. Osteopenia (Z score < 2 S.D.) | Yes | No | Unknown |
| 24. Constipation | Yes | No | Unknown |
| 25. Chronic or recurrent joint pain (at least six times/year) | Yes | No | Unknown |
| 26. Non-Hodgkin intestinal lymphoma | Yes | No | Unknown |
| 27. Infertility and/or multiple miscarriage | Yes | No | Unknown |
| 28. Other autoimmune disorders (such as systemic erythematosus lupus, etc.) with confirmed diagnosis at II or III level regional hospital | Yes | No | Unknown |
| 29. Dermatitis herpetiformis (even if only suspected) | Yes | No | Unknown |
| SEX No. | FEMALES 10,025 | MALES 9988 | ||||
|---|---|---|---|---|---|---|
| AGE RANGE (Years) | 3–6 | 7–10 | 11–14 | 3–6 | 7–10 | 11–14 |
| No. | 3921 | 4304 | 1800 | 3972 | 4377 | 1639 |
| % | 39.1 | 43.3 | 17.1 | 39.7 | 43.8 | 16.4 |
| ETHNICITY | ||||||
| CAUCASIAN/OTHER | 3648/273 | 3990/314 | 1676/124 | 3700/272 | 4070/307 | 1512/127 |
| % of Caucasian | 93 | 92.7 | 93.1 | 93.1 | 92.9 | 92.2 |
| CD FAMILIALITY | ||||||
| YES/NO | 421/3500 | 423/3881 | 213/1587 | 400/3572 | 398/3979 | 160/1479 |
| % | 10.7 | 9.6 | 11.8 | 10.1 | 9.1 | 9.7 |
| EMA No. | POSITIVE 122 | NEGATIVE 158 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SEX No. | FEMALES 77 | MALES 45 | FEMALES 91 | MALES 67 | ||||||||
| AGE RANGE (Years) | 3–6 | 7–10 | 11–14 | 3–6 | 7–10 | 11–14 | 3–6 | 7–10 | 11–14 | 3–6 | 7–10 | 11–14 |
| No. | 33 | 33 | 11 | 19 | 17 | 9 | 25 | 37 | 29 | 14 | 37 | 16 |
| ETHNICITY | ||||||||||||
| CAUCASIAN/OTHER | 31/1 | 33/0 | 10/1 | 18/1 | 15/2 | 9/0 | 25/0 | 33/4 | 29/0 | 13/1 | 32/5 | 14/2 |
| CD FAMILIALITY | ||||||||||||
| YES/NO | 7/25 | 4/29 | 2/9 | 4/15 | 4/13 | 1/8 | 3/22 | 7/30 | 7/22 | 4/10 | 3/34 | 4/12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magazzù, G.; Aquilina, S.; Barbara, C.; Bondin, R.; Brusca, I.; Bugeja, J.; Camilleri, M.; Cascio, D.; Costa, S.; Cuzzupè, C.; et al. Recognizing the Emergent and Submerged Iceberg of the Celiac Disease: ITAMA Project—Global Strategy Protocol. Pediatr. Rep. 2022, 14, 293-311. https://doi.org/10.3390/pediatric14020037
Magazzù G, Aquilina S, Barbara C, Bondin R, Brusca I, Bugeja J, Camilleri M, Cascio D, Costa S, Cuzzupè C, et al. Recognizing the Emergent and Submerged Iceberg of the Celiac Disease: ITAMA Project—Global Strategy Protocol. Pediatric Reports. 2022; 14(2):293-311. https://doi.org/10.3390/pediatric14020037
Chicago/Turabian StyleMagazzù, Giuseppe, Samuel Aquilina, Christopher Barbara, Ramon Bondin, Ignazio Brusca, Jacqueline Bugeja, Mark Camilleri, Donato Cascio, Stefano Costa, Chiara Cuzzupè, and et al. 2022. "Recognizing the Emergent and Submerged Iceberg of the Celiac Disease: ITAMA Project—Global Strategy Protocol" Pediatric Reports 14, no. 2: 293-311. https://doi.org/10.3390/pediatric14020037
APA StyleMagazzù, G., Aquilina, S., Barbara, C., Bondin, R., Brusca, I., Bugeja, J., Camilleri, M., Cascio, D., Costa, S., Cuzzupè, C., Duca, A., Fregapane, M., Gentile, V., Giuliano, A., Grifò, A., Grima, A.-M., Ieni, A., Li Calzi, G., Maisano, F., ... Raso, G. (2022). Recognizing the Emergent and Submerged Iceberg of the Celiac Disease: ITAMA Project—Global Strategy Protocol. Pediatric Reports, 14(2), 293-311. https://doi.org/10.3390/pediatric14020037











