Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Questionnaire
2.3. Ethical Considerations
2.4. Data Analysis
3. Results
3.1. Descriptive Analysis: General Characteristics of the Sample
3.2. Previous Interactions with RSV
3.3. General Knowledge Test
3.4. Risk Perception
3.5. Attitudes towards RSV Vaccine
3.6. Univariate Analysis
3.7. Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section 1. Your personal experience with RSV infections: during your clinical practice. | |
| Have you previously managed any RSV case? | [yes] [no] [no answer] |
| Have you previously diagnosed any RSV case? | [yes] [no] [no answer] |
| Have previously required any hospitalization for RSV? | [yes] [no] [no answer] |
| Have you previously required mAb immunoprophylaxis for RSV? | [yes] [no] [no answer] |
| Section 2. At your knowledge (please mark the correct answer) | |
| 1. Nearly all RSV infections occur in infants and children. | [true] [false] [do not know] |
| 2. In most cases, infants acquire RSV infections from their parents. | [true] [false] [do not know] |
| 3. In most cases, RSV evolves in an uncomplicated influenza-like illness. | [true] [false] [do not know] |
| 4. Lower respiratory tract infections from RSV is deprived of specific signs/symptoms. | [true] [false] [do not know] |
| 5. In Europe, RSV season spans from: | |
| November–March | [ ] |
| October–February | [ ] |
| September–January | [ ] |
| 6. SARS-CoV-2 and RSV have the same means of transmission. | [true] [false] [do not know] |
| 7. Safe and effective vaccines against RSV are commercially available. | [true] [false] [do not know] |
| 8. Monoclonal antibodies can be used against RSV only as immunoprophylaxis. | [true] [false] [do not know] |
| 9. Immunoprophylaxis for RSV should be delivered: | |
| Every two months, during RSV season | [ ] |
| Every month, during RSV season | [ ] |
| Only at the beginning of RSV season. | [ ] |
| 10. Globally, RSV causes a total … deaths in children < 1 age: | |
| 43,800 | [ ] |
| 430,800 | [ ] |
| Around 1,000,000 | [ ] |
| 11. According to available figures, RSV causes every year a total of … hospitalizations: | |
| 2 million | [ ] |
| 10 million | [ ] |
| 22 million | [ ] |
| Do not know. | [ ] |
| 12. According to WHO estimated, RSV causes … of lower respiratory tract infections: | |
| 40% | [ ] |
| 60% | [ ] |
| 75% | [ ] |
| 13. RSV infections may cause severe neurological complications. | [true] [false] [do not know] |
| 14. RSV has been acknowledged as a risk factor for adult asthma. | [true] [false] [do not know] |
| 15. Seroprevalence for RSV reaches 100% before 2nd year of age. | [true] [false] [do not know] |
| 16. Maternal antibodies reduce the risk of RSV infections during first 4 months of age. | [true] [false] [do not know] |
| 17. Hospitalization rate for RSV during the first year of age may reach: | |
| 0.5 per 100 | [ ] |
| 1 per 100 | [ ] |
| 5 per 100 | [ ] |
| 18. The majority of patients hospitalized for RSV are affected by chronic respiratory disorders and cardiac malformations. | [true] [false] [do not know] |
| 19. The majority of hospitalizations for RSV occur among pre-term infants. | [true] [false] [do not know] |
| 20. According to available recommendations, mAb should be used only in preterm infants. | [true] [false] [do not know] |
| 21. Around three quarters of all RSV-related deaths occurs in subjects older than 65 years. | [true] [false] [do not know] |
| 22. During SARS-CoV-2 pandemic, global incidence of RSV infections has decreased. | [true] [false] [do not know] |
| 23. To date (December 2021), Italy is affected by a RSV epidemic. | [true] [false] [do not know] |
| 24. RSV natural infection elicit a long-lasting immunity. | [true] [false] [do not know] |
| 25. Severe complications are more likely in RSV than in seasonal influenza infections. | [true] [false] [do not know] |
| 3. Please rate the following items from “not significant” (1) to “very significant” (5) | |
| How do you perceive the frequency of RSV infections? | |
| In infants (age 0 to 8 years) | [1] [2] [3] [4] [5] |
| In adults (age 18 to 64 years) | [1] [2] [3] [4] [5] |
| In elderly (age ≥ 65 years) | [1] [2] [3] [4] [5] |
| How do you perceive the severity of RSV infections? | |
| In infants (age 0 to 8 years) | [1] [2] [3] [4] [5] |
| In adults (age 18 to 64 years) | [1] [2] [3] [4] [5] |
| In elderly (age ≥ 65 years) | [1] [2] [3] [4] [5] |
| 4. Are you favorable towards the implementation of a RSV vaccine in the specific vaccine schedule, if commercially available ?(1 = totally disagree; 5 = totally agree) | [1] [2] [3] [4] [5] |
| 5. In the design of a candidate RSV vaccine, which aspects are of specific importance, from your point of view? (1 = totally disagree; 5 = totally agree) | |
| avoiding natural infection (i.e., mucosal immunity) | [1] [2] [3] [4] [5] |
| avoiding complications (i.e., LRTI) | [1] [2] [3] [4] [5] |
| being efficient also in individuals aged 65 years or more. | [1] [2] [3] [4] [5] |
| 6. Please provide some general information about you | |
| Year of birth: | ______________ |
| Year of medical qualification as GP: | ______________ |
| You identify yourself as: | [male] [female] [no answer] |
| Do you have any previous professional experience in Pediatric settings? | [yes] [no] [no answer] |
| At the moment, how many individuals aged less than 14 years do you assist as GP? | _______________ |
| At the moment, how many medical consultations/visits do you perform by week in individuals aged 14 years or less? | _______________ |
| Variable | Previously Managed Any RSV Case (Average ± SD) | p Value (Mann–Whitney Test) | |
|---|---|---|---|
| Yes (No. = 45) | No (No. = 112) | ||
| GKS (%) | 55.6 ± 11.3 | 52.4 ± 11.2 | 0.111 |
| RPS for infants (%) | 74.5 ± 18.0 | 78.8 ± 20.7 | 0.195 |
| RPS for adults (%) | 37.2 ± 30.5 | 41.5 ± 22.1 | 0.399 |
| RPS for elders (%) | 51.5 ± 22.1 | 64.0 ± 20.2 | 0.002 |
| Variable | Being Favorable towards RSV Vaccines (When Available) (Average ± SD) | p Value (Mann Whitney Test) | |
|---|---|---|---|
| Yes (No. = 144) | No (No. = 13) | ||
| GKS (%) | 52.4 ± 10.8 | 63.7 ± 11.7 | <0.001 |
| RPS–infants (%) | 78.8 ± 20.2 | 64.0 ± 11.3 | <0.001 |
| RPS–adults (%) | 40.4 ± 25.9 | 38.8 ± 5.3 | 0.529 |
| RPS–elders (%) | 60.5 ± 22.1 | 60.3 ± 13.3 | 0.969 |
| Variable | Any Background in Pediatrics (Average ± SD) | p Value (Mann Whitney Test) | |
| Yes (No. = 11) | No (No. = 146) | ||
| GKS (%) | 55.6 ± 9.4 | 53.2 ± 11.4 | 0.425 |
| RPS–infants (%) | 94.5 ± 9.3 | 76.3 ± 20.0 | <0.001 |
| RPS–adults (%) | 19.6 ± 6.1 | 41.8 ± 25.0 | <0.001 |
| RPS–elders (%) | 39.3 ± 18.4 | 62.1 ± 20.9 | <0.001 |
| Variable | Being Favorable towards RSV Vaccine (When Available) | ||
|---|---|---|---|
| Yes No./144, % | No No./13, % | p Value | |
| Male Gender | 54, 37.5% | 8, 61.5% | 0.161 |
| Age > 50 years | 29, 20.1% | 6, 46.2% | 0.070 |
| Seniority ≥ 10 years | 89, 61.8% | 10, 76.9% | 0.434 |
| GKS > median (52.0%) | 63, 43.8% | 13, 100% | <0.001 |
| RPS, infants > median (48.0%) | 57, 39.6% | 0, - | 0.011 |
| RPS, adults > median (48.0%) | 65, 45.1% | 3, 23.1% | 0.213 |
| RPS, elders > median (64.0%) | 38, 26.4% | 3, 23.1% | 1.000 |
| Any background in Pediatrics | 11, 7.6% | 0, - | 0.641 |
| Previously managed any RSV case | 45, 31.3% | 0, - | 0.039 |
| Previously diagnosed any RSV case | 28, 19.4% | 0, - | 0.169 |
| Previously recommended hospitalization for RSV infection | 28, 19.4% | 0, - | 0.169 |
| Previously recommended mAb | 8, 5.6% | 0, - | 0.831 |
References
- Azzari, C.; Baraldi, E.; Bonanni, P.; Bozzola, E.; Coscia, A.; Lanari, M.; Manzoni, P.; Mazzone, T.; Sandri, F.; Checcucci Lisi, G.; et al. Epidemiology and Prevention of Respiratory Syncytial Virus Infections in Children in Italy. Ital. J. Pediatr. 2021, 47, 198. [Google Scholar] [CrossRef] [PubMed]
- Pellegrinelli, L.; Galli, C.; Bubba, L.; Cereda, D.; Anselmi, G.; Binda, S.; Gramegna, M.; Pariani, E. Respiratory Syncytial Virus in Influenza-like Illness Cases: Epidemiology and Molecular Analyses of Four Consecutive Winter Seasons (2014-2015/2017-2018) in Lombardy (Northern Italy). J. Med. Virol. 2020, 92, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2021, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Openshaw, P.J.M.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and Harmful Immunity to RSV Infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef] [PubMed]
- Andeweg, S.P.; Schepp, R.M.; van de Kassteele, J.; Mollema, L.; Berbers, G.A.M.; van Boven, M. Population-Based Serology Reveals Risk Factors for RSV Infection in Children Younger than 5 Years. Sci. Rep. 2021, 11, 8953. [Google Scholar] [CrossRef]
- Mazur, N.I.; Martinón-Torres, F.; Baraldi, E.; Fauroux, B.; Greenough, A.; Heikkinen, T.; Manzoni, P.; Mejias, A.; Nair, H.; Papadopoulos, N.G.; et al. Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: Current Management and New Therapeutics. Lancet Respir. Med. 2015, 3, 888–900. [Google Scholar] [CrossRef]
- Nair, H.; Theodoratou, E.; Rudan, I.; Nokes, D.J.; Ngama HND, M.; Munywoki, P.K.; Dherani, M.; Nair, H.; James Nokes, D.; Gessner, B.D.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.A.; Blount, R.E.; Smadel, J.E. Recovery of Cytopathogenic Agent from Chimpanzees with Coryza. Proc. Soc. Exp. Biol. Med. 1956, 92, 544–549. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef] [Green Version]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Eng. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef]
- Mosalli, R.; Alqarni, S.A.; Khayyat, W.W.; Alsaidi, S.T.; Almatrafi, A.S.; Bawakid, A.S.; Paes, B. Respiratory Syncytial Virus Nosocomial Outbreak in Neonatal Intensive Care: A Review of the Incidence, Management, and Outcomes. Am. J. Infect. Control. 2021; in press. [Google Scholar] [CrossRef] [PubMed]
- Debes, S.; Haug, J.B.; de Blasio, B.F.; Jonassen, C.M.; Dudman, S.G. Etiology of Viral Respiratory Tract Infections in Hospitalized Adults, and Evidence of the High Frequency of Prehospitalization Antibiotic Treatment in Norway. Health Sci. Rep. 2021, 4, 403. [Google Scholar] [CrossRef] [PubMed]
- Obolski, U.; Kassem, E.; Na’amnih, W.; Tannous, S.; Kagan, V.; Muhsen, K. Unnecessary Antibiotic Treatment of Children Hospitalized with RSV-Bronchiolitis: Risk Factors and Prescription Patterns. J. Glob. Antimicrob. Resist. 2021, 27, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Nourbakhsh, S.; Shoukat, A.; Zhang, K.; Poliquin, G.; Halperin, D.; Sheffield, H.; Halperin, S.A.; Langley, J.M.; Moghadas, S.M. Effectiveness and Cost-Effectiveness of RSV Infant and Maternal Immunization Programs: A Case Study of Nunavik, Canada. EClinicalMedicine 2021, 41, 101141. [Google Scholar] [CrossRef] [PubMed]
- Paes, B.A.; Frcpc, M.; Mitchell, I.; Mb Frcpc, M.A.; Banerji, A.; Mph, M.D.; Lanctôt, K.L.; Langley, J.M.; Bosco, D.; Paes, A. A Decade of Respiratory Syncytial Virus Epidemiology and Prophylaxis: Translating Evidence into Everyday Clinical Practice Case Presentation. Can. Respir. J. 2011, 18, e10–e19. [Google Scholar] [CrossRef] [PubMed]
- Arriola, C.S.; Kim, L.; Langley, G.; Anderson, E.J.; Openo, K.; Martin, A.M.; Lynfield, R.; Bye, E.; Como-Sabetti, K.; Reingold, A.; et al. Estimated Burden of Community-Onset Respiratory Syncytial Virus-Associated Hospitalizations among Children Aged <2 Years in the United States, 2014–2015. J. Pediatr. Infect. Dis. Soc. 2020, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, I.; Li, A.; Bjornson, C.L.; Lanctot, K.L.; Paes, B.A. Respiratory Syncytial Virus Immunoprophylaxis with Palivizumab: 12-Year Observational Study of Usage and Outcomes in Canada. Am. J. Perinatol. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Viguria, N.; Navascués, A.; Juanbeltz, R.; Echeverría, A.; Ezpeleta, C.; Castilla, J. Effectiveness of Palivizumab in Preventing Respiratory Syncytial Virus Infection in High-Risk Children. Hum. Vaccines Immunother. 2021, 17, 1867–1872. [Google Scholar] [CrossRef]
- Chida-Nagai, A.; Sato, H.; Sato, I.; Shiraishi, M.; Sasaki, D.; Izumi, G.; Yamazawa, H.; Cho, K.; Manabe, A.; Takeda, A. Risk Factors for Hospitalisation Due to Respiratory Syncytial Virus Infection in Children Receiving Prophylactic Palivizumab. Eur. J. Pediatr. 2021, 181, 539–547. [Google Scholar] [CrossRef]
- Zylbersztejn, A.; Almossawi, O.; Gudka, N.; Tompsett, D.; de Stavola, B.; Standing, J.F.; Smyth, R.; Hardelid, P. Access to Palivizumab among Children at High Risk of Respiratory Syncytial Virus Complications in English Hospitals. Br. J. Clin. Pharmacol. 2021, 88, 1246–1257. [Google Scholar] [CrossRef]
- Batista, J.D.L.; Ferreira, M.A.P.; Xavier, C.D.S.; de Souza, I.T.A.; Cruz, L.N.; Polanczyk, C.A. A Post-Incorporation Study on the Use of Palivizumab in the Brazilian Public Health System. Rev. Inst. Med. Trop. Sao Paulo 2021, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sullender, W.M. Respiratory Syncytial Virus Genetic and Antigenic Diversity. Clin. Microbiol. Rev. 2000, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Piedimonte, G.; Perez, M.K. Respiratory Syncytial Virus Infection and Bronchiolitis Practice Gaps. Pediatrics Rev. 2014, 35, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; de Conto, F.; Buttrini, M.; Piccolo, G.; Montecchini, S.; Maccari, C.; Martinelli, M.; di Maio, A.; Ferraglia, F.; Pinardi, F.; et al. Human Respiratory Viruses, Including SARS-CoV-2, Circulating in the Winter Season 2019–2020 in Parma, Northern Italy. Int. J. Infect. Dis. 2021, 102, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Sherman, A.C.; Babiker, A.; Sieben, A.J.; Pyden, A.; Steinberg, J.; Kraft, C.S.; Koelle, K.; Kanjilal, S. The Effect of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Mitigation Strategies on Seasonal Respiratory Viruses: A Tale of 2 Large Metropolitan Centers in the United States. Clin. Infect. Dis. 2021, 72, E154–E157. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, I.; Artama, M.; Mäkelä, L.; Backman, K.; Heiskanen-Kosma, T.; Renko, M. Effect of Social Distancing Due to the COVID-19 Pandemic on the Incidence of Viral Respiratory Tract Infections in Children in Finland during Early 2020. Pediatric Infect. Dis. J. 2020, 39, E423–E427. [Google Scholar] [CrossRef]
- van Brusselen, D.; de Troeyer, K.; ter Haar, E.; vander Auwera, A.; Poschet, K.; van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; van Herendael, B.; et al. Bronchiolitis in COVID-19 Times: A Nearly Absent Disease? Eur. J. Pediatr. 2021, 180, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Britton, P.N.; Hu, N.; Saravanos, G.; Shrapnel, J.; Davis, J.; Snelling, T.; Dalby-Payne, J.; Kesson, A.M.; Wood, N.; Macartney, K.; et al. COVID-19 Public Health Measures and Respiratory Syncytial Virus. Lancet Child Adolesc. Health 2020, 4, e42–e43. [Google Scholar] [CrossRef]
- Hatter, L.; Eathorne, A.; Hills, T.; Bruce, P.; Beasley, R. Respiratory Syncytial Virus: Paying the Immunity Debt with Interest. Lancet Child Adolesc. Health 2021, 5, e44–e45. [Google Scholar] [CrossRef]
- Foley, D.A.; Phuong, L.K.; Peplinski, J.; Lim, S.M.; Lee, W.H.; Farhat, A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; et al. Examining the Interseasonal Resurgence of Respiratory Syncytial Virus in Western Australia. Arch. Dis. Child. 2021, 107, e7. [Google Scholar] [CrossRef]
- Foley, D.A.; Yeoh, D.K.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Moore, H.C.; Blyth, C.C. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019-Related Public Health Measures. Clin. Infect. Dis. 2021, 73, E2829–E2830. [Google Scholar] [CrossRef] [PubMed]
- Betsch, C.; Wicker, S. Personal Attitudes and Misconceptions, Not Official Recommendations Guide Occupational Physicians’ Vaccination Decisions. Vaccine 2014, 32, 4478–4484. [Google Scholar] [CrossRef] [PubMed]
- Hurley, L.P.; Allison, M.A.; Kim, L.; O’Leary, S.T.; Crane, L.A.; Brtnikova, M.; Beaty, B.L.; Allen, K.E.; Poser, S.; Lindley, M.C.; et al. Primary Care Physicians’ Perspectives on Respiratory Syncytial Virus (RSV) Disease in Adults and a Potential RSV Vaccine for Adults. Vaccine 2019, 37, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.H. Respiratory Syncytial Virus Infection and Palivizumab: Are Families Receiving Accurate Information? Am. J. Perinatol. 2010, 27, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Reeves, R.M.; Hardelid, P.; Panagiotopoulos, N.; Minaji, M.; Warburton, F.; Pebody, R. Burden of Hospital Admissions Caused by Respiratory Syncytial Virus (RSV) in Infants in England: A Data Linkage Modelling Study. J. Infect. 2019, 78, 468–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbati, F.; Moriondo, M.; Pisano, L.; Calistri, E.; Lodi, L.; Ricci, S.; Giovannini, M.; Canessa, C.; Indolfi, G.; Azzari, C. Epidemiology of Respiratory Syncytial Virus-Related Hospitalization over a 5-Year Period in Italy: Evaluation of Seasonality and Age Distribution before Vaccine Introduction. Vaccines 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rainisch, G.; Adhikari, B.; Meltzer, M.I.; Langley, G. Estimating the Impact of Multiple Immunization Products on Medically-Attended Respiratory Syncytial Virus (RSV) Infections in Infants. Vaccine 2020, 38, 251–257. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, Regional, and National Disease Burden Estimates of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children in 2015: A Systematic Review and Modelling Study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.; Hall, C.B.; Katkin, J.P.; Shi, N.; Masaquel, A.S.; McLaurin, K.K.; Mahadevia, P.J. Healthcare Costs within a Year of Respiratory Syncytial Virus among Medicaid Infants. Pediatr. Pulmonol. 2010, 45, 772–781. [Google Scholar] [CrossRef]
- Shi, T.; Arnott, A.; Semogas, I.; Falsey, A.R.; Openshaw, P.; Wedzicha, J.A.; Campbell, H.; Nair, H. The Etiological Role of Common Respiratory Viruses in Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222, S563–S569. [Google Scholar] [CrossRef]
- Staadegaard, L.; Caini, S.; Wangchuk, S.; Thapa, B.; de Almeida, W.A.F.; de Carvalho, F.C.; Njouom, R.; Fasce, R.A.; Bustos, P.; Kyncl, J.; et al. The Global Epidemiology of RSV in Community and Hospitalized Care: Findings from 15 Countries. Open Forum Infect. Dis. 2021, 8, ofab159. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Lopardo, G.; Scarpellini, B.; Stein, R.T.; Ribeiro, D. Systematic Review on Respiratory Syncytial Virus Epidemiology in Adults and the Elderly in Latin America. Int. J. Infect. Dis. 2020, 90, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccò, M.; Gualerzi, G.; Ranzieri, S.; Ferraro, P.; Bragazzi, N.L. Knowledge, Attitudes, Practices (KAP) of Italian Occupational Physicians towards Tick Borne Encephalitis. Trop. Med. Infect. Dis. 2020, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Razio, B.; Panato, C.; Poletti, L.; Signorelli, C. Knowledge, Attitudes and Practices of Agricultural Workers towards Tetanus Vaccine: A Field Report. Ann. Ig 2017, 29, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Ranzieri, S.; Balzarini, F.; Vezzosi, L.; Marchesi, F.; Valente, M.; Peruzzi, S. A Pilot Study on Knowledge, Attitudes and Beliefs of Medical Professionals on Invasive Fungal Infections. J. Mycol. Med. 2020, 31, 101103. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Balzarini, F.; Ranzieri, S. Mandate or Not Mandate: Knowledge, Attitudes, and Practices of Italian Occupational Physicians towards SARS-CoV-2 Immunization at the Beginning of Vaccination Campaign. Vaccines 2021, 9, 889. [Google Scholar] [CrossRef]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Balzarini, F.; Capozzi, V.A.; Volpi, L. Knowledge, Attitudes, Beliefs and Practices of Obstetrics-Gynecologists on Seasonal Influenza and Pertussis Immunizations in Pregnant Women: Preliminary Results from North-Western Italy. Miner. Ginecol. 2019, 71, 288–297. [Google Scholar] [CrossRef]
- Ricco, M.; Gualerzi, G.; Ranzieri, S. Personal Beliefs and Misconceptions, Not Evidence Guide General Practitioners in the Managing of Travelers’ Diarrhea: Results from a Pilot Study (North-Western Italy, 2019). Med. Mal. Infect. 2020, 51, 266–272. [Google Scholar] [CrossRef]
- Yates, F.J.; Stone, E.R. The Risk Construct. In Risk-Taking Behaviour; Yates, F.J., Ed.; John Wiley & Sons: Chichester, UK, 1992; pp. 1–25. ISBN 0471922501. [Google Scholar]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Demont, C.; Petrica, N.; Bardoulat, I.; Duret, S.; Watier, L.; Chosidow, A.; Lorrot, M.; Kieffer, A.; Lemaitre, M. Economic and Disease Burden of RSV-Associated Hospitalizations in Young Children in France, from 2010 through 2018. BMC Infect. Dis. 2021, 21, 730. [Google Scholar] [CrossRef]
- Halabi, K.C.; Saiman, L.; Zachariah, P. The Epidemiology of Respiratory Syncytial Virus in New York City during the COVID-19 Pandemic Compared with Previous Years. J. Pediatr. 2021, 242, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Lumley, S.F.; Richens, N.; Lees, E.; Cregan, J.; Kalimeris, E.; Oakley, S.; Morgan, M.; Segal, S.; Dawson, M.; Walker, A.S.; et al. Changes in Paediatric Respiratory Infections at a UK Teaching Hospital 2016-2021; Impact of the SARS-CoV-2 Pandemic. J. Infect. 2021, 84, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Varela, F.H.; Scotta, M.C.; Polese-Bonatto, M.; Sartor, I.T.S.; Ferreira, C.F.; Fernandes, I.R.; Zavaglia, G.O.; de Almeida, W.A.F.; Arakaki-Sanchez, D.; Pinto, L.A.; et al. Absence of Detection of RSV and Influenza during the COVID-19 Pandemic in a Brazilian Cohort: Likely Role of Lower Transmission in the Community. J. Glob. Health 2021, 11, 05007. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, G.; la Vecchia, A.; Umbrello, G.; di Pietro, G.; Bono, P.; Scalia, S.; Pinzani, R.; Tagliabue, C.; Bosis, S.; Agostoni, C.; et al. Disappearance of Seasonal Respiratory Viruses in Children Under Two Years Old during COVID-19 Pandemic: A Monocentric Retrospective Study in Milan, Italy. Front. Pediatr. 2021, 9, 721005. [Google Scholar] [CrossRef] [PubMed]
- de Francesco, M.A.; Pollara, C.; Gargiulo, F.; Giacomelli, M.; Caruso, A. Circulation of Respiratory Viruses in Hospitalized Adults before and during the COVID-19 Pandemic in Brescia, Italy: A Retrospective Study. Int. J. Environ. Res. Public Health 2021, 18, 9525. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, A.; Donà, D.; Barbieri, E.; Giaquinto, C.; Bont, L.J.; Baraldi, E. COVID-19 Lesson for Respiratory Syncytial Virus (RSV): Hygiene Works. Children 2021, 8, 1144. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N.; Ujiie, M. Resurgence of Respiratory Syncytial Virus Infections during COVID-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef] [PubMed]
- Binns, E.; Koenraads, M.; Hristeva, L.; Flamant, A.; Baier-Grabner, S.; Loi, M.; Lempainen, J.; Osterheld, E.; Ramly, B.; Chakakala-Chaziya, J.; et al. Influenza and Respiratory Syncytial Virus during the COVID-19 Pandemic: Time for a New Paradigm? Pediatr. Pulmonol. 2021, 57, 38–42. [Google Scholar] [CrossRef]
- Lively, J.Y.; Curns, A.T.; Weinberg, G.Y.; Edwards, K.M.; Staat, M.A.; Prill, M.M.; Gerber, S.I.; Lengley, G.E. Respiratory Syncytial Virus–Associated Outpatient Visits Among Children Younger Than 24 Months. J. Pediatr. Infect. Dis. Soc. 2019, 8, 284–286. [Google Scholar] [CrossRef]
- Byington, C.L.; Wilkes, J.; Korgenski, K.; Sheng, X. Respiratory Syncytial Virus-Associated Mortality in Hospitalized Infants and Young Children. Pediatrics 2015, 135, e24–e31. [Google Scholar] [CrossRef] [Green Version]
- Giles, M.L.; Buttery, J.; Davey, M.A.; Wallace, E. Pregnant Women’s Knowledge and Attitude to Maternal Vaccination Including Group B Streptococcus and Respiratory Syncytial Virus Vaccines. Vaccine 2019, 37, 6743–6749. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.R.; Calvert, A.; Metz, J.; Kilich, E.; Macleod, R.; Beadon, K.; Heath, P.T.; Khalil, A.; Finn, A.; Snape, M.D.; et al. Attitudes of Pregnant Women and Healthcare Professionals toward Clinical Trials and Routine Implementation of Antenatal Vaccination against Respiratory Syncytial Virus: A Multicenter Questionnaire Study. Pediatr. Infect. Dis. J. 2019, 38, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.E.; Beekmann, S.E.; Polgreen, P.; Poser, S.; St Pierre, J.; Santibañez, S.; Gerber, S.I.; Kim, L. Survey of Diagnostic Testing for Respiratory Syncytial Virus (RSV) in Adults: Infectious Disease Physician Practices and Implications for Burden Estimates. Diagn. Microbiol. Infect. Dis. 2018, 92, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.C.; Ryan, P.; Howard, D.E.; Feldman, K.A. Understanding Knowledge, Attitudes, and Behaviors Toward West Nile Virus Prevention: A Survey of High-Risk Adults in Maryland. Vector-Borne Zoonotic Dis. 2018, 18, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Corace, K.M.; Srigley, J.A.; Hargadon, D.P.; Yu, D.; MacDonald, T.K.; Fabrigar, L.R.; Garber, G.E. Using Behavior Change Frameworks to Improve Healthcare Worker Influenza Vaccination Rates: A Systematic Review. Vaccine 2016, 34, 3235–3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazio, R.H.; Zanna, M.P.; Cooper, J. Direct Experience and Attitude-Behavior Consistency: An Information Processing Analysis. Personal. Soc. Psychol. Bull. 1978, 4, 48–51. [Google Scholar] [CrossRef]
- McLaurin, K.K.; Farr, A.M.; Wade, S.W.; Diakun, D.R.; Stewart, D.L. Respiratory Syncytial Virus Hospitalization Outcomes and Costs of Full-Term and Preterm Infants. J. Perinatol. 2016, 36, 990–996. [Google Scholar] [CrossRef] [Green Version]
- Bouzid, D.; Vila, J.; Hansen, G.; Manissero, D.; Pareja, J.; Rao, S.N.; Visseaux, B. Systematic Review on the Association between Respiratory Virus Real-Time PCR Cycle Threshold Values and Clinical Presentation or Outcomes. J. Antimicrob. Chemother. 2021, 76, III33–III49. [Google Scholar] [CrossRef]
- Auvinen, R.; Syrjänen, R.; Ollgren, J.; Nohynek, H.; Skogberg, K. Clinical Characteristics and Population-Based Attack Rates of Respiratory Syncytial Virus versus Influenza Hospitalizations among Adults—An Observational Study. Influenza Other Respir. Viruses 2021, 16, 276–288. [Google Scholar] [CrossRef]
- Yung, C.F.; Lee, K.S.; Thein, T.L.; Tan, L.K.; Gan, V.C.; Wong, J.G.X.; Lye, D.C.; Ng, L.C.; Leo, Y.S. Dengue Serotype-Specific Differences in Clinical Manifestation, Laboratory Parameters and Risk of Severe Disease in Adults, Singapore. Am. J. Trop. Med. Hyg. 2015, 92, 999–1005. [Google Scholar] [CrossRef]
- Hall, C.B.; Long, C.E.; Schnabel, K.C. Respiratory Syncytial Virus Infections in Previously Healthy Working Adults. Clin. Infect. Dis. 2001, 33, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Biagi, C.; Dondi, A.; Scarpini, S.; Rocca, A.; Vandini, S.; Poletti, G.; Lanari, M. Current State and Challenges in Developing Respiratory Syncytial Virus Vaccines. Vaccines 2020, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Britton, P.N.; King, C.L.; Booy, R. The Immunogenicity and Safety of Respiratory Syncytial Virus Vaccines in Development: A Systematic Review. Influenza Other Respir. Viruses 2021, 15, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Graham, B.S.; Modjarrad, K.; McLellan, J.S. Novel Antigens for RSV Vaccines. Curr. Opin. Immunol. 2015, 35, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiervang, E.; Goodman, R. Advantages and Limitations of Web-Based Surveys: Evidence from a Child Mental Health Survey. Soc. Psychiatry Psychiatr. Epidemiol. 2011, 46, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, S.; Lei, W.; Zhao, Y.; Liu, H.; Yao, D.; Xu, Y.; Lv, Q.; Hao, G.; Xu, Y.; et al. Knowledge, Attitudes, and Practices Regarding Zika: Paper and Internet Based Survey in Zhejiang, China. JMIR Public Health Surveill 2017, 3, e81. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Vaccinations of Health Care Workers: A Cross Sectional Pilot Study in North-Eastern Italy. Int. J. Occup. Med. Environ. Health 2017, 30, 775–790. [Google Scholar] [CrossRef]
- Riccò, M.; Cattani, S.; Casagranda, F.; Gualerzi, G.; Signorelli, C. Knowledge, Attitudes, Beliefs and Practices of Occupational Physicians towards Seasonal Influenza Vaccination: A Cross-Sectional Study from North-Eastern Italy. J. Prev. Med. Hyg. 2017, 58, E141–E154. [Google Scholar]
- Riccò, M.; Vezzosi, L.; Gualerzi, G.; Bragazzi, N.L.; Balzarini, F. Pertussis Immunization in Healthcare Workers Working in Pediatric Settings: Knowledge, Attitudes and Practices (KAP) of Occupational Physicians. Preliminary Results from a Web-Based Survey (2017). J. Prev. Med. Hyg. 2020, 61, E66. [Google Scholar]
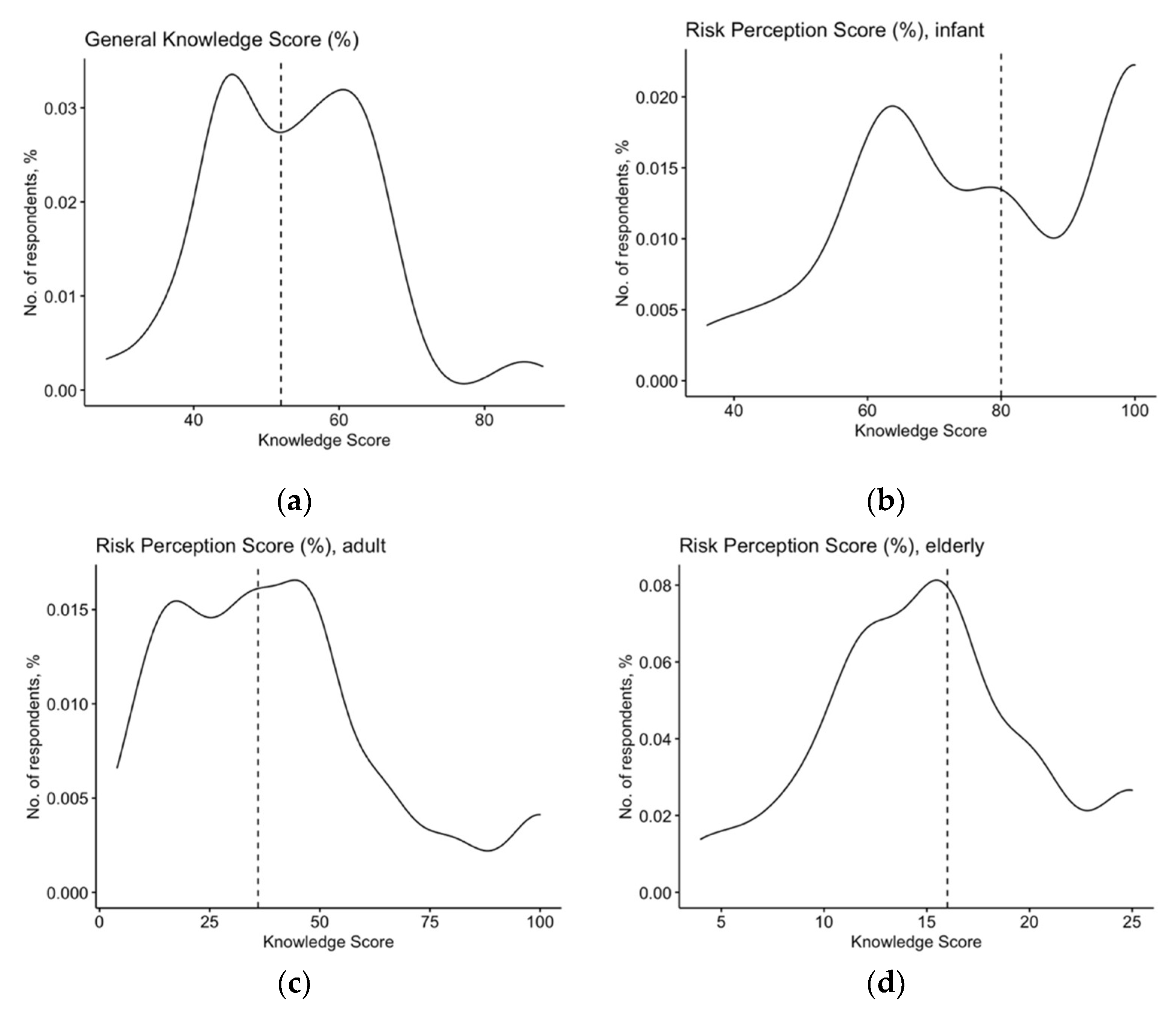
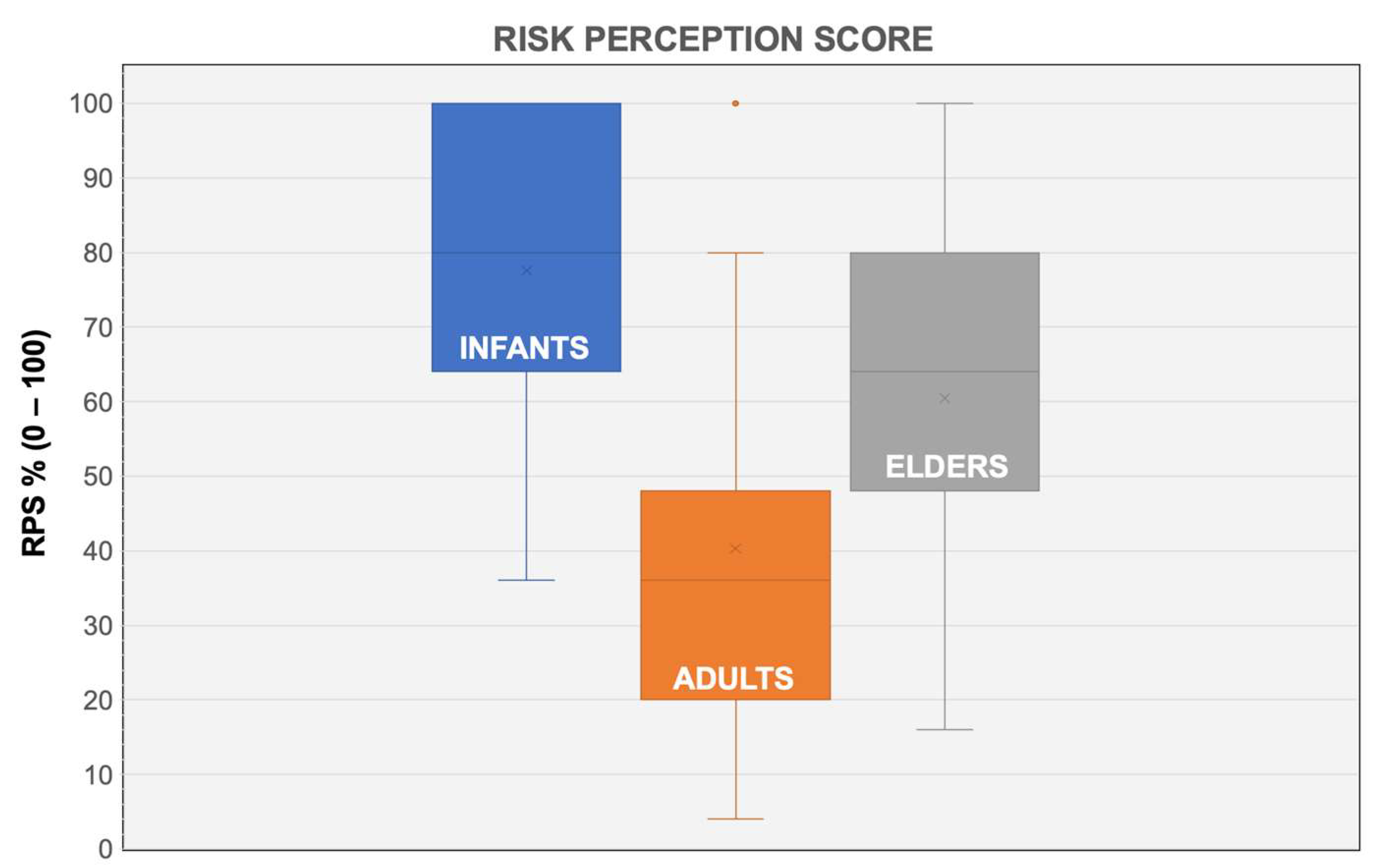
| Variable | No./157% | Average ± SD |
|---|---|---|
| Gender | ||
| Male | 62, 39.5% | |
| Female | 79, 60.5% | |
| Age (years) | 43.2 ± 10.7 | |
| Age ≥ 50 years | 35, 22.3% | |
| Seniority as GP | 16.9 ± 10.9 | |
| Seniority ≥ 10 years | 99, 63.1% | |
| Any occupational background in Pediatrics | 11, 7.0% | |
| Previously managed RSV cases | 45, 28.7% | |
| Previously diagnosed RSV cases | 28, 17.8% | |
| Previously required hospitalization for RSV | 28, 17.8% | |
| Previously required mAb immunoprophylaxis for RSV | 8, 5.1% | |
| Acknowledging RSV infection as frequent/very frequent in | ||
| infants | 138, 87.9% | |
| adults | 67, 42.7% | |
| elderly | 94, 59.9% | |
| Acknowledging RSV infection as severe/very severe in… | ||
| infants | 141, 89.8% | |
| adults | 54, 34.4% | |
| elderly | 124, 79.0% | |
| General Knowledge Score (%) | 53.4 ± 11.3 | |
| General Knowledge Score > median (52.0%) | 76, 48.4% | |
| Risk Perception Score for infants | 77.6 ± 20.0 | |
| Risk Perception Score for infants > median (80.0%) | 57, 36.3% | |
| Risk Perception Score for adults | 40.3 ± 24.8 | |
| Risk Perception Score for adults > median (48.0%) | 68, 43.3% | |
| Risk Perception Score for elderly | 60.5 ± 21.5 | |
| Risk Perception Score for elderly > median (64.0%) | 41, 26.1% | |
| Favorable/Highly favorable towards an RSV vaccination when made available | 144, 91.7% | |
| Acknowledging as significant/very significant aspects for candidate RSV vaccines | ||
| Avoiding natural infection (i.e., mucosal immunity) | 141, 89.8% | |
| Avoiding complications (i.e., LRTI) | 154, 98.1% | |
| Being efficient also in individuals aged 65 years or more | 104, 66.2% |
| Statement | Correct Answer | Total (No./157) |
|---|---|---|
| Nearly all RSV infections occur in infants and children. | False | 61, 38.9% |
| In most cases, infants acquire RSV infections from their parents. | False | 88, 56.1% |
| In most cases, RSV evolves in an uncomplicated influenza-like illness. | True | 138, 87.9% |
| Lower respiratory tract infections from RSV is deprived of specific signs/symptoms. | True | 72, 45.9% |
| In Europe, RSV season spans from: | ||
| November–March | True | 97, 61.8% |
| October–February | False | 47, 29.9% |
| September–January | False | 13, 8.3% |
| SARS-CoV-2 and RSV have the same means of transmission. | True | 157, 100% |
| Safe and effective vaccines against RSV are commercially available. | False | 128, 81.5% |
| Monoclonal antibodies can be used against RSV only as immunoprophylaxis. | True | 65, 41.4% |
| Immunoprophylaxis for RSV should be delivered: | ||
| Every two months, during RSV season | False | 28, 17.8% |
| Every month, during RSV season | True | 56, 35.7% |
| Only at the beginning of RSV season | False | 73, 46.5% |
| Globally, RSV causes a total … deaths in children < 1 age: | ||
| 43,800 | True | 71, 45.2% |
| 430,800 | False | 73, 46.5% |
| Around 1,000,000 | False | 13, 8.3% |
| According to available figures, RSV causes every year a total of … hospitalizations: | ||
| 2 million | True | 62, 39.5% |
| 10 million | False | 75, 47.8% |
| 22 million | False | 16, 10.2% |
| Do not know | - | 4, 2.5% |
| According to WHO estimated, RSV causes … of lower respiratory tract infections: | ||
| 40% | False | 92, 58.6% |
| 60% | True | 54, 34.4% |
| 75% | False | 11, 7.0% |
| RSV infections may cause severe neurological complications. | True | 117, 74.5% |
| RSV has been acknowledged as a risk factor for adult asthma. | True | 133, 84.7% |
| Seroprevalence for RSV reaches 100% before 2nd year of age. | True | 84, 53.5% |
| Maternal antibodies reduce the risk of RSV infections during first 4 months of age. | False | 12, 7.6% |
| Hospitalization rate for RSV during the first year of age may reach: | ||
| 0.5 per 100 | True | 32, 20.4% |
| 1 per 100 | False | 53, 33.8% |
| 5 per 100 | False | 72, 45.9% |
| The majority of patients hospitalized for RSV are affected by chronic respiratory disorders and cardiac malformations. | False | 47, 29.9% |
| The majority of hospitalizations for RSV occur among pre-term infants. | False | 31, 19.7% |
| According to available recommendations, mAb should be used only in preterm infants. | True | 53, 33.8% |
| Around three quarters of all RSV-related deaths occurs in subjects older than 65 years. | True | 34, 21.7% |
| During SARS-CoV-2 pandemic, global incidence of RSV infections has decreased. | True | 111, 70.7% |
| To date (December 2021), Italy is affected by an RSV epidemic. | True | 132, 84.1% |
| RSV natural infection elicit a long-lasting immunity. | False | 86, 54.8% |
| Severe complications are more likely in RSV than in seasonal influenza infections. | True | 139, 88.5% |
| Variable | GKS | RPS for Infants | RPS for Adults | RPS for Elders |
|---|---|---|---|---|
| GKS | - | −0.140 (p = 0.081) | −0.005 (p = 0.952) | −0.122 (p = 0.127) |
| RPS for infants | −0.140 (p = 0.081) | - | −0.194 (p = 0.015) | −0.168 (p = 0.036) |
| RPS for adults | −0.005 (p = 0.952) | −0.194 (p = 0.015) | - | −0.610 (p < 0.001) |
| RPS for elders | −0.122 (p = 0.127) | −0.168 (p = 0.036) | −0.610 (p < 0.001) | - |
| Variable | Risk Perception for Infants | |||
|---|---|---|---|---|
| High Concern No./57% | Low Concern No./100% | p Value | aOR (95% CI) | |
| Male gender | 13, 22.8% | 49, 49.0% | 0.002 | 0.472 (0.201; 1.107) |
| Age > 50 years | 9, 15.8% | 26, 26.0% | 0.201 | - |
| Seniority ≥ 10 years | 37, 64.9% | 62, 62.0% | 0.848 | - |
| GKS > median (52.0%) | 22, 38.6% | 54, 54.0% | 0.091 | - |
| RPS, adults > median (48.0%) | 22, 38.6% | 46, 46.0% | 0.464 | - |
| RPS, elders > median (64.0%) | 10, 17.5% | 31, 31.0% | 0.098 | - |
| Any background in pediatrics | 8, 14.0% | 3, 3.0% | 0.023 | 55.398 (6.796; 451.604) |
| Previously managed any RSV case | 10, 17.5% | 35, 35.0% | 0.032 | 0.114 (0.024; 0.552) |
| Previously diagnosed any RSV case | 12, 21.1% | 16, 16.0% | 0.563 | - |
| Previously recommended hospitalization for RSV infection | 4, 7.0% | 24, 24.0% | 0.014 | 0.240 (0.066; 0.869) |
| Previously recommended mAb | 4, 7.0% | 4, 4.0% | 0.653 | - |
| Favorable/Highly favorable towards RSV vaccine | 57, 100% | 87, 87.0% | 0.011 | 4.728 (1.999; 11.187) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riccò, M.; Ferraro, P.; Peruzzi, S.; Zaniboni, A.; Ranzieri, S. Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021). Pediatr. Rep. 2022, 14, 147-165. https://doi.org/10.3390/pediatric14020021
Riccò M, Ferraro P, Peruzzi S, Zaniboni A, Ranzieri S. Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021). Pediatric Reports. 2022; 14(2):147-165. https://doi.org/10.3390/pediatric14020021
Chicago/Turabian StyleRiccò, Matteo, Pietro Ferraro, Simona Peruzzi, Alessandro Zaniboni, and Silvia Ranzieri. 2022. "Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021)" Pediatric Reports 14, no. 2: 147-165. https://doi.org/10.3390/pediatric14020021
APA StyleRiccò, M., Ferraro, P., Peruzzi, S., Zaniboni, A., & Ranzieri, S. (2022). Respiratory Syncytial Virus: Knowledge, Attitudes and Beliefs of General Practitioners from North-Eastern Italy (2021). Pediatric Reports, 14(2), 147-165. https://doi.org/10.3390/pediatric14020021








