Therapeutic Options for Childhood Absence Epilepsy
Abstract
1. Introduction
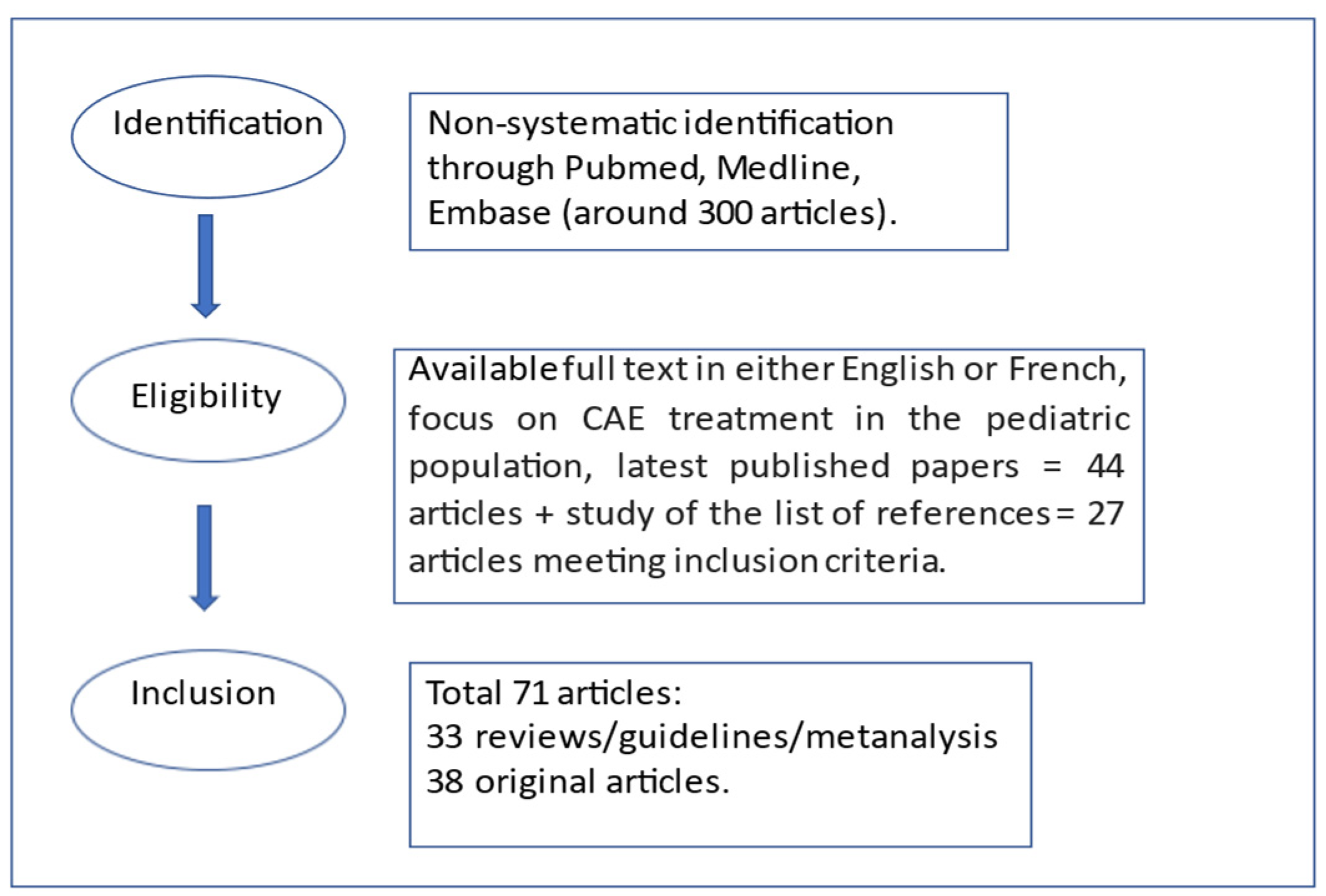
2. Materials and Methods
3. Childhood Absence Epilepsy Therapeutic Options
3.1. First Line Treatment
3.1.1. Ethosuximide
3.1.2. Valproic Acid
3.1.3. Lamotrigine
3.2. Associations of Antiepileptic Drugs
3.3. New Frontiers in Therapy-Resistant CAE Forms
3.4. Long Term Prognosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Matricardi, S.; Verrotti, A.; Chiarelli, F.; Cerminara, C.; Curatolo, P. Current advances in childhood absence epilepsy. Pediatr. Neurol. 2014, 50, 205–212. [Google Scholar] [CrossRef]
- Verrotti, A.; D’Alonzo, R.; Rinaldi, V.E.; Casciato, S.; D’Aniello, A.; Di Gennaro, G. Childhood absence epilepsy and benign epilepsy with centro-temporal spikes: A narrative review analysis. World J. Pediatr. 2017, 13, 106–111. [Google Scholar] [CrossRef]
- Hughes, J.R. Absence seizures: A review of recent reports with new concepts. Epilepsy Behav. 2009, 15, 404–412. [Google Scholar] [CrossRef]
- Guilhoto, L.M. Absence epilepsy: Continuum of clinical presentation and epigenetics? Seizure 2017, 44, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Garzon, P.; Lemelle, L.; Auvin, S. Childhood absence epilepsy: An update. Arch. Pediatr. Organe Off. Soc. Francaise Pediatr. 2016, 23, 1176–1183. [Google Scholar]
- Verrotti, A.; Laino, D.; Rinaldi, V.E.; Suppiej, A.; Giordano, L.; Toldo, I.; Margari, L.; Parisi, P.; Rizzo, R.; Matricardi, S.; et al. Clinical dissection of childhood occipital epilepsy of Gastaut and prognostic implication. Eur. J. Neurol. 2016, 23, 241–246. [Google Scholar] [CrossRef]
- Caraballo, R.H.; Sologuestua, A.; Grañana, N.; Adi, J.N.; Cersósimo, R.O.; Mazza, E.; Foster, O.; Fejerman, N. Idiopathic occipital and absence epilepsies appearing in the same children. Pediatr. Neurol. 2004, 30, 24–28. [Google Scholar] [CrossRef]
- Giordano, L.; Tambucci, R.; Cocco, I.E.; Angriman, M.; Coppola, G.; Operto, F.F.; Farello, G.; Savasta, S.; Belcastro, V.; Verrotti, A. Infantile spasms followed by childhood absence epilepsy: A case series. Seizure 2020, 74, 77–80. [Google Scholar] [CrossRef]
- Verrotti, A.; Casciato, S.; Spalice, A.; Carotenuto, M.; Striano, P.; Parisi, P.; Zamponi, N.; Savasta, S.; Rinaldi, V.E.; D’Alonzo, R.; et al. Coexistence of childhood absence epilepsy and benign epilepsy with centrotemporal spikes: A case series. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2017, 21, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Leresche, N. Childhood absence epilepsy: Genes, channels, neurons and networks. Nat. Rev. Neurosci. 2002, 3, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, O. Genes and molecular mechanisms involved in the epileptogenesis of idiopathic absence epilepsies. Seizure 2012, 21, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Thakran, S.; Guin, D.; Singh, P.; Singh, P.; Kukal, S.; Rawat, C.; Yadav, S.; Kushwaha, S.S.; Srivastava, A.K.; Hasija, Y.; et al. Genetic Landscape of Common Epilepsies: Advancing towards Precision in Treatment. Int. J. Mol. Sci. 2020, 21, 7784. [Google Scholar] [CrossRef]
- Glauser, T.A.; Holland, K.; O’Brien, V.P.; Keddache, M.; Martin, L.J.; Clark, P.O.; Cnaan, A.; Dlugos, D.; Hirtz, D.G.; Shinnar, S.; et al. Pharmacogenetics of antiepileptic drug efficacy in childhood absence epilepsy. Ann. Neurol. 2017, 81, 444–453. [Google Scholar] [CrossRef]
- Tenney, J.R.; Kadis, D.S.; Agler, W.; Rozhkov, L.; Altaye, M.; Xiang, J.; Vannest, J.; Glauser, T.A. Ictal connectivity in childhood absence epilepsy: Associations with outcome. Epilepsia 2018, 59, 971–981. [Google Scholar] [CrossRef]
- Glauser, T.A.; Cnaan, A.; Shinnar, S.; Hirtz, D.G.; Dlugos, D.; Masur, D.; Clark, P.O.; Capparelli, E.V.; Adamson, P.C. Childhood Absence Epilepsy Study Group Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N. Engl. J. Med. 2010, 362, 790–799. [Google Scholar] [CrossRef]
- Vining, E.P.G.; Thio, L.L. Absence in childhood absence epilepsy: The horse is out of the barn. Neurology 2013, 81, 1564–1565. [Google Scholar] [CrossRef] [PubMed]
- Auvin, S. Advancing pharmacologic treatment options for pharmacologic treatment options for children with epilepsy. Expert Opin. Pharmacother. 2016, 17, 1475–1482. [Google Scholar] [CrossRef]
- Shinnar, R.C.; Shinnar, S.; Cnaan, A.; Clark, P.; Dlugos, D.; Hirtz, D.G.; Hu, F.; Liu, C.; Masur, D.; Weiss, E.F.; et al. Pretreatment behavior and subsequent medication effects in childhood absence epilepsy. Neurology 2017, 89, 1698–1706. [Google Scholar] [CrossRef]
- Matricardi, S.; Deleo, F.; Ragona, F.; Rinaldi, V.E.; Pelliccia, S.; Coppola, G.; Verrotti, A. Neuropsychological profiles and outcomes in children with new onset frontal lobe epilepsy. Epilepsy Behav. 2016, 55, 79–83. [Google Scholar] [CrossRef]
- Stafstrom, C.E.; Sun, L.R.; Kossoff, E.H.; Dabrowski, A.K.; Singhi, S.; Kelley, S.A. Diagnosing and managing childhood absence epilepsy by telemedicine. Epilepsy Behav. 2021, 115, 107404. [Google Scholar] [CrossRef] [PubMed]
- Arsov, T.; Mullen, S.A.; Damiano, J.A.; Lawrence, K.M.; Huh, L.L.; Nolan, M.; Young, H.; Thouin, A.; Dahl, H.-H.M.; Berkovic, S.F.; et al. Early onset absence epilepsy: 1 in 10 cases is caused by GLUT1 deficiency. Epilepsia 2012, 53, e204–e207. [Google Scholar] [CrossRef]
- Wheless, J.W.; Clarke, D.F.; Arzimanoglou, A.; Carpenter, D. Treatment of pediatric epilepsy: European expert opinion, 2007. Epileptic Disord. Int. Epilepsy J. Videotape 2007, 9, 353–412. [Google Scholar]
- Kessler, S.K.; McGinnis, E. A Practical Guide to Treatment of Childhood Absence Epilepsy. Paediatr. Drugs 2019, 21, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H. Topiramate monotherapy for childhood absence seizures: An open label pilot study. Seizure 2002, 11, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Wilfong, A.; Schultz, R. Zonisamide for absence seizures. Epilepsy Res. 2005, 64, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Cerminara, C.; Domizio, S.; Mohn, A.; Franzoni, E.; Coppola, G.; Zamponi, N.; Parisi, P.; Iannetti, P.; Curatolo, P. Levetiracetam in absence epilepsy. Dev. Med. Child Neurol. 2008, 50, 850–853. [Google Scholar] [CrossRef]
- Kanner, A.M.; Ashman, E.; Gloss, D.; Harden, C.; Bourgeois, B.; Bautista, J.F.; Abou-Khalil, B.; Burakgazi-Dalkilic, E.; Llanas Park, E.; Stern, J.; et al. Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs I: Treatment of new-onset epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2018, 91, 74–81. [Google Scholar] [PubMed]
- Berg, A.T.; Levy, S.R.; Testa, F.M.; Blumenfeld, H. Long-term seizure remission in childhood absence epilepsy: Might initial treatment matter? Epilepsia 2014, 55, 551–557. [Google Scholar] [CrossRef]
- Brigo, F.; Igwe, S.C. Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents. Cochrane Database Syst. Rev. 2017, 2, CD003032. [Google Scholar] [CrossRef] [PubMed]
- Posner, E.B.; Mohamed, K.; Marson, A.G. Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents. Cochrane Database Syst. Rev. 2003, CD003032, Update in Cochrane Database Syst. Rev. 2005, CD003032. [Google Scholar]
- Coulter, D.A.; Huguenard, J.R.; Prince, D.A. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann. Neurol. 1989, 25, 582–593. [Google Scholar] [CrossRef]
- Smith, G.A.; McKauge, L.; Dubetz, D.; Tyrer, J.H.; Eadie, M.J. Factors influencing plasma concentrations of ethosuximide. Clin. Pharmacokinet. 1979, 4, 38–52. [Google Scholar] [CrossRef]
- Vining, E.P.G. Ethosuximide in childhood absence epilepsy--older and better. N. Engl. J. Med. 2010, 362, 843–845. [Google Scholar] [CrossRef] [PubMed]
- Romoli, M.; Mazzocchetti, P.; D’Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic Acid and Epilepsy: From Molecular Mechanisms to Clinical Evidences. Curr. Neuropharmacol. 2019, 17, 926–946. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Matricardi, S.; Rinaldi, V.E.; Prezioso, G.; Coppola, G. Neuropsychological impairment in childhood absence epilepsy: Review of the literature. J. Neurol. Sci. 2015, 359, 59–66. [Google Scholar] [CrossRef]
- Zaccara, G.; Franciotta, D.; Perucca, E. Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia 2007, 48, 1223–1244. [Google Scholar] [CrossRef]
- Leach, M.J.; Marden, C.M.; Miller, A.A. Pharmacological studies on lamotrigine, a novel potential antiepileptic drug: II. Neurochemical studies on the mechanism of action. Epilepsia 1986, 27, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.M.; Enlow, T.; Holmes, G.L.; Manasco, P.; Concannon, S.; Chen, C.; Womble, G.; Casale, E.J. Lamictal (lamotrigine) monotherapy for typical absence seizures in children. Epilepsia 1999, 40, 973–979. [Google Scholar] [CrossRef]
- Holmes, G.L.; Frank, L.M.; Sheth, R.D.; Philbrook, B.; Wooten, J.D.; Vuong, A.; Kerls, S.; Hammer, A.E.; Messenheimer, J. Lamotrigine monotherapy for newly diagnosed typical absence seizures in children. Epilepsy Res. 2008, 82, 124–132. [Google Scholar] [CrossRef]
- Schlumberger, E.; Chavez, F.; Palacios, L.; Rey, E.; Pajot, N.; Dulac, O. Lamotrigine in treatment of 120 children with epilepsy. Epilepsia 1994, 35, 359–367. [Google Scholar] [CrossRef]
- Cnaan, A.; Shinnar, S.; Arya, R.; Adamson, P.C.; Clark, P.O.; Dlugos, D.; Hirtz, D.G.; Masur, D.; Glauser, T.A. Childhood Absence Epilepsy Study Group Second monotherapy in childhood absence epilepsy. Neurology 2017, 88, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Auvin, S. Management of childhood epilepsy. Presse Med. Paris Fr. 1983 2011, 40, 287–292. [Google Scholar]
- Pisani, F.; Narbone, M.C.; Trunfio, C.; Fazio, A.; La Rosa, G.; Oteri, G.; Di Perri, R. Valproic acid-ethosuximide interaction: A pharmacokinetic study. Epilepsia 1984, 25, 229–233. [Google Scholar] [CrossRef]
- Brodie, M.J.; Yuen, A.W. Lamotrigine substitution study: Evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res. 1997, 26, 423–432. [Google Scholar] [CrossRef]
- Mullen, S.A.; Suls, A.; De Jonghe, P.; Berkovic, S.F.; Scheffer, I.E. Absence epilepsies with widely variable onset are a key feature of familial GLUT1 deficiency. Neurology 2010, 75, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Ragona, F.; Matricardi, S.; Castellotti, B.; Patrini, M.; Freri, E.; Binelli, S.; Granata, T. Refractory absence epilepsy and glut1 deficiency syndrome: A new case report and literature review. Neuropediatrics 2014, 45, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, A.; Noebels, J.L. Monogenic models of absence epilepsy: Windows into the complex balance between inhibition and excitation in thalamocortical microcircuits. Prog. Brain Res. 2014, 213, 223–252. [Google Scholar]
- Panayiotopoulos, C.P. Treatment of typical absence seizures and related epileptic syndromes. Paediatr. Drugs 2001, 3, 379–403. [Google Scholar] [CrossRef] [PubMed]
- Hitiris, N.; Brodie, M.J. Evidence-based treatment of idiopathic generalized epilepsies with older antiepileptic drugs. Epilepsia 2005, 46 (Suppl. S9), 149–153. [Google Scholar] [CrossRef]
- Striano, P.; Minetti, C. Epilepsy: Old drugs do the trick in childhood absence epilepsy. Nat. Rev. Neurol. 2010, 6, 420–421. [Google Scholar] [CrossRef]
- Noyer, M.; Gillard, M.; Matagne, A.; Hénichart, J.P.; Wülfert, E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur. J. Pharmacol. 1995, 286, 137–146. [Google Scholar] [CrossRef]
- Rigo, J.-M.; Hans, G.; Nguyen, L.; Rocher, V.; Belachew, S.; Malgrange, B.; Leprince, P.; Moonen, G.; Selak, I.; Matagne, A.; et al. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br. J. Pharmacol. 2002, 136, 659–672. [Google Scholar] [CrossRef]
- Carunchio, I.; Pieri, M.; Ciotti, M.T.; Albo, F.; Zona, C. Modulation of AMPA receptors in cultured cortical neurons induced by the antiepileptic drug levetiracetam. Epilepsia 2007, 48, 654–662. [Google Scholar] [CrossRef]
- Fattore, C.; Boniver, C.; Capovilla, G.; Cerminara, C.; Citterio, A.; Coppola, G.; Costa, P.; Darra, F.; Vecchi, M.; Perucca, E. A multicenter, randomized, placebo-controlled trial of levetiracetam in children and adolescents with newly diagnosed absence epilepsy. Epilepsia 2011, 52, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Auvin, S.; Chhun, S.; Berquin, P.; Ponchel, E.; Delanoë, C.; Chiron, C. Aggravation of absence seizure related to levetiracetam. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2011, 15, 508–511. [Google Scholar] [CrossRef]
- Biton, V.; Montouris, G.D.; Ritter, F.; Riviello, J.J.; Reife, R.; Lim, P.; Pledger, G. A randomized, placebo-controlled study of topiramate in primary generalized tonic-clonic seizures. Topiramate YTC Study Group. Neurology 1999, 52, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Piña-Garza, J.E.; Schwarzman, L.; Wiegand, F.; Hulihan, J. A pilot study of topiramate in childhood absence epilepsy. Acta Neurol. Scand. 2011, 123, 54–59. [Google Scholar] [CrossRef]
- Posner, E. Pharmacological treatment of childhood absence epilepsy. Expert Rev. Neurother. 2006, 6, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Lőrincz, M.L.; McCafferty, C.; Lambert, R.C.; Leresche, N.; Di Giovanni, G.; David, F. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain J. Neurol. 2020, 143, 2341–2368. [Google Scholar] [CrossRef]
- Trinka, E.; Lattanzi, S.; Carpenter, K.; Corradetti, T.; Nucera, B.; Rinaldi, F.; Shankar, R.; Brigo, F. Exploring the Evidence for Broad-Spectrum Effectiveness of Perampanel: A Systematic Review of Clinical Data in Generalised Seizures. CNS Drugs 2021, 35, 821–837. [Google Scholar] [CrossRef]
- Stockings, E.; Zagic, D.; Campbell, G.; Weier, M.; Hall, W.D.; Nielsen, S.; Herkes, G.K.; Farrell, M.; Degenhardt, L. Evidence for cannabis and cannabinoids for epilepsy: A systematic review of controlled and observational evidence. J. Neurol. Neurosurg. Psychiatry 2018, 89, 741–753. [Google Scholar] [CrossRef]
- Luijtelaar, G.; van Zobeiri, M.; Lüttjohann, A.; Depaulis, A. Experimental Treatment Options in Absence Epilepsy. Curr. Pharm. Des. 2017, 23, 5577–5592. [Google Scholar] [CrossRef] [PubMed]
- Groomes, L.B.; Pyzik, P.L.; Turner, Z.; Dorward, J.L.; Goode, V.H.; Kossoff, E.H. Do patients with absence epilepsy respond to ketogenic diets? J. Child Neurol. 2011, 26, 160–165. [Google Scholar] [CrossRef]
- Clemens, Z.; Kelemen, A.; Fogarasi, A.; Tóth, C. Childhood absence epilepsy successfully treated with the paleolithic ketogenic diet. Neurol. Ther. 2013, 2, 71–76. [Google Scholar] [CrossRef]
- Thammongkol, S.; Vears, D.F.; Bicknell-Royle, J.; Nation, J.; Draffin, K.; Stewart, K.G.; Scheffer, I.E.; Mackay, M.T. Efficacy of the ketogenic diet: Which epilepsies respond? Epilepsia 2012, 53, e55–e59. [Google Scholar] [CrossRef] [PubMed]
- Marchiò, M.; Roli, L.; Lucchi, C.; Costa, A.M.; Borghi, M.; Iughetti, L.; Trenti, T.; Guerra, A.; Biagini, G. Ghrelin Plasma Levels After 1 Year of Ketogenic Diet in Children With Refractory Epilepsy. Front. Nutr. 2019, 6, 112. [Google Scholar] [CrossRef]
- Costa, A.-M.; Marchiò, M.; Bruni, G.; Bernabei, S.M.; Cavalieri, S.; Bondi, M.; Biagini, G. Evaluation of E-Health Applications for Paediatric Patients with Refractory Epilepsy and Maintained on Ketogenic Diet. Nutrients 2021, 13, 1240. [Google Scholar] [CrossRef]
- Arya, R.; Greiner, H.M.; Lewis, A.; Mangano, F.T.; Gonsalves, C.; Holland, K.D.; Glauser, T.A. Vagus nerve stimulation for medically refractory absence epilepsy. Seizure 2013, 22, 267–270. [Google Scholar] [CrossRef][Green Version]
- Franzoni, E.; Matricardi, S.; Di Pisa, V.; Capovilla, G.; Romeo, A.; Tozzi, E.; Pruna, D.; Salerno, G.G.; Zamponi, N.; Accorsi, P.; et al. Refractory absence seizures: An Italian multicenter retrospective study. Eur. J. Paediatr. Neurol. 2015, 19, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Nabbout, R.; Andrade, D.M.; Bahi-Buisson, N.; Cross, H.; Desquerre, I.; Dulac, O.; Granata, T.; Hirsch, E.; Navarro, V.; Ouss, L.; et al. Outcome of childhood-onset epilepsy from adolescence to adulthood: Transition issues. Epilepsy Behav. 2017, 69, 161–169. [Google Scholar] [CrossRef]
- Caplan, R.; Siddarth, P.; Stahl, L.; Lanphier, E.; Vona, P.; Gurbani, S.; Koh, S.; Sankar, R.; Shields, W.D. Childhood absence epilepsy: Behavioral, cognitive, and linguistic comorbidities. Epilepsia 2008, 49, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
| Older therapeutic options for resistant CAE | Efficacy and characteristics |
| Clobazam, Clonazepam and Acetazolamide | Clonazepam most frequently used but benzodiazepines have invalidating side effects and possible development of tolerance [58]. Only used in association with first line CAE therapy. Acetazolamide rarely used due to important adverse effects (kidney stones) [58]. |
| New therapeutic options for resistant CAE | Efficacy and characteristics |
| Levetiracetam | Contrasting data regarding efficacy; possibly used in monotherapy [26,55]. Can be associated with VPA,LTG, ETX [69]. Most promising drug for future studies [58]. |
| Topiramate | Not efficacious in monotherapy [57]. Can be associated with VPA,LTG, ETX [69]. |
| Zonisamide | Possibly used in monotherapy [25]; studies are necessary for possible associations with other antiepileptic drugs. |
| Experimental therapeutic options for resistant CAE | Efficacy and characteristics |
| Perampanel; EpidiolexVR | Perampanel as adjunctive therapy in idiopathic generalized epilepsy with absences, no evidence yet for treatment of CAE [60]. Epidiolex VR only in animal models [59]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rinaldi, V.E.; Di Cara, G.; Mencaroni, E.; Verrotti, A. Therapeutic Options for Childhood Absence Epilepsy. Pediatr. Rep. 2021, 13, 658-667. https://doi.org/10.3390/pediatric13040078
Rinaldi VE, Di Cara G, Mencaroni E, Verrotti A. Therapeutic Options for Childhood Absence Epilepsy. Pediatric Reports. 2021; 13(4):658-667. https://doi.org/10.3390/pediatric13040078
Chicago/Turabian StyleRinaldi, Victoria Elisa, Giuseppe Di Cara, Elisabetta Mencaroni, and Alberto Verrotti. 2021. "Therapeutic Options for Childhood Absence Epilepsy" Pediatric Reports 13, no. 4: 658-667. https://doi.org/10.3390/pediatric13040078
APA StyleRinaldi, V. E., Di Cara, G., Mencaroni, E., & Verrotti, A. (2021). Therapeutic Options for Childhood Absence Epilepsy. Pediatric Reports, 13(4), 658-667. https://doi.org/10.3390/pediatric13040078







