Clinical and Brain Imaging Findings in a Child with Vitamin B12 Deficiency
Abstract
:1. Introduction
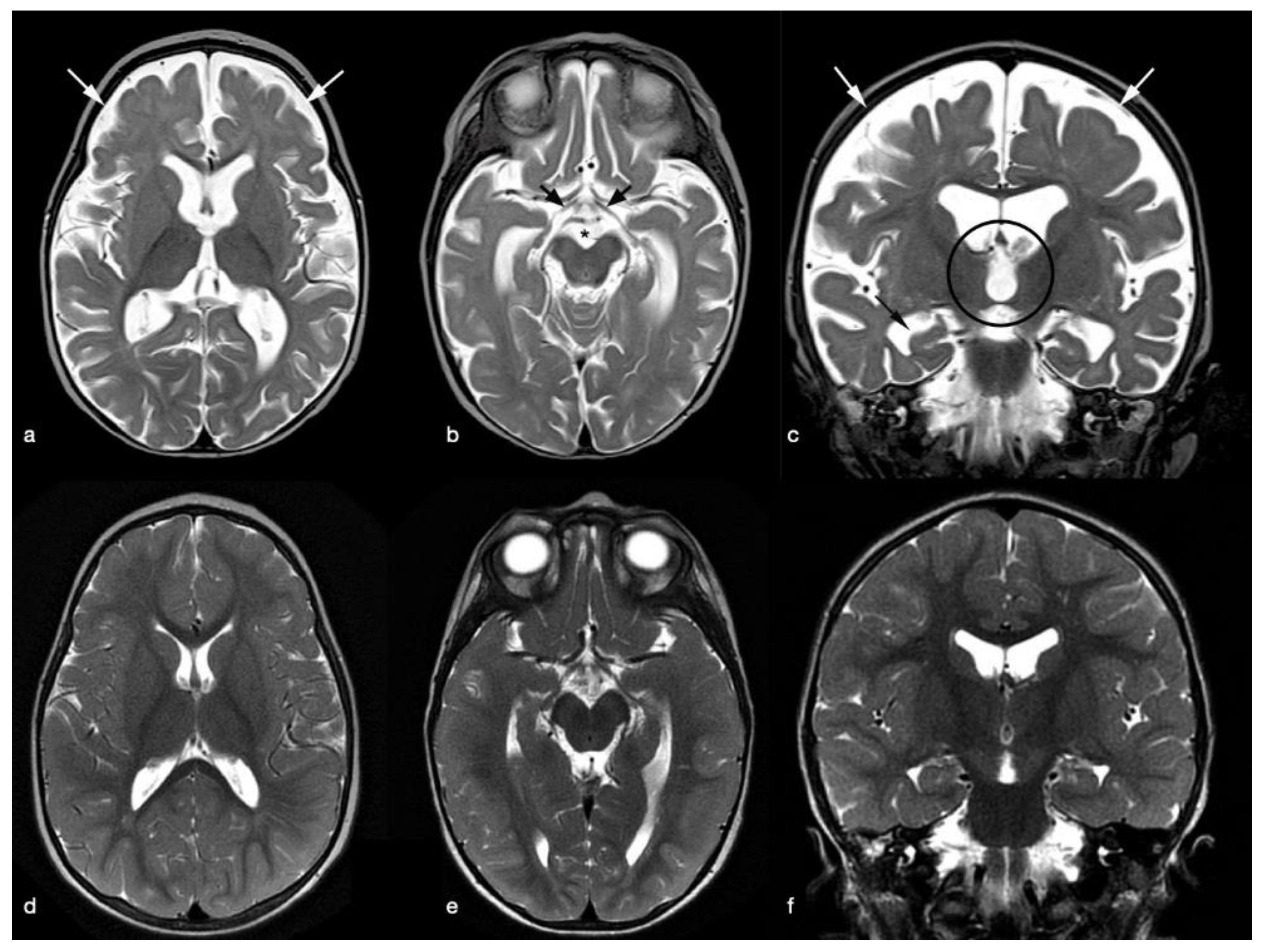
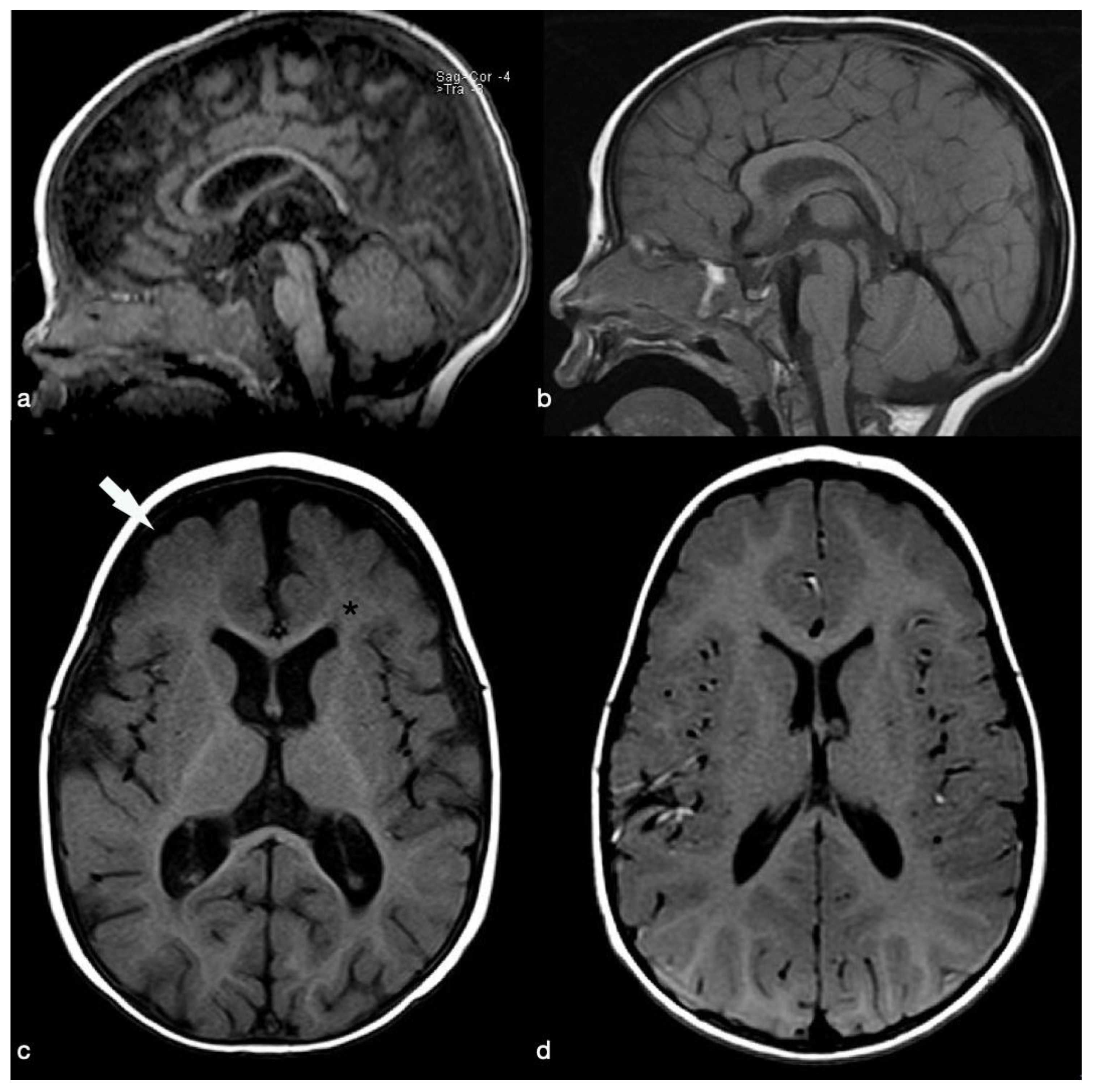
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sachdev, H.P.S.; Shah, D. Vitamin B12 (Cobalamin). In Nelson Textbook of Pediatrics, 19th ed.; Kliegman, R.M., Stanton, B.F., St. Geme, J.W., Schor, N.F., Behrman, R.E., Eds.; Saunders: Philadelphia, PA, USA, 2011; pp. 197–198. [Google Scholar]
- Grattan-Smith, P.J.; Wilcken, B.; Procopis, P.G.; Wise, G.A. The neurological syndrome of infantile cobalamin deficiency: Developmental regression and involuntary movements. Mov. Disord. 1997, 12, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Casella, E.B.; Valente, M.; De Navarro, J.M.; Kok, F. Vitamin B12 deficiency in infancy as a cause of developmental regression. Brain Dev. 2005, 27, 592–594. [Google Scholar] [CrossRef]
- Kocaoglu, C.; Akin, F.; Caksen, H.; Böke, S.B.; Arslan, S.; Aygün, S. Cerebral atrophy in a vitamin B12-deficient infant of a vegetarian mother. J. Health Popul. Nutr. 2014, 32, 367–371. [Google Scholar] [PubMed]
- Honzik, T.; Adamovicova, M.; Smolka, V.; Magner, M.; Hruba, E.; Zeman, J. Clinical presentation and metabolic consequences in 40 breastfed infants with nutritional vitamin B12 deficiency--what have we learned? Eur. J. Paediatr. Neurol. 2010, 14, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Glaser, K.; Girschick, H.J.; Schropp, C.; Speer, C.P. Psychomotor development following early treatment of severe infantile vitamin B12 deficiency and West syndrome—Is everything fine? A case report and review of literature. Brain Dev. 2015, 37, 347–351. [Google Scholar] [PubMed]
- Graham, S.M.; Arvela, O.M.; Wise, G.A. Long-term neurologic consequences of nutritional vitamin B12 deficiency in infants. J. Pediatr. 1992, 121, 710–714. [Google Scholar] [CrossRef]
- von Schenck, U.; Bender-Götze, C.; Koletzko, B. Persistence of neurological damage induced by dietary vitamin B-12 deficiency in infancy. Arch. Dis. Child. 1997, 77, 137–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørke Monsen, A.L.; Ueland, P.M.; Vollset, S.E.; Guttormsen, A.B.; Markestad, T.; Solheim, E.; Refsum, H. Determinants of cobalamin status in newborns. Pediatrics 2001, 108, 624–630, Erratum in Pediatrics 2002, 110, 853. [Google Scholar]
- Avci, Z.; Turul, T.; Aysun, S.; Unal, I. Involuntary movements and magnetic resonance imaging findings in infantile cobalamine (vitamin B12) deficiency. Pediatrics 2003, 112, 684–686. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.F.; Matsukura, M.; Fukui, K.; Watanabe, Y.; Matsumoto, N.; Kira, R. West Syndrome in an Infant With Vitamin B12 Deficiency Born to Autoantibodies Positive Mother. Front. Pediatr. 2019, 20, 531. [Google Scholar] [CrossRef] [PubMed]
- Pavone, P.; Sullo, F.; Falsaperla, R.; Greco, F.; Crespo, A.; Calvo, A.; Caraballo, R. Vitamin B12 Deficiency and West Syndrome: An Uncommon but Preventable Cause of Neurological Disorder. Report on Three Cases, One of Them with Late Onset during Vitamin B12 Treatment. Neuropediatrics 2021, 52, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.S.; Pons, R.; Ghaoui, R.; Sue, C.M. Genetic mimics of cerebral palsy. Mov. Disord. 2019, 34, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Taskesen, M.; Yaramis, A.; Pirinccioglu, A.G.; Ekici, F. Cranial magnetic resonance imaging findings of nutritional vitamin B12 deficiency in 15 hypotonic infants. Eur. J. Paediatr. Neurol. 2012, 16, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Acıpayam, C.; Güneş, H.; Güngör, O.; Ipek, S.; Sarışık, N.; Demir, N.Ş. Cerebral atrophy in 21 hypotonic infants with severe vitamin B12 deficiency. J. Paediatr. Child Health 2020, 56, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Dubaj, C.; Czyż, K.; Furmaga-Jabłońska, W. Vitamin B(12) deficiency as a cause of severe neurological symptoms in breast fed infant—A case report. Ital. J. Pediatr. 2020, 46, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Parameters | Admission | Discharge | 10-Months Follow-Up |
|---|---|---|---|
| WBC | 3 × 109/L (6–16) * | 8.1 × 109/L | 8.2 × 109/L |
| RBC | 1.5 × 1012/L (4–5.1) * | 3.9 × 1012/L | 4.97 × 1012/L |
| HB | 4.8 g/dL (10.3–13) * | 11.4 g/dL | 12.2 g/dL |
| HTC | 15% (30 × 38.5) * | 37.2% | 36.2% |
| MCV | 100 fL (70–90) * | 93 fL | 72.8 |
| MCH | 32 pg (23–29) * | 28 pg | 23.9 pg |
| RDW | 25.4% (<15) * | 18% | 15.7% |
| Reticulocytes | 16 × 109/L (27–99) * | 132 × 109/L | 87 × 109/L |
| Vitamin B12 | <100 pg/mL (197–866) * | 630 pg/mL | 736 pg/mL |
| Bilirubin, Total | 1.4 mg/dL (0–0.5) * | 0.4 mg/dL | 0.3 mg/dL |
| Bilirubin, Direct | 0.3 mg/dl (0–0.3) | 0.1 mg/dL | 0.1 mg/dL |
| LDH | 1602 U/L (0–470) * | 1049 U/L | 550 U/L |
| Haptoglobin | <0.078 g/L (0–2.27) | 0.24 g/L | 0.5 g/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feraco, P.; Incandela, F.; Franceschi, R.; Gagliardo, C.; Bellizzi, M. Clinical and Brain Imaging Findings in a Child with Vitamin B12 Deficiency. Pediatr. Rep. 2021, 13, 583-588. https://doi.org/10.3390/pediatric13040069
Feraco P, Incandela F, Franceschi R, Gagliardo C, Bellizzi M. Clinical and Brain Imaging Findings in a Child with Vitamin B12 Deficiency. Pediatric Reports. 2021; 13(4):583-588. https://doi.org/10.3390/pediatric13040069
Chicago/Turabian StyleFeraco, Paola, Francesca Incandela, Roberto Franceschi, Cesare Gagliardo, and Maria Bellizzi. 2021. "Clinical and Brain Imaging Findings in a Child with Vitamin B12 Deficiency" Pediatric Reports 13, no. 4: 583-588. https://doi.org/10.3390/pediatric13040069
APA StyleFeraco, P., Incandela, F., Franceschi, R., Gagliardo, C., & Bellizzi, M. (2021). Clinical and Brain Imaging Findings in a Child with Vitamin B12 Deficiency. Pediatric Reports, 13(4), 583-588. https://doi.org/10.3390/pediatric13040069









