Herpes Zoster in an Immunocompetent Child without a History of Varicella
Abstract
1. Introduction
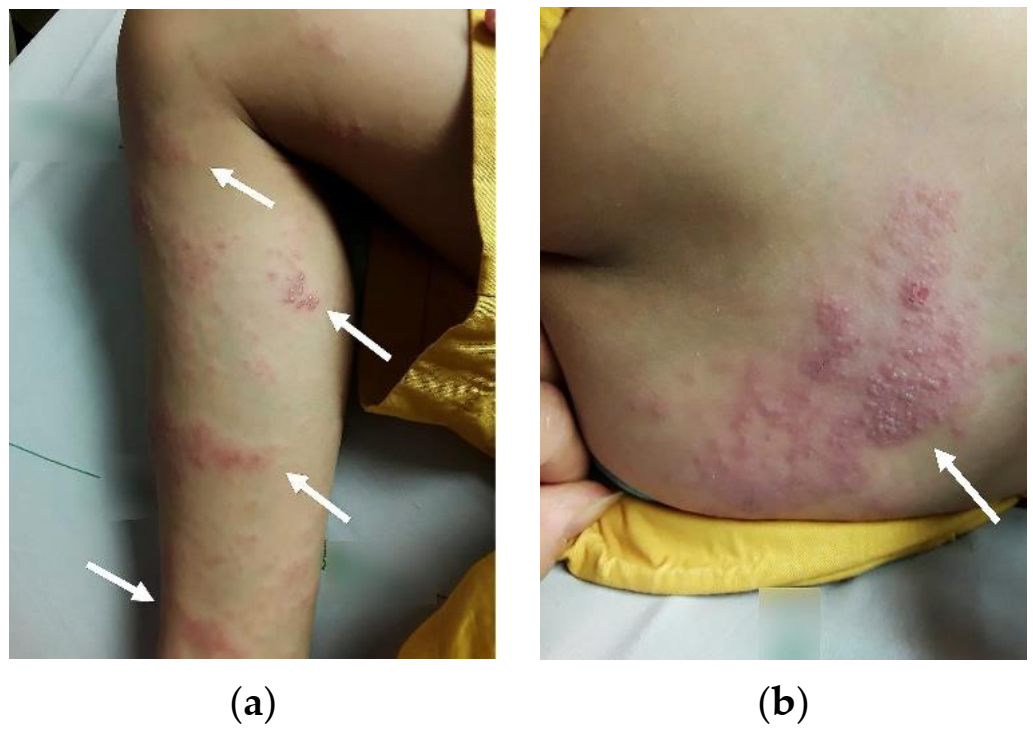
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kliegman, R.M. Nelson Textbook of Pediatrics, 21st ed.; Elsevier: Philadelphia, MO, USA, 2019. [Google Scholar]
- Chun, C.; Weinmann, S.; Riedlinger, K.; Mullooly, J.P.; Houston, H.; Schmid, D.S.; Seward, J.F. Laboratory characteristics of suspected herpes zoster in vaccinated children. Pediatr. Infect. Dis. J. 2011, 30, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Guess, H.A.; Broughton, D.D.; Melton, L.J., 3rd; Kurland, L.T. Epidemiology of herpes zoster in children and adolescents: A population-based study. Pediatrics 1985, 76, 512–517. [Google Scholar] [PubMed]
- Leung, A.K.; Robson, W.L.; Leong, A.G. Herpes zoster in childhood. J. Pediatr. Health Care 2006, 20, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chao, Y.H.; Wu, K.H.; Yen, T.Y.; Hsu, Y.L.; Hsieh, T.H.; Wei, H.M.; Wu, J.L.; Muo, C.H.; Hwang, K.P.; et al. Increased risk of herpes zoster in children with cancer: A nationwide population-based cohort study. Medicine 2016, 95, e4037. [Google Scholar] [CrossRef] [PubMed]
- Aktas, H.; Erdal, S.A.; Guvenc, U. Herpes Zoster in children: Evaluation of the sixty cases. Dermatol. Ther. 2019, 32, e13087. [Google Scholar] [CrossRef]
- Na, G.Y. Herpes zoster in three healthy children immunized with varicella vaccine (Oka/Biken); the causative virus differed from vaccine strain on PCR analysis of the IV variable region (R5) and of a PstI-site region. Br. J. Dermatol. 1997, 137, 255–258. [Google Scholar]
- Liang, M.G.; Heidelberg, K.A.; Jacobson, R.M.; McEvoy, M.T. Herpes zoster after varicella immunization. J. Am. Acad. Dermatol. 1998, 38, 761–763. [Google Scholar] [CrossRef]
- Kohl, S.; Rapp, J.; La Russa, P.; Gershon, A.A.; Steinberg, S.P. Natural varicella-zoster virus reactivation shortly after varicella immunization in a child. Pediatr. Infect Dis. J. 1999, 18, 1112–1113. [Google Scholar] [CrossRef]
- Uebe, B.; Sauerbrei, A.; Burdach, S.; Horneff, G. Herpes zoster by reactivated vaccine varicella zoster virus in a healthy child. Eur. J. Pediatr. 2002, 161, 442–444. [Google Scholar] [CrossRef]
- Feder, H.M., Jr.; Hoss, D.M. Herpes zoster in otherwise healthy children. Pediatr. Infect. Dis. J. 2004, 451–457, 458–560. [Google Scholar] [CrossRef]
- Binder, N.R.; Holland, G.N.; Hosea, S.; Silverberg, M.L. Herpes zoster ophthalmicus in an otherwise-healthy child. J. AAPOS 2005, 9, 597–598. [Google Scholar] [CrossRef]
- Levin, M.J.; DeBiasi, R.L.; Bostik, V.; Schmid, D.S. Herpes zoster with skin lesions and meningitis caused by 2 different genotypes of the Oka varicella-zoster virus vaccine. J. Infect. Dis. 2008, 198, 1444–1447. [Google Scholar] [CrossRef]
- Obieta, M.P.; Jacinto, S.S. Herpes zoster after varicella vaccination in a healthy young child. Int. J. Dermatol. 2008, 47, 640–641. [Google Scholar] [CrossRef]
- Ota, K.; Kim, V.; Lavi, S.; Ford-Jones, E.L.; Tipples, G.; Scolnik, D.; Tellier, R. Vaccine-strain varicella zoster virus causing recurrent herpes zoster in an immunocompetent 2-year-old. Pediatr. Infect. Dis. J. 2008, 27, 847–848. [Google Scholar] [CrossRef]
- Iyer, S.; Mittal, M.K.; Hodinka, R.L. Herpes zoster and meningitis resulting from reactivation of varicella vaccine virus in an immunocompetent child. Ann. Emerg. Med. 2009, 53, 792–795. [Google Scholar] [CrossRef]
- Lin, P.; Yoon, M.K.; Chiu, C.S. Herpes zoster keratouveitis and inflammatory ocular hypertension 8 years after varicella vaccination. Ocul. Immunol. Inflamm. 2009, 17, 33–35. [Google Scholar] [CrossRef]
- Chouliaras, G.; Spoulou, V.; Quinlivan, M.; Breuer, J.; Theodoridou, M. Vaccine-associated herpes zoster ophthalmicus [correction of opthalmicus] and encephalitis in an immunocompetent child. Pediatrics 2010, 125, e969–e972. [Google Scholar] [CrossRef]
- Han, J.Y.; Hanson, D.C.; Way, S.S. Herpes zoster and meningitis due to reactivation of varicella vaccine virus in an immunocompetent child. Pediatr. Infect. Dis. J. 2011, 30, 266–268. [Google Scholar] [CrossRef]
- Esposito, S.; Bosis, S.; Pinzani, R.; Morlacchi, L.; Senatore, L.; Principi, N. A case of meningitis due to varicella zoster virus reactivation in an immunocompetent child. Ital. J. Pediatr. 2013, 39, 72. [Google Scholar] [CrossRef]
- Ryu, W.Y.; Kim, N.Y.; Kwon, Y.H.; Ahn, H.B. Herpes zoster ophthalmicus with isolated trochlear nerve palsy in an otherwise healthy 13-year-old girl. J. AAPOS 2014, 18, 193–195. [Google Scholar] [CrossRef]
- Iwasaki, S.; Motokura, K.; Honda, Y.; Mikami, M.; Hata, D.; Hata, A. Vaccine-strain herpes zoster found in the trigeminal nerve area in a healthy child: A case report. J. Clin. Virol. 2016, 85, 44–47. [Google Scholar] [CrossRef]
- Peterson, N.; Goodman, S.; Peterson, M.; Peterson, W. Herpes zoster in children. Cutis 2016, 98, 94–95. [Google Scholar]
- Dreyer, S.; Hemarajata, P.; Hogeling, M.; Henderson, G.P. Pediatric vaccine-strain herpes zoster: A case series. Pediatr. Dermatol. 2017, 34, 665–667. [Google Scholar] [CrossRef]
- Guffey, D.J.; Koch, S.B.; Bomar, L.; Huang, W.W. Herpes zoster following varicella vaccination in children. Cutis 2017, 99, 207–211. [Google Scholar]
- Oliveira, K.; Fonseca, J.; Moreira, D.; Vila Real, M. Varicella-zoster virus meningitis in an immunocompetent paediatric patient. Neurologia 2018, 33, 623–624. [Google Scholar] [CrossRef]
- Ashi, A.; Ali, A.; Alzahrani, M.; Ali, J.; Albar, R. Herpes Zoster Eruption in an Otherwise Healthy Child: A Case Report. Cureus 2019, 11, e5194. [Google Scholar] [CrossRef]
- Harrington, W.E.; Mato, S.; Burroughs, L.; Carpenter, P.A.; Gershon, A.; Schmid, D.S.; Englund, J.A. Vaccine Oka Varicella Meningitis in Two Adolescents. Pediatrics 2019, 144, e20191522. [Google Scholar] [CrossRef]
- Moodley, A.; Swanson, J.; Grose, C.; Bonthius, D.J. Severe Herpes Zoster Following Varicella Vaccination in Immunocompetent Young Children. J. Child Neurol. 2019, 34, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pelekouda, E.; Papagiannis, D.; Tsiaousi, I.; Maltezou, H.C. Herpes zoster after vaccination with one dose varicella vaccine to a 4-year-old child. Infez. Med. 2019, 27, 449–451. [Google Scholar] [PubMed]
- Plachouri, K.M.; Gkentzi, D.; Varvarigou, A.; Georgiou, S.; Dimitriou, G. Herpes Zoster Onset 9 Years after First Varicella Zoster Vaccination in an 11-year-old Child—A Case Report. Curr. Pediatr. Rev. 2019, 15, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, R.; Minami, K.; Ogawa, A.; Okada, H.; Terakawa, R.; Koike, Y.; Ogura, S.; Takeuchi, K.; Higuchi, T. Herpes zoster and meningitis in an immunocompetent child: A case report. J. Med. Case Rep. 2019, 13, 182. [Google Scholar] [CrossRef]
- Quesada, D.; Morsky, L.; Aguiniga-Navarrete, P.; Garrett, M.B. Pediatric Herpes Zoster. Clin. Pract. Cases Emerg. Med. 2020, 4, 32–34. [Google Scholar] [CrossRef]
- Heusel, E.H.; Grose, C. Twelve Children with Varicella Vaccine Meningitis: Neuropathogenesis of Reactivated Live Attenuated Varicella Vaccine Virus. Viruses 2020, 12, 1078. [Google Scholar] [CrossRef]
- Kangro, H.O.; Ward, A.; Argent, S.; Heath, R.B.; Cradock-Watson, J.E.; Ridehalgh, M.K. Detection of specific IgM in varicella and herpes zoster by antibody-capture radioimmunoassay. Epidemiol. Infect. 1988, 101, 187–195. [Google Scholar] [CrossRef]
- Min, S.W.; Kim, Y.S.; Nahm, F.S.; Yoo, D.H.; Choi, E.; Lee, P.B.; Choo, H.; Park, Z.Y.; Yang, C.S. The positive duration of varicella zoster immunoglobulin M antibody test in herpes zoster. Medicine 2016, 95, e4616. [Google Scholar] [CrossRef]
- Körholz, J.; Richter, N.; Schäfer, J.; Schuetz, C.; Roesler, J. A case of recurrent herpes simplex 2 encephalitis, VZV reactivations, and dominant partial interferon-gamma-receptor-1 deficiency supports relevance of IFNgamma for antiviral defense in humans. Mol. Cell Pediatr. 2020, 7, 14. [Google Scholar] [CrossRef]
- Dorman, S.E.; Picard, C.; Lammas, D.; Heyne, K.; van Dissel, J.T.; Baretto, R.; Rosenzweig, S.D.; Newport, M.; Levin, M.; Roesler, J.; et al. Clinical features of dominant and recessive interferon gamma receptor 1 deficiencies. Lancet 2004, 364, 2113–2121. [Google Scholar] [CrossRef]
| Reference | Age | Sex | Age at Vaccination | Varicella History | Interval between Vaccination and HZ | Dermatomes/Regions with Lesions | VZV Strain | IgM | IgG |
|---|---|---|---|---|---|---|---|---|---|
| Na, G. Y. et al. (1997) | 4 y 10 m | M | 2 y | No | 2 y 10 m | R’t S3 | Wild type | - | + |
| 3 y 1 m | F | 1 y 5 m | No | 1 y 8 m | L’t S3–4 | Wild type | - | + | |
| 5 y 5 m | M | 3 y 5 m | No | 2 y | R’t T2 | Wild type | - | ND | |
| 2 y 6 m | F | 1 y | No | 1 y 6 m | L’t T10 | ND | - | + | |
| 3 y 9 m | F | 2 y 5 m | No | 1 y 3 m | R’t L3–4 | ND | - | + | |
| 4 y 2 m | M | 2 y 4 m | No | 1 y 10 m | R’t S1–2 | ND | - | + | |
| Liang, M. G. et al. (1998) | 1 y 7 m | F | 1 y 3 m | Yes (7 m) | 4 m | L’t C6–7 | Vaccine | ND | ND |
| Kohl, S. et al. (1999) | 6 y | M | 6 y | No | 12 d | L’t T2 | Wild type | ND | ND |
| Uebe, B. et al. (2002) | 2 y 3 m | F | 11 m | No; contact history with her sister with varicella | 1 y 4 m | R’t C6–C8 | Vaccine | - | + |
| Feder, H. M. et al. (2004) | 6 y | F | N/A | Yes (3 m) | N/A | R’t V2–3 | ND | ND | ND |
| 3 y | F | N/A | Yes (6 m) | N/A | L’t L1–2 | ND | ND | ND | |
| 8 y | F | N/A | Yes (7 y) | N/A | R’t T6 | ND | ND | ND | |
| 3 y | M | 1 y | Yes (6 m) | 2 y | L’t V1 | ND | ND | ND | |
| 5y | F | 1 y 3 m | N/A | 3 y 9 m | L’t L2 | Wild type | ND | ND | |
| Ota, K. et al. (2008) | 2 y 4 m | M | 1 y 1 m | N/A | 1 y 3 m | L’t chest and upper limb | Vaccine | ND | + |
| Levin, M. J. et al. (2008) | 8 y | M | 1 y | No | 7 y | R’t shoulder and meningitis | Vaccine (skin and CSF) | + | + |
| Iyer, S. et al. (2009) | 9 y | M | 1 y | No | 8 y | L’t C5–6 & meningitis | Vaccine (Skin and CSF) | ND | ND |
| Chouliaras G. et al. (2010) | 3 y6 m | F | 1 y 8 m | No; contact history at 1 y 3 m | 1 y 10 m | R’t V1 and encephalitis | Vaccine (CSF) | ND | ND |
| Han, J. Y. et al. (2011) | 7 y | M | 1 y | No | 6 y | R’t arm and meningitis | Vaccine (Skin and CSF) | ND | ND |
| Iwasaki, S. et al. (2016) | 2 y | F | 1 y 5 m | No | 7 m | L’t V1–2 | Vaccine | + | + |
| Dreyer, S. et al. (2017) | 3 y | M | 1 y | N/A | 2 y | R’t L2 | Vaccine | ND | ND |
| 2 y | F | 1 y | No | 1 y | L’t L4 | Vaccine | ND | ND | |
| Moodley, A. et al. (2018) | 3 y 3 m | M | 1 y 8 m | No | 1 y 7 m | L’t L4-S1 | Wild type variant of vaccine | ND | ND |
| 1 y 8 m | M | 1y 1 m | No | 7 m | R’t L3 | ND | + | + | |
| 3 y 6 m | M | 1 y | No | 2 y 6 m | R’t thigh | ND | N/A | N/A | |
| Pelekouda, E. et al. (2019) | 4 y | F | 1 y 3 m | No | 2 y 9 m | R’t C4–5 & T1 | ND | - | ND |
| Yasuda, R. et al. (2019) | 11 y | F | N/A | Yes (2 y) | N/A | L’t chest and meningitis | ND | - | + |
| Harrington, W. E. et al. (2019) | 14 y | M | 1 y and 4 y | No | ? | L’t L1–2 and meningitis | Vaccine (Skin and CSF) | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, B.-S.; Hung, C.-J.J.; Lue, K.-H. Herpes Zoster in an Immunocompetent Child without a History of Varicella. Pediatr. Rep. 2021, 13, 162-167. https://doi.org/10.3390/pediatric13020022
Shang B-S, Hung C-JJ, Lue K-H. Herpes Zoster in an Immunocompetent Child without a History of Varicella. Pediatric Reports. 2021; 13(2):162-167. https://doi.org/10.3390/pediatric13020022
Chicago/Turabian StyleShang, Bing-Shiau, Cheng-Jui Jamie Hung, and Ko-Huang Lue. 2021. "Herpes Zoster in an Immunocompetent Child without a History of Varicella" Pediatric Reports 13, no. 2: 162-167. https://doi.org/10.3390/pediatric13020022
APA StyleShang, B.-S., Hung, C.-J. J., & Lue, K.-H. (2021). Herpes Zoster in an Immunocompetent Child without a History of Varicella. Pediatric Reports, 13(2), 162-167. https://doi.org/10.3390/pediatric13020022








