Comparative Virulence Gene Profiling of Campylobacter jejuni and Campylobacter coli Isolates from Avian and Human Sources in Egypt
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Bacterial Isolation
2.2. Molecular Typing and Virulence Gene Detection of Campylobacter Species
2.3. Gene Expression of Seven Campylobacter Virulence Genes in Chicken Samples Versus Human Samples
2.4. Analysis of the SYBR Green RT-PCR for Gene Expression
2.5. Statistical Analysis
2.6. Ethical Statement
3. Results
3.1. Isolation and Molecular Typing of Campylobacter Species
3.2. Prevalence of Campylobacter Virulence Genes and tetO Gene
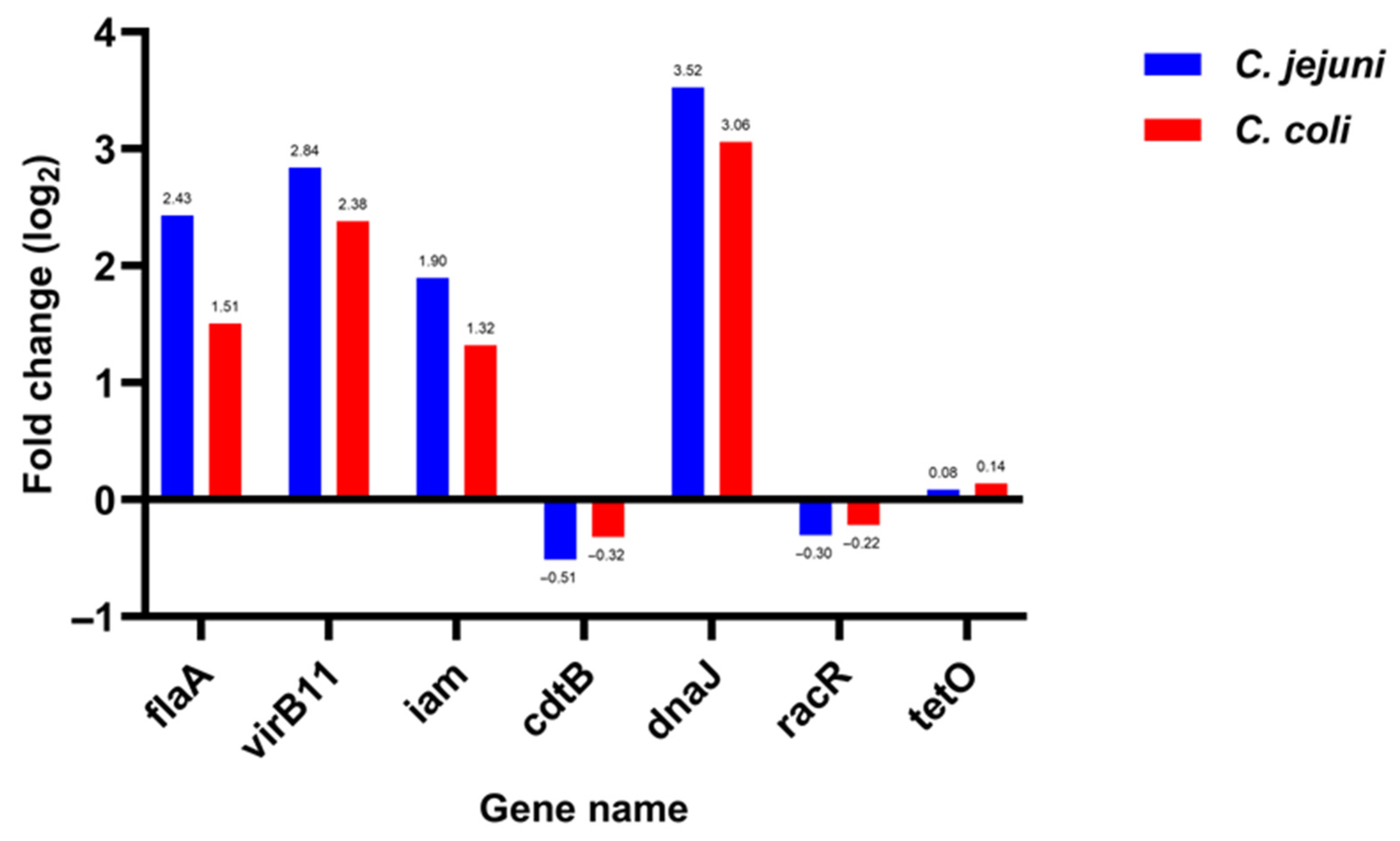
3.3. Expression of Seven Campylobacter Virulence Genes in Chicken Samples Versus Human Samples
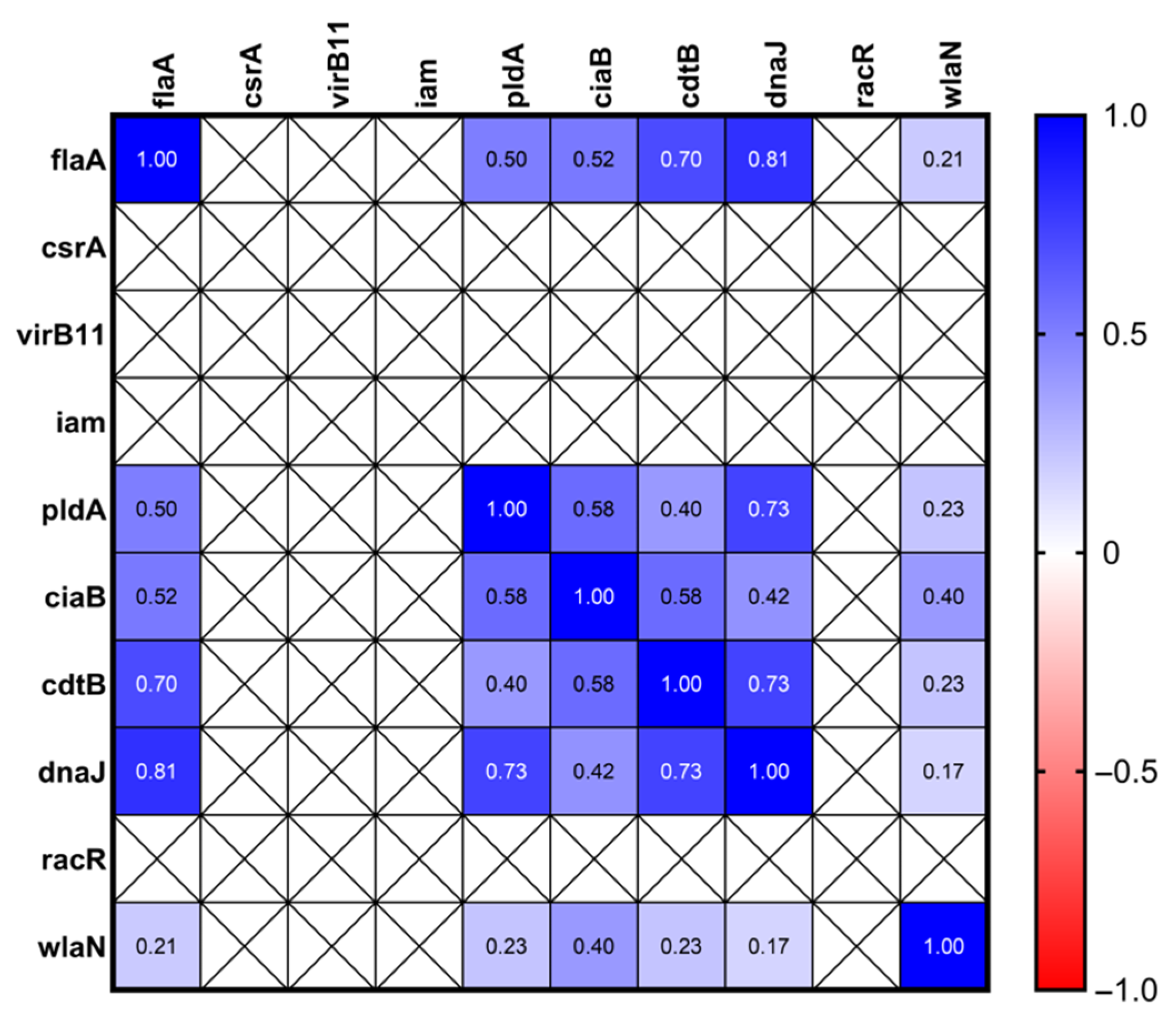
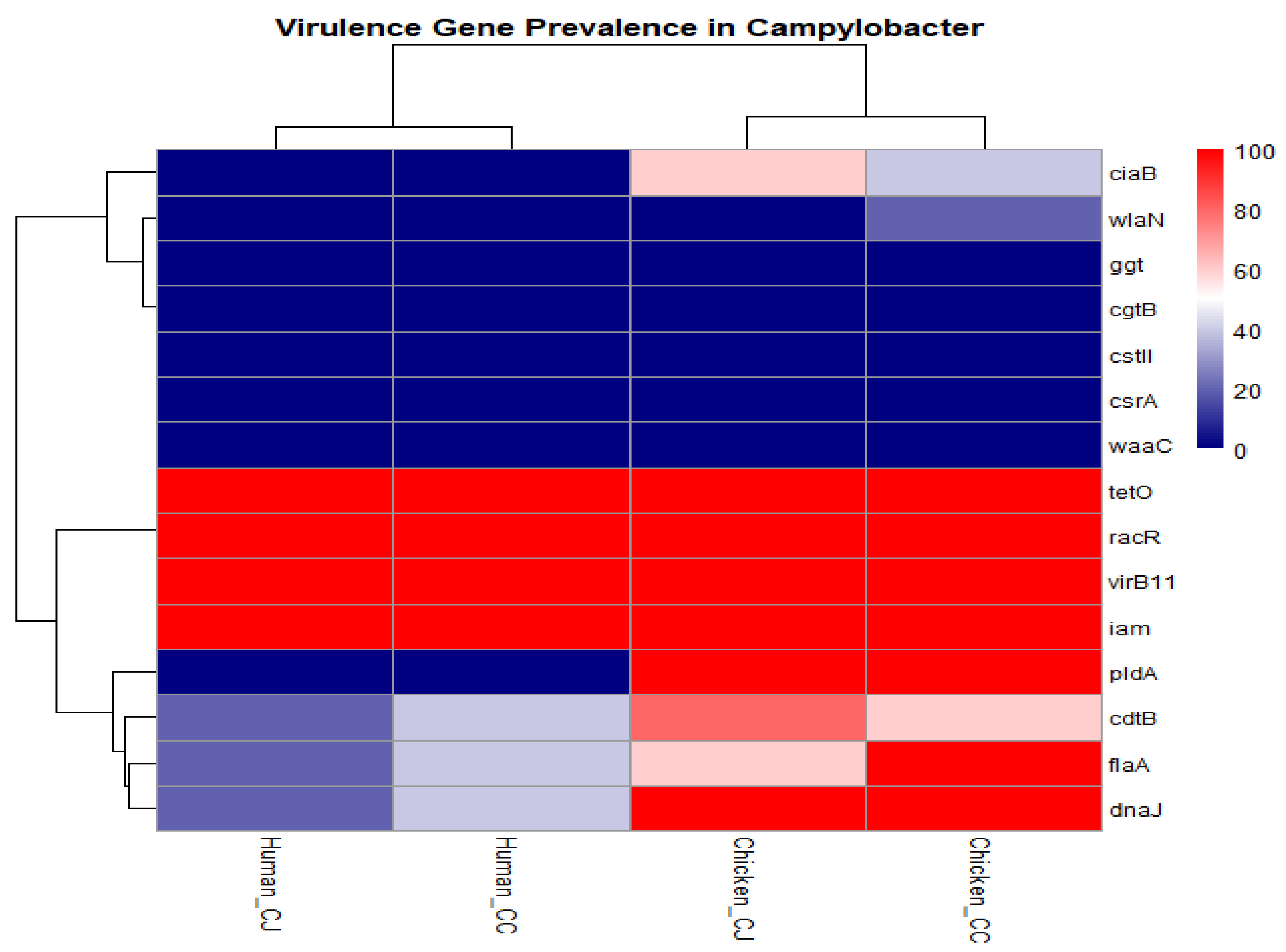
3.4. Hierarchical Clustering Heatmap of Prevalence of 15 Virulence Genes in Different Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: A data synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Khoshbakht, R.; Tabatabaei, M.; Shirzad Aski, H.; Hosseinzadeh, S. Occurrence of virulence genes and strain diversity of thermophilic campylobacters isolated from cattle and sheep faecal samples. Iran. J. Vet. Res. 2014, 15, 138–144. [Google Scholar]
- OIE. Chapter 2.9.3. Infection with Campylobacter jejuni and C. coli. In OIE Terrestrial Manual; WOAH: Paris, France, 2024. [Google Scholar]
- Noormohamed, A.; Fakhr, M.K. Prevalence and antimicrobial susceptibility of Campylobacter spp. in Oklahoma conventional and organic retail poultry. Open Microbiol. J. 2014, 8, 130–137. [Google Scholar] [CrossRef][Green Version]
- Sierra-Arguello, Y.M.; Perdoncini, G.; Rodrigues, L.B.; dos Santos, L.R.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.d.S.; Gomes, M.J.P.; Nascimento, V.P.D. Identification of pathogenic genes in Campylobacter jejuni isolated from broiler carcasses and broiler slaughterhouses. Sci. Rep. 2021, 11, 4588. [Google Scholar] [CrossRef]
- Fields, J.A.; Li, J.; Gulbronson, C.J.; Hendrixson, D.R.; Thompson, S.A. Campylobacter jejuni CsrA Regulates Metabolic and Virulence Associated Proteins and Is Necessary for Mouse Colonization. PLoS ONE 2016, 11, e0156932. [Google Scholar] [CrossRef] [PubMed]
- Bacon, D.J.; Alm, R.A.; Burr, D.H.; Hu, L.; Kopecko, D.J.; Ewing, C.P.; Guerry, P. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect. Immun. 2000, 68, 4384–4390. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, M.; Grudlewska-Buda, K.; Śpica, D.; Skowron, K.; Ćwiklińska-Jurkowska, M.; Szady-Grad, M.; Indykiewicz, P.; Wiktorczyk-Kapischke, N.; Klawe, J.J. Genetic relatedness, virulence, and drug susceptibility of Campylobacter isolated from water and wild birds. Front. Cell. Infect. Microbiol. 2022, 12, 1005085. [Google Scholar] [CrossRef]
- Datta, S.; Niwa, H.; Itoh, K. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 2003, 52, 345–348. [Google Scholar] [CrossRef]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The biology of the cytolethal distending toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef]
- Grant, K.A.; Belandia, I.U.; Dekker, N.; Richardson, P.T.; Park, S.F. Molecular characterization of pldA, the structural gene for a phospholipase A from Campylobacter coli, and its contribution to cell-associated hemolysis. Infect. Immun. 1997, 65, 1172–1180. [Google Scholar] [CrossRef]
- Konkel, M.E.; Klena, J.D.; Rivera-Amill, V.; Monteville, M.R.; Biswas, D.; Raphael, B.; Mickelson, J. Secretion of virulence proteins from Campylobacter jejuni is dependent on a functional flagellar export apparatus. J. Bacteriol. 2004, 186, 3296–3303. [Google Scholar] [CrossRef]
- Chansiripornchai, N.; Sasipreeyajan, J. PCR detection of four virulence-associated genes of Campylobacter jejuni isolates from Thai broilers and their abilities of adhesion to and invasion of INT-407 cells. J. Vet. Med. Sci. 2009, 71, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Brás, A.M.; Chatterjee, S.; Wren, B.W.; Newell, D.G.; Ketley, J.M. A novel Campylobacter jejuni two-component regulatory system important for temperature-dependent growth and colonization. J. Bacteriol. 1999, 181, 3298–3302. [Google Scholar] [CrossRef] [PubMed]
- Louwen, R.; Heikema, A.; van Belkum, A.; Ott, A.; Gilbert, M.; Ang, W.; Endtz, H.P.; Bergman, M.P.; Nieuwenhuis, E.E. The sialylated lipooligosaccharide outer core in Campylobacter jejuni is an important determinant for epithelial cell invasion. Infect. Immun. 2008, 76, 4431–4438. [Google Scholar] [CrossRef]
- Barnes, I.H.; Bagnall, M.C.; Browning, D.D.; Thompson, S.A.; Manning, G.; Newell, D.G. Gamma-glutamyl transpeptidase has a role in the persistent colonization of the avian gut by Campylobacter jejuni. Microb. Pathog. 2007, 43, 198–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rizal, A.; Kumar, A.; Vidyarthi, A.S. Prevalence of pathogenic genes in Campylobacter jejuni isolated from poultry and human. Internet J. Food Saf. 2010, 12, 29–34. [Google Scholar]
- Awad, A.; Yeh, H.Y.; Ramadan, H.; Rothrock, M.J. Genotypic characterization, antimicrobial susceptibility and virulence determinants of Campylobacter jejuni and Campylobacter coli isolated from pastured poultry farms. Front. Microbiol. 2023, 14, 1271551. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Abd El-Aziz, N.K.; Samir, M.; El-Naenaeey, E.-s.Y.; Abo Remela, E.M.; Mosbah, R.A.; Bendary, M.M. Genetic diversity of Campylobacter jejuni isolated from avian and human sources in Egypt. Front. Microbiol. 2019, 10, 2353. [Google Scholar] [CrossRef]
- Mouftah, S.F.; Pascoe, B.; Calland, J.K.; Mourkas, E.; Tonkin, N.; Lefevre, C.; Deuker, D.; Smith, S.; Wickenden, H.; Hitchings, M.D. Local accessory gene sharing among Egyptian Campylobacter potentially promotes the spread of antimicrobial resistance. Microb. Genom. 2022, 8, 834. [Google Scholar] [CrossRef]
- Sainato, R.; ElGendy, A.; Poly, F.; Kuroiwa, J.; Guerry, P.; Riddle, M.S.; Porter, C.K. Epidemiology of Campylobacter Infections among Children in Egypt. Am. J. Trop. Med. Hyg. 2018, 98, 581–585. [Google Scholar] [CrossRef]
- Steele, T.W.; McDermott, S.N. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 1984, 16, 263–265. [Google Scholar] [CrossRef]
- Barakat, A.M.A.; El-Razik, K.A.A.; Elfadaly, H.A.; Rabie, N.S.; Sadek, S.A.S.; Almuzaini, A.M. Prevalence, molecular detection, and virulence gene profiles of Campylobacter species in humans and foods of animal origin. Vet. World 2020, 13, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Elbrissi, A.; Sabeil, Y.A.; Khalifa, K.A.; Enan, K.; Khair, O.M.; El Hussein, A.M. Isolation, identification and differentiation of Campylobacter spp. using multiplex PCR assay from goats in Khartoum State, Sudan. Trop. Anim. Health Prod. 2017, 49, 575–581. [Google Scholar] [CrossRef]
- Gonzalez, I.; Grant, K.A.; Richardson, P.T.; Park, S.F.; Collins, M.D. Specific identification of the enteropathogens Campylobacter jejuni and Campylobacter coli by using a PCR test based on the ceuE gene encoding a putative virulence determinant. J. Clin. Microbiol. 1997, 35, 759–763. [Google Scholar] [CrossRef]
- Shin, E.; Lee, Y. Comparison of three different methods for Campylobacter isolation from porcine intestines. J. Microbiol. Biotechnol. 2009, 19, 647–650. [Google Scholar]
- Wang, G.; Clark, C.G.; Taylor, T.M.; Pucknell, C.; Barton, C.; Price, L.; Woodward, D.L.; Rodgers, F.G. Colony multiplex PCR assay for identification and differentiation of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus subsp. fetus. J. Clin. Microbiol. 2002, 40, 4744–4747. [Google Scholar] [CrossRef]
- Zheng, J.; Meng, J.; Zhao, S.; Singh, R.; Song, W. Adherence to and invasion of human intestinal epithelial cells by Campylobacter jejuni and Campylobacter coli isolates from retail meat products. J. Food Prot. 2006, 69, 768–774. [Google Scholar] [CrossRef]
- González-Hein, G.; Huaracán, B.; García, P.; Figueroa, G. Prevalence of virulence genes in strains of Campylobacter jejuni isolated from human, bovine and broiler. Braz. J. Microbiol. 2013, 44, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Pratt, A.; Korolik, V. Tetracycline resistance of Australian Campylobacter jejuni and Campylobacter coli isolates. J. Antimicrob. Chemother. 2005, 55, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Mendoza, D.; Martínez-Flores, I.; Santamaría, R.I.; Lozano, L.; Bustamante, V.H.; Pérez-Morales, D. Genomic analysis reveals the genetic determinants associated with antibiotic resistance in the zoonotic pathogen Campylobacter spp. Distributed Globally. Front. Microbiol. 2020, 11, 513070. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Y.; Huang, K.; Zhu, C.; Yin, Y. Application of real-time PCR for quantitative detection of Campylobacter jejuni in poultry, milk and environmental water. FEMS Immunol. Med. Microbiol. 2003, 38, 265–271. [Google Scholar] [CrossRef]
- Thibodeau, A.; Fravalo, P.; Yergeau, É.; Arsenault, J.; Lahaye, L.; Letellier, A. Chicken caecal microbiome modifications induced by Campylobacter jejuni colonization and by a non-antibiotic feed additive. PLoS ONE 2015, 10, e0131978. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Zishiri, O.T. Genetic characterisation of virulence genes associated with adherence, invasion and cytotoxicity in Campylobacter spp. isolated from commercial chickens and human clinical cases. Onderstepoort J. Vet. Res. 2018, 85, e1–e9. [Google Scholar] [CrossRef]
- Kinga Wieczorek, J.O. Molecular characterization of Campylobacter spp. isolated from poultry faeces and carcasses in Poland. Acta Vet. Brno 2011, 80, 19–27. [Google Scholar] [CrossRef]
- Guerry, P. Campylobacter flagella: Not just for motility. Trends Microbiol. 2007, 15, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.M.; Prioleau, M.D.; Borchardt, M.A.; Hynds, P.D. Epidemiological evidence of groundwater contribution to global enteric disease, 1948–2015. Hydrogeol. J. 2017, 25, 981–1001. [Google Scholar] [CrossRef]
- Cho, H.-H.; Kim, S.-H.; Min, W.; Ku, B.-K.; Kim, Y.-H. Prevalence of virulence and cytolethal distending toxin (CDT) genes in thermophilic Campylobacter spp. from dogs and humans in Gyeongnam and Busan, Korea. Korean J. Vet. Res. 2014, 54, 39–48. [Google Scholar] [CrossRef]
- El-Adawy, H.; Hotzel, H.; Tomaso, H.; Neubauer, H.; Taboada, E.N.; Ehricht, R.; Hafez, H.M. Detection of genetic diversity in Campylobacter jejuni isolated from a commercial turkey flock using flaA typing, MLST analysis and microarray assay. PLoS ONE 2013, 8, e51582. [Google Scholar] [CrossRef]
- Lima, L.M.; Perdoncini, G.; Borges, K.A.; Furian, T.Q.; Salle, C.T.P.; Moraes, H.L.D.S.; Nascimento, V.P.D. Prevalence and distribution of pathogenic genes in Campylobacter jejuni isolated from poultry and human sources. J. Infect. Dev. Ctries. 2022, 16, 1466–1472. [Google Scholar] [CrossRef]
- Stintzi, A. Gene expression profile of Campylobacter jejuni in response to growth temperature variation. J. Bacteriol. 2003, 185, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.A.; Thompson, S.A. Campylobacter jejuni CsrA Mediates Oxidative Stress Responses, Biofilm Formation, and Host Cell Invasion. J. Bacteriol. 2008, 190, 3411–3416. [Google Scholar] [CrossRef]
- Fields, J.A.; Thompson, S.A. Campylobacter jejuni CsrA complements an Escherichia coli csrA mutation for the regulation of biofilm formation, motility and cellular morphology but not glycogen accumulation. BMC Microbiol. 2012, 12, 233. [Google Scholar] [CrossRef]
- Otigbu, A.C.; Clarke, A.M.; Fri, J.; Akanbi, E.O.; Njom, H.A. Antibiotic sensitivity profiling and virulence potential of Campylobacter jejuni isolates from estuarine water in the Eastern Cape Province, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 925. [Google Scholar] [CrossRef] [PubMed]
- Aljazzar, A.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; El-Gharreb, W.R.; Abdel-Raheem, S.M.; Ibrahim, A.M.; Abdelaziz, A.M.; Ibrahim, D. Prevalence and Antimicrobial Susceptibility of Campylobacter Species with Particular Focus on the Growth Promoting, Immunostimulant and Anti-Campylobacter jejuni Activities of Eugenol and Trans-Cinnamaldehyde Mixture in Broiler Chickens. Animals 2022, 12, 905. [Google Scholar] [CrossRef]
- Wieczorek, K.; Osek, J. Identification of virulence genes in Campylobacter jejuni and C. coli isolates by PCR. Bull. Vet. Inst. Puławy 2008, 52, 211–216. [Google Scholar]
- Krutkiewicz, A.; Klimuszko, D. Genotyping and PCR detection of potential virulence genes in Campylobacter jejuni and Campylobacter coli isolates from different sources in Poland. Folia Microbiol. 2010, 55, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Jribi, H.; Sellami, H.; Hassena, A.B.; Gdoura, R. Prevalence of putative virulence genes in Campylobacter and Arcobacter species isolated from poultry and poultry by-products in Tunisia. J. Food Prot. 2017, 80, 1705–1710. [Google Scholar] [CrossRef]
- Biswas, D.; Hannon, S.J.; Townsend, H.; Potter, A.; Allan, B.J. Genes coding for virulence determinants of Campylobacter jejuni in human clinical and cattle isolates from Alberta, Canada, and their potential role in colonization of poultry. Int. Microbiol. 2011, 14, 25–32. [Google Scholar]
- Frazão, M.R.; Medeiros, M.I.C.; da Silva Duque, S.; Falcão, J.P. Pathogenic potential and genotypic diversity of Campylobacter jejuni: A neglected food-borne pathogen in Brazil. J. Med. Microbiol. 2017, 66, 350–359. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Ruiz-Palacios, G.M.; Ramos-Cervantes, P.; Cervantes, L.-E.; Jiang, X.; Pickering, L.K. Molecular characterization of invasive and noninvasive Campylobacter jejuni and Campylobacter coli isolates. J. Clin. Microbiol. 2001, 39, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Atkinson, G.C.; Thakor, N.S.; Allas, U.; Lu, C.C.; Chan, K.Y.; Tenson, T.; Schulten, K.; Wilson, K.S.; Hauryliuk, V.; et al. Mechanism of tetracycline resistance by ribosomal protection protein Tet(O). Nat. Commun. 2013, 4, 1477. [Google Scholar] [CrossRef]
- Hassanain, N.A. Antimicrobial resistant Campylobacter jejuni isolated from humans and animals in Egypt. Glob. Vet. 2011, 6, 195–200. [Google Scholar]
- Bardoň, J.; Pudová, V.; Koláčková, I.; Karpíšková, R.; Röderová, M.; Kolář, M. Virulence and antibiotic resistance genes in Campylobacter spp. in the Czech Republic. Epidemiol. Mikrobiol. Imunol. 2017, 66, 59–66. [Google Scholar]
- Godschalk, P.C.; van Belkum, A.; van den Braak, N.; van Netten, D.; Ang, C.W.; Jacobs, B.C.; Gilbert, M.; Endtz, H.P. PCR-restriction fragment length polymorphism analysis of Campylobacter jejuni genes involved in lipooligosaccharide biosynthesis identifies putative molecular markers for Guillain-Barré syndrome. J. Clin. Microbiol. 2007, 45, 2316–2320. [Google Scholar] [CrossRef]
- Kordinas, V.; Nicolaou, C.; Ioannidis, A.; Papavasileiou, E.; John Legakis, N.; Chatzipanagiotou, S. Prevalence of four virulence genes in Campylobacter jejuni determined by PCR and sequence analysis. Mol. Diagn. 2005, 9, 211–215. [Google Scholar] [CrossRef]
- Linton, D.; Gilbert, M.; Hitchen, P.G.; Dell, A.; Morris, H.R.; Wakarchuk, W.W.; Gregson, N.A.; Wren, B.W. Phase variation of a β-1, 3 galactosyltransferase involved in generation of the ganglioside GM1-like lipo-oligosaccharide of Campylobacter jejuni. Mol. Microbiol. 2000, 37, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Nahar, N.; Rashid, R. Genotypic Analysis of the Virulence and Antibiotic Resistance Genes in Campylobacter species in silico. J. Bioanal. Biomed. 2018, 10, 13–23. [Google Scholar] [CrossRef]
- Talukder, K.A.; Aslam, M.; Islam, Z.; Azmi, I.J.; Dutta, D.K.; Hossain, S.; Nur-E-Kamal, A.; Nair, G.B.; Cravioto, A.; Sack, D.A. Prevalence of virulence genes and cytolethal distending toxin production in Campylobacter jejuni isolates from diarrheal patients in Bangladesh. J. Clin. Microbiol. 2008, 46, 1485–1488. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, A.; Tracz, D.M.; Nonaka, L.; Ngo, T.M.; Connell, S.R.; Taylor, D.E. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet (O)-mediated tetracycline resistance. Antimicrob. Agents Chemother. 2004, 48, 3442–3450. [Google Scholar] [CrossRef]
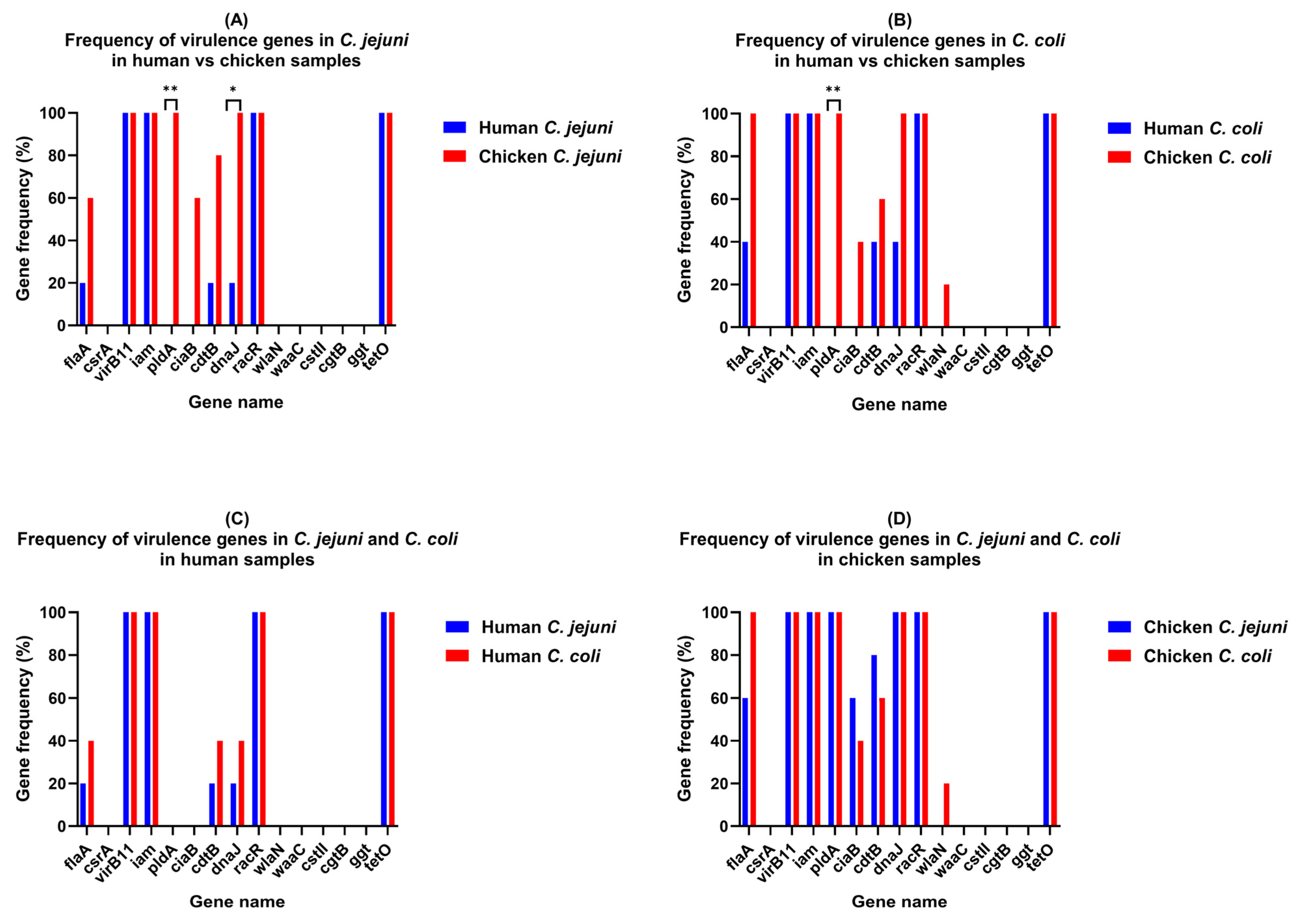
| Gene | C. coli (%) | p-Value | C. jejuni (%) | p-Value | ||
|---|---|---|---|---|---|---|
| Human | Chicken | Human | Chicken | |||
| flaA | 2 (40) | 5 (100) | 0.1667 | 1 (20) | 3 (60) | 0.5238 |
| csrA | 0 | 0 | >0.9999 | 0 | 0 | >0.9999 |
| virB11 | 5 (100) | 5 (100) | >0.9999 | 5 (100) | 5 (100) | >0.9999 |
| iam | 5 (100) | 5 (100) | >0.9999 | 5 (100) | 5 (100) | >0.9999 |
| pldA | 0 | 5 (100) | 0.0079 | 0 | 5 (100) | 0.0079 |
| ciaB | 0 | 2 (40) | 0.4444 | 0 | 3 (60) | 0.1667 |
| cdtB | 2 (40) | 3 (60) | >0.9999 | 1 (20) | 4 (80) | 0.2063 |
| dnaJ | 2 (40) | 5 (100) | 0.1667 | 1 (20) | 5 (100) | 0.0476 |
| racR | 5 (100) | 5 (100) | >0.9999 | 5 (100) | 5 (100) | >0.9999 |
| wlaN | 0 | 1 (20) | >0.9999 | 0 | 0 | >0.9999 |
| waaC | 0 | 0 | >0.9999 | 0 | 0 | >0.9999 |
| cstII | 0 | 0 | >0.9999 | 0 | 0 | >0.9999 |
| cgtB | 0 | 0 | >0.9999 | 0 | 0 | >0.9999 |
| ggt | 0 | 0 | >0.9999 | 0 | 0 | >0.9999 |
| tetO | 5 (100) | 5 (100) | >0.9999 | 5 (100) | 5 (100) | >0.9999 |
| Gene | C. jejuni | C. coli |
|---|---|---|
| flaA | 2.43 | 1.51 |
| virB11 | 2.84 | 2.38 |
| iam | 1.9 | 1.32 |
| cdtB | −0.51 | −0.32 |
| dnaJ | 3.52 | 3.06 |
| racR | −0.3 | −0.22 |
| tetO | 0.08 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekky, A.; Issa, M.R.; Hashish, A.; Hassan, W.; Wahdan, A.; Hisham, I.; Enany, S.; Enany, M. Comparative Virulence Gene Profiling of Campylobacter jejuni and Campylobacter coli Isolates from Avian and Human Sources in Egypt. Microbiol. Res. 2025, 16, 209. https://doi.org/10.3390/microbiolres16090209
Mekky A, Issa MR, Hashish A, Hassan W, Wahdan A, Hisham I, Enany S, Enany M. Comparative Virulence Gene Profiling of Campylobacter jejuni and Campylobacter coli Isolates from Avian and Human Sources in Egypt. Microbiology Research. 2025; 16(9):209. https://doi.org/10.3390/microbiolres16090209
Chicago/Turabian StyleMekky, Amr, Mohamed R. Issa, Amro Hashish, Wafaa Hassan, Ali Wahdan, Islam Hisham, Shymaa Enany, and Mohamed Enany. 2025. "Comparative Virulence Gene Profiling of Campylobacter jejuni and Campylobacter coli Isolates from Avian and Human Sources in Egypt" Microbiology Research 16, no. 9: 209. https://doi.org/10.3390/microbiolres16090209
APA StyleMekky, A., Issa, M. R., Hashish, A., Hassan, W., Wahdan, A., Hisham, I., Enany, S., & Enany, M. (2025). Comparative Virulence Gene Profiling of Campylobacter jejuni and Campylobacter coli Isolates from Avian and Human Sources in Egypt. Microbiology Research, 16(9), 209. https://doi.org/10.3390/microbiolres16090209






