Herd-Level Prevalence of Hepatitis E Virus in Greek Pig Farms
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. HEV RNA Real-Time PCR Detection
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrik, J.; Lozano, M.; Seed, C.R.; Faddy, H.M.; Keller, A.J.; Scuracchio, P.S.P.; Wendel, S.; Andonov, A.; Fearon, M.; Delage, G.; et al. Hepatitis E. Vox Sang. 2016, 110, 93–130. [Google Scholar] [CrossRef]
- Perez-Gracia, M.T.; García, M.; Suay, B.; Mateos-Lindemann, M.L. Current knowledge on hepatitis E. J. Clin. Transl. Hepatol. 2015, 3, 117–126. [Google Scholar] [CrossRef]
- Kumar, S.; Subhadra, S.; Singh, B.; Panda, B.K. Hepatitis E virus: The current scenario. Int. J. Infect. Dis. 2013, 17, 228–233. [Google Scholar] [CrossRef]
- Ahmad, T.; Jin, H.; Dhama, K.; Yatoo, M.I.; Tiwari, R.; Bilal, M.; Dhawan, M.; Emran, T.B.; Alestad, J.H.; Alhani, H.M.; et al. Hepatitis E virus in pigs and the environment: An updated review of public health concerns. Narra J. 2022, 2, 78. [Google Scholar] [CrossRef]
- Izopet, J.; Xia, N. Hepatitis E Vaccines. In Plotkin’s Vaccines, 8th ed.; Orenstein, W., Offit, P., Edwards, K.M., Plotkin, S., Eds.; Elsevier: Philadelphia, PA, USA, 2023; Chapter 29; ISBN 9780323790581. [Google Scholar]
- Purdy, M.A.; Drexler, J.F.; Meng, X.-J.; Norder, H.; Okamoto, H.; Van Der Poel, W.H.M.; Reuter, G.; De Souza, W.M.; Ulrich, R.G.; Smith, D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 001778. [Google Scholar] [CrossRef]
- Prpić, J.; Baymakova, M. Hepatitis E Virus (HEV) Infection among Humans and Animals: Epidemiology, Clinical Characteristics, Treatment, and Prevention. Pathogens 2023, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Emerson, S.U.; Nguyen, H.; Torian, U.; Purcell, R.H. ORF3 Protein of Hepatitis E Virus Is Not Required for Replication, Virion Assembly, or Infection of Hepatoma Cells In Vitro. J. Virol. 2006, 80, 10457–10464. [Google Scholar] [CrossRef]
- Sooryanarain, H.; Meng, X.J. Swine hepatitis E virus: Cross-species infection, pork safety and chronic infection. Virus Res. 2020, 284, 197985. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Ratho, R.K.; Kumar, S.; Saxena, S.K.; Bora, I.; Thakur, P. Viral hepatitis E and chronicity: A growing public health concern. Front. Microbiol. 2020, 11, 577339. [Google Scholar] [CrossRef]
- Hakim, M.S.; Wang, W.; Bramer, W.M.; Geng, J.; Huang, F.; de Man, R.A.; Peppelenbosch, M.P.; Pan, Q. The global burden of hepatitis E outbreaks: A systematic review. Liver Int. 2017, 37, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Carratalà, A.; Joost, S. Population density and water balance influence the global occurrence of hepatitis E epidemics. Sci. Rep. 2019, 9, 10042. [Google Scholar] [CrossRef] [PubMed]
- Kamani, L.; Padhani, Z.A.; Das, J.K. Hepatitis E: Genotypes, Strategies to Prevent and Manage, and the Existing Knowledge Gaps. JGH Open 2021, 5, 1127–1134. [Google Scholar] [CrossRef]
- Di Profio, F.; Sarchese, V.; Palombieri, A.; Fruci, P.; Lanave, G.; Robetto, S.; Martella, V.; Di Martino, B. Current Knowledge of Hepatitis E Virus (HEV) Epidemiology in Ruminants. Pathogens 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Teng, J.L.L.; Chiu, T.H.; Lau, S.K.P.; Woo, P.C.Y. Hepatitis E virus genotypes and evolution: Emergence of camel hepatitis E variants. Int. J. Mol. Sci. 2017, 18, 869. [Google Scholar] [CrossRef] [PubMed]
- Primadharsini, P.P.; Nagashima, S.; Okamoto, H. Genetic variability and evolution of hepatitis E virus. Viruses 2019, 11, 456. [Google Scholar] [CrossRef]
- Monini, M.; Di Bartolo, I.; De Sabato, L.; Ianiro, G.; Agostinelli, F.; Ostanello, F. Hepatitis E Virus (HEV) in Heavy Pigs in Slaughterhouses of Northern Italy: Investigation of Seroprevalence, Viraemia, and Faecal Shedding. Animals 2023, 13, 2942. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Cao, K.-Y.; Wernery, U.; Schountz, T.; Chiu, T.H.; Tsang, A.K.L.; Wong, P.-C.; Wong, E.Y.M.; et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg. Infect. Dis. 2016, 22, 2219–2221. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tsang, A.K.L.; Joseph, M.; Wong, E.Y.M.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R.; et al. New Hepatitis E Virus Genotype in Camels, the Middle East. Emerg. Infect. Dis. 2014, 20, 1044–1048. [Google Scholar] [CrossRef]
- Proietto, S.; Leedom Larson, K.R. Hepatitis E Virus; Swine Health Information Center and Center for Food Security and Public Health: Ames, IA, USA, 2016; Available online: http://www.cfsph.iastate.edu/pdf/shic-factsheet-hepatitise-virus (accessed on 28 June 2025).
- Sridhar, S.; Lau, S.K.; Woo, P.C. Hepatitis E: A disease of reemerging importance. J. Formos. Med. Assoc. 2015, 114, 681–690. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernandez Escamez, P.S.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Norrung, B.; et al. Public health risks associated with hepatitis E virus (HEV) as a food-borne pathogen. EFSA J. 2017, 15, e04886. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Khuroo, N.S. Transmission of Hepatitis E Virus in Developing Countries. Viruses 2016, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Meester, M.; Tobias, T.J.; Bouwknegt, M.; Kusters, N.E.; Stegeman, J.A.; van der Poel, W.H.M. Infection dynamics and persistence of hepatitis E virus on pig farms—A review. Porc. Health Manag. 2021, 7, 16. [Google Scholar] [CrossRef]
- González, M.M.; Sanabria, L.P.; Castaño-Osorio, J.C. Hepatitis E Virus: A review of the current status and perspectives. Infectio 2022, 26, 181–188. [Google Scholar] [CrossRef]
- Hakze-van der Honing, R.W.; van Coillie, E.; Antonis, A.F.; van der Poel, W.H. First isolation of hepatitis E virus genotype 4 in Europe through swine surveillance in The Netherlands and Belgium. PLoS ONE 2011, 6, e22673. [Google Scholar] [CrossRef] [PubMed]
- Songtanin, B.; Molehin, A.J.; Brittan, K.; Manatsathit, W.; Nugent, K. Hepatitis E Virus Infections: Epidemiology, Genetic Diversity, and Clinical Considerations. Viruses 2023, 15, 1389. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H.R.; Izopet, J. Transmission and Epidemiology of Hepatitis E Virus Genotype 3 and 4 Infections. Cold Spring Harb. Perspect. Med. 2018, 8, a032144. [Google Scholar] [CrossRef]
- Meng, X.J.; Purcell, R.H.; Halbur, P.G.; Lehman, J.R.; Webb, D.M.; Tsareva, T.S.; Haynes, J.S.; Thacker, B.J.; Emerson, S.U. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA 1997, 94, 9860–9865. [Google Scholar] [CrossRef]
- Meng, X.J. Emerging and re-emerging swine viruses. Transbound Emerg. Dis. 2012, 59, 85–102. [Google Scholar] [CrossRef]
- Ahmed, R.; Nasheri, N. Animal reservoirs for hepatitis E virus within the Paslahepevirus genus. Vet. Microbiol. 2023, 278, 109618. [Google Scholar] [CrossRef]
- Salines, M.; Andraud, M.; Rose, N. From the epidemiology of hepatitis E virus (HEV) within the swine reservoir to public health risk mitigation strategies: A comprehensive review. Vet. Res. 2017, 48, 31. [Google Scholar] [CrossRef]
- Baumann-Popczyk, A.; Popczyk, B.; Golab, E.; Rozej-Bielicka, W.; Sadkowska-Todys, M. A cross-sectional study among Polish hunters: Seroprevalence of hepatitis E and analysis of factors contributing to HEV infections. Med. Microbiol. Immunol. 2017, 206, 367–378. [Google Scholar] [CrossRef]
- Li, P.; Ji, Y.; Li, Y.; Ma, Z.; Pan, Q. Estimating the global prevalence of hepatitis E virus in swine and pork products. One Health 2022, 14, 100362. [Google Scholar] [CrossRef] [PubMed]
- Meester, M.; Bouwknegt, M.; Hakze-van der Honing, R.; Vernooij, H.; Houben, M.; van Oort, S.; van der Poel, W.H.M.; Stegeman, A.; Tobias, T. Repeated cross-sectional sampling of pigs at slaughter indicates varying age of hepatitis E virus infection within and between pig farms. Vet. Res. 2022, 53, 50. [Google Scholar] [CrossRef]
- Asimoula, S.; Tzika, E.; Alexopoulos, C.; Kyriakis, S.C.; Froesner, G. First report of serological evidence of hepatitis E virus infection in swine in northern Greece. Acta Vet. 2009, 59, 205–211. [Google Scholar] [CrossRef][Green Version]
- La Bella, G.; Basanisi, M.G.; Nobili, G.; Coppola, R.; Damato, A.M.; Donatiello, A.; Occhiochiuso, G.; Romano, A.C.; Toce, M.; Palazzo, L.; et al. Evidence of Circulation and Phylogenetic Analysis of Hepatitis E Virus (HEV) in Wild Boar in South-East Italy. Viruses 2023, 15, 2021. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, D.; Ward, P.; Gagné, M.-J.; Poitras, E.; Müller, P.; Trottier, Y.-L.; Simard, C.; Houde, A. Presence of hepatitis E virus in a naturally infected swine herd from nursery to slaughter. Int. J. Food Microbiol. 2007, 117, 160–166. [Google Scholar] [CrossRef]
- De Deus, N.; Casas, M.; Peralta, B.; Nofrarías, M.; Pina, S.; Martín, M.; Segalés, J. Hepatitis E virus infection dynamics and organic distribution in naturally infected pigs in a farrow-to-finish farm. Vet. Microbiol. 2008, 132, 19–28. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, C.; Li, M.; Harrison, T.J.; Qiao, Z.; Feng, Y.; Ma, Z.; Wang, Y. Infection dynamics of hepatitis E virus in naturally infected pigs in a Chinese farrow-to-finish farm. Infect. Genet. Evol. 2011, 11, 1727–1731. [Google Scholar] [CrossRef]
- Krog, J.S.; Larsen, L.E.; Breum, S.O. Tracing hepatitis E virus in pigs from birth to slaughter. Front. Vet. Sci. 2019, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Motoya, T.; Umezawa, M.; Goto, K.; Doi, I.; Nagata, N.; Ikeda, Y.; Sakuta, A.; Sasaki, N.; Ishii, K. High prevalence of hepatitis E virus infection among domestic pigs in Ibaraki prefecture, Japan. BMC Vet. Res. 2019, 15, 87. [Google Scholar] [CrossRef]
- Meng, X.J.; Wiseman, B.; Elvinger, F.; Guenette, D.K.; Toth, T.E.; Engle, R.E.; Emerson, S.U.; Purcell, R.H. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and in normal blood donors in the United States and other countries. J. Clin. Microbiol. 2002, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Galiana, C.; Fernández-Barredo, S.; García, A.; Gómez, M.T.; Pérez-Gracia, M.T. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am. J. Trop. Med. Hyg. 2008, 78, 1012–1015. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, A.; Mohn, U.; Lange, J.; Motz, M.; Wenzel, J.J.; Jilg, W.; Walther, M.; Straube, E.; Wutzler, P.; Zell, R. Prevalence of hepatitis E virus-specific antibodies in humans with occupational exposure to swine in Portugal. Med. Microbiol. Immunol. 2012, 201, 239–244. [Google Scholar] [CrossRef]
- Lee, J.T.; Shao, P.L.; Chang, L.Y.; Xia, N.S.; Chen, P.J.; Lu, C.Y.; Huang, L.M. Seroprevalence of hepatitis E virus infection among swine farmers and the general population in rural Taiwan. PLoS ONE 2013, 8, e67180. [Google Scholar] [CrossRef]
- Kogias, D.; Skeva, A.; Smyrlis, A.; Mourvati, E.; Kantartzi, K.; Romanidou, G.; Kalientzidou, M.; Rekari, V.; Konstantinidou, E.; Kiorteve, P.; et al. Hepatitis E Virus (HEV) Infection in Hemodialysis Patients: A Multicenter Epidemiological Cohort Study in North-Eastern Greece. Pathogens 2023, 12, 667. [Google Scholar] [CrossRef]
- Meng, X.J.; Baldwin, C.A.; Elvinger, F.; Halbur, P.; Wilson, C.A. Swine Hepatitis E Virus. In Diseases of Swine, 9th ed.; Straw, B.E., D’Allaire, S., Taylor, D.J., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 537–545. [Google Scholar]
- Bautista, D.; Villar, L.A.; Reyes, R.; Cleves, M.A.; Gelves, M.; Lozano-Para, A.; Bueno-Ariza, N.; Orostegui, M.; Martinez-Vega, R.A.; Diaz-Galvis, M. Sensitivity and efficiency of RNA sample pooling for real-time quantitative polymerase chain reaction testing for SARS-CoV-2. J. Public Health Emerg. 2022, 6, 25. [Google Scholar] [CrossRef]
- Handous, I.; Hannachi, N.; Marzouk, M.; Hazgui, O.; Ben-Alaya, N.B.E.B.; Bougadida, J. Pooling nasopharyngeal swab specimens to increase testing capacity for SARS-CoV-2 by real-time RT-PCR. Biol. Proced. Online 2021, 23, 15. [Google Scholar] [CrossRef]
- Abdelrazik, A.M.; El-Said, M.N.; Abdelaziz, H.M. Evaluation of pooling strategy of SARS-CoV-2 RT-PCR in limited resources setting in Egypt at low prevalence. Trop. Anim. Health Prod. 2023, 55, 416. [Google Scholar] [CrossRef] [PubMed]
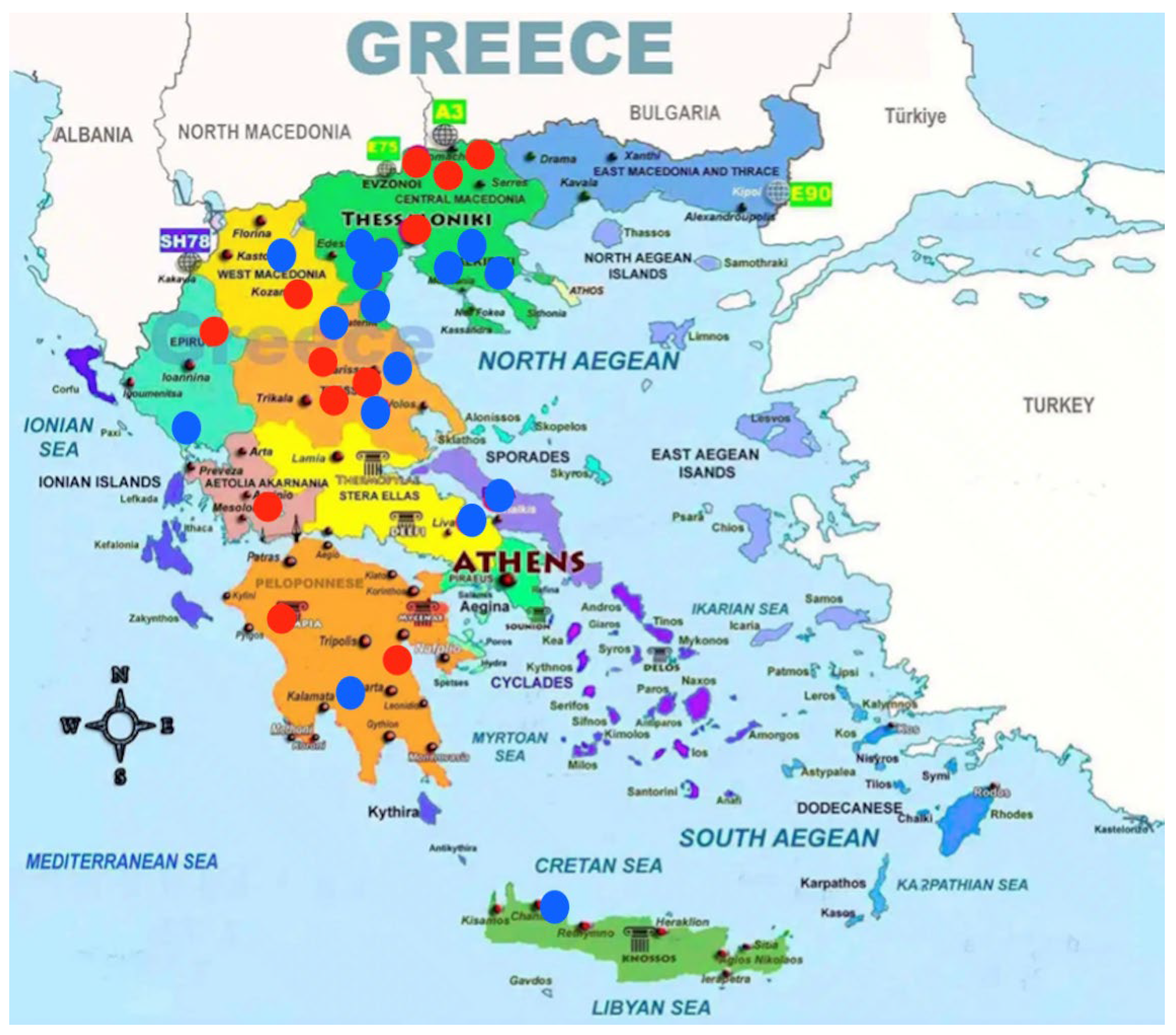
| Region | Number of Sampled Farms | Number of Sampled Pigs | HEV Positive Farms | HEV Negative Farms | % Percentage of HEV Positive Farms |
|---|---|---|---|---|---|
| Macedonia | 13 | 130 | 5 | 8 | 38.5 |
| Epirus | 2 | 20 | 1 | 1 | 50 |
| Thessaly | 6 | 60 | 3 | 3 | 50 |
| Sterea Hellas | 3 | 30 | 1 | 2 | 33.3 |
| Peloponnese | 3 | 30 | 2 | 1 | 66.6 |
| Crete | 1 | 10 | 0 | 1 | 0 |
| TOTAL | 28 | 280 | 12 | 16 | 42.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamelou, E.; Papageorgiou, K.; Stoikou, A.; Chatzopoulos, D.; Papadopoulos, D.; Giantsis, I.A.; Billinis, C.; Petridou, E.; Kritas, S.K. Herd-Level Prevalence of Hepatitis E Virus in Greek Pig Farms. Microbiol. Res. 2025, 16, 208. https://doi.org/10.3390/microbiolres16090208
Stamelou E, Papageorgiou K, Stoikou A, Chatzopoulos D, Papadopoulos D, Giantsis IA, Billinis C, Petridou E, Kritas SK. Herd-Level Prevalence of Hepatitis E Virus in Greek Pig Farms. Microbiology Research. 2025; 16(9):208. https://doi.org/10.3390/microbiolres16090208
Chicago/Turabian StyleStamelou, Efthymia, Konstantinos Papageorgiou, Aikaterini Stoikou, Dimitrios Chatzopoulos, Dimitrios Papadopoulos, Ioannis A. Giantsis, Charalambos Billinis, Evanthia Petridou, and Spyridon K. Kritas. 2025. "Herd-Level Prevalence of Hepatitis E Virus in Greek Pig Farms" Microbiology Research 16, no. 9: 208. https://doi.org/10.3390/microbiolres16090208
APA StyleStamelou, E., Papageorgiou, K., Stoikou, A., Chatzopoulos, D., Papadopoulos, D., Giantsis, I. A., Billinis, C., Petridou, E., & Kritas, S. K. (2025). Herd-Level Prevalence of Hepatitis E Virus in Greek Pig Farms. Microbiology Research, 16(9), 208. https://doi.org/10.3390/microbiolres16090208








