The Prevalence of ESKAPE Pathogens and Their Drug Resistance Profiles in Aquatic Environments Around the World
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Study Selection
2.4. Quality Assessment of the Studies
2.5. Data Collection and Analysis
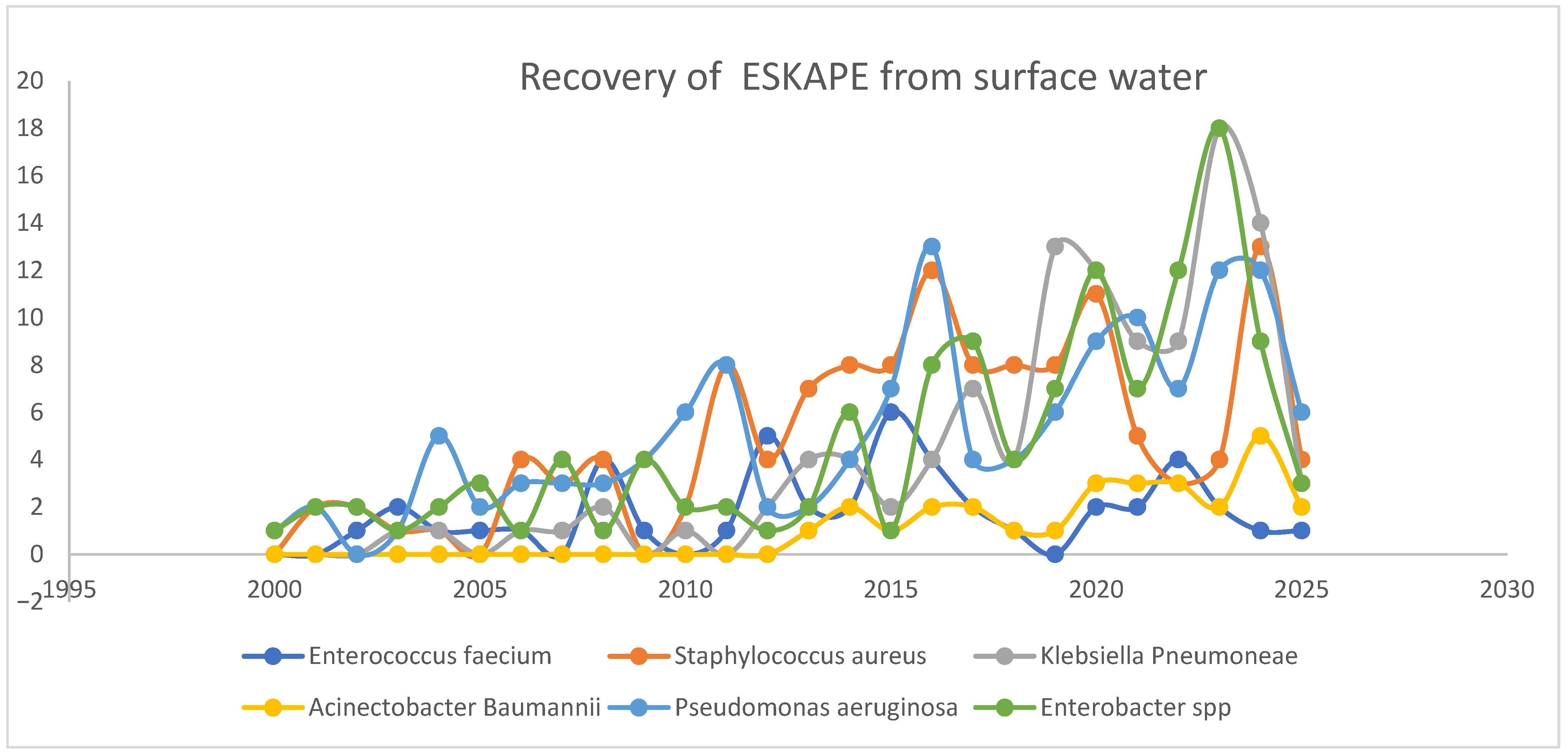
3. Results
3.1. Eligibility of Assessment Outcomes
3.2. Characteristics and Quality of the Study
4. Discussion
4.1. Enterococcus faecium/faecalis
4.2. Staphylococcus aureus
4.3. Klebsiella pneumoniae
4.4. Acinetobacter baumannii
4.5. Pseudomonas aeruginosa
4.6. Enterobacter spp.
- Data collected for the analysis of the prevalence of individual ESKAPE pathogens in water systems in various regions are unevenly distributed across the globe.
- Important data, such as VGs, ARGs, and MDR, were not included in some of the studies analyzed, limiting the available information on the patterns and mechanisms of resistance of the ESKAPE pathogens in the studied literature.
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- WHO. Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 November 2024).
- CDC. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/antimicrobial-resistance/data-research/threats/index.html (accessed on 1 November 2024).
- Maraki, S.; Mavromanolaki, V.E.; Kasimati, A.; Iliaki-Giannakoudaki, E.; Stafylaki, D. The evolving epidemiology of antimicrobial resistance of ESKAPE pathogens isolated in the intensive care unit of a Greek university hospital. Diagn. Microbiol. Infect. Dis. 2025, 112, 116804. [Google Scholar] [CrossRef]
- Dominey-Howes, D.; Bajorek, B.; Michael, C.A.; Betteridge, B.; Iredell, J.; Labbate, M. Applying the emergency risk management process to tackle the crisis of antibiotic resistance. Front. Microbiol. 2015, 6, 129597. [Google Scholar] [CrossRef]
- WHO. WHO Bacterial Priority Pathogens List; WHO: Geneva, Switzerland, 2024; Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (accessed on 1 September 2024).
- Pipitò, L.; Rubino, R.; D’Agati, G.; Bono, E.; Mazzola, C.V.; Urso, S.; Zinna, G.; Distefano, S.A.; Firenze, A.; Bonura, C.; et al. Antimicrobial Resistance in ESKAPE Pathogens: A Retrospective Epidemiological Study at the University Hospital of Palermo, Italy. Antibiotics 2025, 14, 186. [Google Scholar] [CrossRef]
- Safdar, N.; Saleem, S.; Salman, M.; Tareq, A.H.; Ishaq, S.; Ambreen, S.; Hameed, A.; Habib, M.B.; Ali, T.M. Economic burden of antimicrobial resistance on patients in Pakistan. Front. Public Health 2025, 13, 1481212. [Google Scholar] [CrossRef]
- Hampton, T. Report Reveals Scope of US Antibiotic Resistance Threat. JAMA 2013, 310, 1661–1663. [Google Scholar] [CrossRef]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Do Thi Thuy, N.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015, a population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Weist, K.; Högberg, L.D. ECDC publishes 2015 surveillance data on antimicrobial resistance and antimicrobial consumption in Europe. Eurosurveillance 2016, 21, 30401. [Google Scholar] [CrossRef] [PubMed]
- Gasser, M.; Zingg, W.; Cassini, A.; Kronenberg, A. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in Switzerland. Lancet Infect. Dis. 2019, 19, 17–18. [Google Scholar] [CrossRef]
- Kariuki, S.; Kering, K.; Wairimu, C.; Onsare, R.; Mbae, C. Antimicrobial Resistance Rates and Surveillance in Sub-Saharan Africa: Where Are We Now? Infect. Drug Resist. 2022, 15, 3589. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final paper_with cover.pdf (accessed on 23 August 2025).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Trespidi, G.; Barbieri, G.; Arshad, A.; Israyilova, A.; Buroni, S. The Evolution of Antimicrobial Resistance in Acinetobacter baumannii and New Strategies to Fight It. Antibiotics 2025, 14, 85. [Google Scholar] [CrossRef]
- Ravi, K.; Singh, B. ESKAPE: Navigating the Global Battlefield for Antimicrobial Resistance and Defense in Hospitals. Bacteria 2024, 3, 76–98. [Google Scholar] [CrossRef]
- European Commission. A European One Health Action Plan Against Antimicrobial Resistance (AMR). European Commission: Brussels, Belgium, 2017. Available online: https://health.ec.europa.eu/document/download/353f40d1-f114-4c41-9755-c7e3f1da5378_en?filename=amr_2017_action-plan.pdf (accessed on 1 September 2024).
- Savin, M.; Bierbaum, G.; Hammerl, J.A.; Heinemann, C.; Parcina, M.; Sib, E.; Voigt, A.; Kreyenschmidt, J. ESKAPe bacteria and extended-spectrum-β-lactamase-producing Escherichia coli isolated from wastewater and process water from German poultry slaughterhouses. Appl. Environ. Microbiol. 2020, 86, e02748-19. [Google Scholar] [CrossRef]
- Severino, N.; Reyes, C.; Fernandez, Y.; Azevedo, V.; De Francisco, L.E.; Ramos, R.T.; Maroto-Martín, L.O.; Franco, E.F. Bacterial Foodborne Diseases in Central America and the Caribbean: A Systematic Review. Microbiol. Res. 2025, 16, 78. [Google Scholar] [CrossRef]
- Mukwevho, F.N.; Mbanga, J.; Bester, L.A.; Ismail, A.; Essack, S.Y.; Abia, A.L.K. Potential environmental transmission of antibiotic-resistant Escherichia coli and Enterococcus faecium harbouring multiple antibiotic resistance genes and mobile genetic elements in surface waters close to informal settlements: A tale of two cities. Sci. Total Environ. 2025, 976, 179321. [Google Scholar] [CrossRef]
- Solís-Soto, L.; Castro-Delgado, Z.L.; García, S.; Heredia, N.; Avila-Sosa, R.; Dávila-Aviña, J.E. Pathogenic bacteria and their antibiotic resistance profile in irrigation water in farms from Mexico. J. Water Sanit. Hyg. Dev. 2024, 14, 565–571. [Google Scholar] [CrossRef]
- Nathan, L.S.; Pathak, R.K.; Paul, A. Incidence of Multi Drug Resistant Enterococcus spp. at Sangam Ghat at Prayagraj, India. Biochem. Cell. Arch. 2023, 23, 511–515. [Google Scholar] [CrossRef]
- Cinthi, M.; Coccitto, S.N.; Morroni, G.; D’achille, G.; Brenciani, A.; Giovanetti, E. Detection of an Enterococcus faecium Carrying a Double Copy of the PoxtA Gene from Freshwater River, Italy. Antibiotics 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.D.R.; Furlan, J.P.R.; Gallo, I.F.L.; Ramos, M.S.; Savazzi, E.A.; Stehling, E.G. Occurrence of multidrug-resistant Enterococcus faecium isolated from environmental samples. Lett. Appl. Microbiol. 2021, 73, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Biggel, M.; Nüesch-Inderbinen, M.; Raschle, S.; Stevens, M.J.A.; Stephan, R. Spread of vancomycin-resistant Enterococcus faecium ST133 in the aquatic environment in Switzerland. J. Glob. Antimicrob. Resist. 2021, 27, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Barrett, J.B.; Frye, J.G.; Jackson, C.R. Antimicrobial Resistance Gene Detection and Plasmid Typing Among Multidrug Resistant Enterococci Isolated from Freshwater Environment. Microorganism 2020, 8, 1338. [Google Scholar] [CrossRef]
- Pérez-Etayo, L.; González, D.; Leiva, J.; Vitas, A.I. Multidrug-Resistant Bacteria Isolated from Different Aquatic Environments in the North of Spain and South of France. Microorganism 2020, 8, 1425. [Google Scholar] [CrossRef]
- Ekwanzala, M.D.; Dewar, J.B.; Kamika, I.; Momba, M.N.B. Comparative genomics of vancomycin-resistant Enterococcus spp. revealed common resistome determinants from hospital wastewater to aquatic environments. Sci. Total Environ. 2020, 719, 137275. [Google Scholar] [CrossRef]
- Matlou, D.P.; Bissong, M.E.A.T.; Tchatchouang, C.D.K.; Adem, M.R.; Foka, F.E.T.; Kumar, A.; Ateba, C.N. Virulence profiles of vancomycin-resistant enterococci isolated from surface and ground water utilized by humans in the North West Province, South Africa: A public health perspective. Environ. Sci. Pollut. Res. 2019, 26, 15105–15114. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Tyski, S.; Wolinowska, R.; Grzybowska, W.; Zaręba, T.; Drobniewska, A.; Wroczyński, P.; Nałęcz-Jawecki, G. Occurrence of antimicrobial agents, drug-resistant bacteria, and genes in the sewage-impacted Vistula River (Poland). Environ. Sci. Pollut. Res. 2018, 25, 5788–5807. [Google Scholar] [CrossRef]
- Sacramento, A.G.; Cerdeira, L.T.; De Almeida, L.M.; Zanella, R.C.; Pires, C.; Sato, M.I.Z.; Costa, E.A.S.; Roberto, N.P.; Mamizuka, E.M.; Lincopan, N. Environmental dissemination of vanA-containing Enterococcus faecium strains belonging to hospital-associated clonal lineages. J. Antimicrob. Chemother. 2016, 71, 264–266. [Google Scholar] [CrossRef]
- Lata, P.; Ram, S.; Shanker, R. Multiplex PCR based genotypic characterization of pathogenic vancomycin resistant Enterococcus faecalis recovered from an Indian river along a city landscape. SpringerPlus 2016, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, M.; Iguchi, A.; Suzuki, Y. Identification of Enterococcus faecium and Enterococcus faecalis as vanC-type Vancomycin-Resistant Enterococci (VRE) from sewage and river water in the provincial city of Miyazaki, Japan. J. Environ. Sci. Health Part A 2015, 50, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bessa, L.J.; Barbosa-Vasconcelos, A.; Mendes, Â.; Vaz-Pires, P.; Da Costa, P.M. High prevalence of multidrug-resistant Escherichia coli and Enterococcus spp. in river water, upstream and downstream of a wastewater treatment plant. J. Water Health 2014, 12, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Hajiesmaili, R.; Talebjannat, M.; Yahyapour, Y. Identification and Antimicrobial Resistance of Enterococcus. spp. Isolated from the River and Coastal Waters in Northern Iran. Sci. World J. 2014, 2014, 287458. [Google Scholar] [CrossRef]
- De Niederhäusern, S.; Bondi, M.; Anacarso, I.; Iseppi, R.; Sabia, C.; Bitonte, F.; Messi, P. Antibiotics and heavy metals resistance and other biological characters in enterococci isolated from surface water of Monte Cotugno Lake (Italy). J. Environ. Sci. Health Part A 2013, 48, 939–946. [Google Scholar] [CrossRef]
- Middleton, J.H.; Chamberlain, D. Antibiotic Resistance and Virulence Factor Expression of Enterococcus faecalis and Enterococcus faecium isolates from the Upper Passaic River, Morris County, NJ. New Jersey Acad. Sci. 2013, 58, 1–9. [Google Scholar]
- Rathnayake, I.U.; Hargreaves, M.; Huygens, F. Antibiotic resistance and virulence traits in clinical and environmental Enterococcus faecalis and Enterococcus faecium isolates. Syst. Appl. Microbiol. 2012, 35, 326–333. [Google Scholar] [CrossRef]
- Lanthier, M.; Scott, A.; Zhang, Y.; Cloutier, M.; Durie, D.; Henderson, V.C.; Wilkes, G.; Lapen, D.; Topp, E. Distribution of selected virulence genes and antibiotic resistance in Enterococcus species isolated from the South Nation River drainage basin, Ontario, Canada. J. Appl. Microbiol. 2011, 110, 407–421. [Google Scholar] [CrossRef]
- Mondragón, V.A.; Llamas-Pérez, D.F.; González-Guzmán, G.E.; Márquez-González, A.R.; Padilla-Noriega, R.; Durán-Avelar, M.D.J.; Franco, B. Identification of Enterococcus faecalis bacteria resistant to heavy metals and antibiotics in surface waters of the Mololoa River in Tepic, Nayarit, Mexico. Environ. Monit. Assess. 2011, 183, 329–340. [Google Scholar] [CrossRef]
- dos Santos, I.R.; da Silva, I.N.M.; de Oliveira Neto, J.R.; de Oliveira, N.R.L.; de Sousa, A.R.V.; de Melo, A.M.; De Paula, J.A.M.; Do Amaral, C.L.; Silveira-Lacerda, E.D.P.; Da Cunha, L.C.; et al. The presence of antibiotics and multidrug-resistant Staphylococcus aureus reservoir in a low-order stream spring in central Brazil. Braz. J. Microbiol. 2023, 54, 997–1007. [Google Scholar] [CrossRef]
- Gerken, T.J.; Roberts, M.C.; Dykema, P.; Melly, G.; Lucas, D.; De Los Santos, V.; Gonzalez, J.; Butaye, P.; Wiegner, T.N. Environmental Surveillance and Characterization of Antibiotic Resistant Staphylococcus aureus at Coastal Beaches and Rivers on the Island of Hawaiʻi. Antibiotics 2021, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Vieira-Pinto, M.; Saraiva, C.; Manageiro, V.; Reis, L.; Ferreira, E.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Prevalence and Characteristics of Multidrug-Resistant Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) CC398 Isolated from Quails (Coturnix Coturnix Japonica) Slaughtered for Human Consumption. Animals 2021, 11, 2038. [Google Scholar] [CrossRef]
- Ramaite, K.; Deogratias Ekwanzala, M.; Dewar, J.B.; Ndombo, M.; Momba, B. Human-Associated Methicillin-Resistant Staphylococcus aureus Clonal Complex 80 Isolated from Cattle and Aquatic Environments. Antibiotics 2021, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Ramessar, K.; Olaniran, A.O. Antibiogram and molecular characterization of methicillin-resistant Staphylococcus aureus recovered from treated wastewater effluent and receiving surface water in Durban, South Africa. World J. Microbiol. Biotechnol. 2019, 35, 142. [Google Scholar] [CrossRef]
- Lepuschitz, S.; Mach, R.; Springer, B.; Allerberger, F.; Ruppitsch, W. Draft genome sequence of a community-acquired methicillin-resistant Staphylococcus aureus USA300 isolate from a river sample. Genome Announc. 2017, 5, e01166-17. [Google Scholar] [CrossRef]
- Akanbi, O.E.; Njom, H.A.; Fri, J.; Otigbu, A.C.; Clarke, A.M. Antimicrobial Susceptibility of Staphylococcus aureus Isolated from Recreational Waters and Beach Sand in Eastern Cape Province of South Africa. Int. J. Environ. Res. Public Health 2017, 14, 1001. [Google Scholar] [CrossRef]
- Skariyachan, S.; Mahajanakatti, A.B.; Grandhi, N.J.; Prasanna, A.; Sen, B.; Sharma, N.; Vasist, K.S.; Narayanappa, R. Environmental monitoring of bacterial contamination and antibiotic resistance patterns of the fecal coliforms isolated from Cauvery River, a major drinking water source in Karnataka, India. Environ. Monit. Assess. 2015, 187, 279. [Google Scholar] [CrossRef]
- Porrero, M.C.; Harrison, E.; Fernández-Garayzábal, J.F.; Paterson, G.K.; Díez-Guerrier, A.; Holmes, M.A.; Domínguez, L. Detection of mecC-Methicillin-resistant Staphylococcus aureus isolates in river water: A potential role for water in the environmental dissemination. Environ. Microbiol. Rep. 2014, 6, 705–708. [Google Scholar] [CrossRef]
- Sood, A.; Pandey, P.; Bisht, S.; Sharma, S. Anthropogenic activities as a source of high prevalence of antibiotic resistant Staphylococcus aureus in the River Ganga. Appl. Ecol. Environ. Res. 2014, 12, 33–48. [Google Scholar] [CrossRef]
- Levin-Edens, E.; Soge, O.O.; No, D.; Stiffarm, A.; Meschke, J.S.; Roberts, M.C. Methicillin-resistant Staphylococcus aureus from Northwest marine and freshwater recreational beaches. FEMS Microbiol. Ecol. 2012, 79, 412–420. [Google Scholar] [CrossRef]
- Soge, O.O.; Meschke, J.S.; No, D.B.; Roberts, M.C. Characterization of methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococcus spp. isolated from US West Coast public marine beaches. J. Antimicrob. Chemother. 2009, 64, 1148–1155. [Google Scholar] [CrossRef]
- Araújo, S.; Silva, V.; de Lurdes Enes Dapkevicius, M.; Pereira, J.E.; Martins, Â.; Igrejas, G.; Poeta, P. Comprehensive Profiling of Klebsiella in Surface Waters from Northern Portugal: Understanding Patterns in Prevalence, Antibiotic Resistance, and Biofilm Formation. Water 2024, 16, 1297. [Google Scholar] [CrossRef]
- Xiao, Z.; Qin, Y.; Han, L.; Liu, Y.; Wang, Z.; Huang, Y.; Ma, Y.; Zou, Y. Effects of wastewater treatment plant effluent on microbial risks of pathogens and their antibiotic resistance in the receiving river. Environ. Pollut. 2024, 345, 123461. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, R.K.; Dixit, S.; Behera, D.U.; Subudhi, E. NDM-5-carrying Klebsiella pneumoniae ST437 belonging to high-risk clonal complex (CC11) from an urban river in eastern India. 3 Biotech 2023, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Zhou, Z.; Berglund, B.; Zheng, B.; Meng, M.; Zhao, L.; Zhang, H.; Wang, Z.; Wu, T.; Li, Q.; et al. Persistent transmission of carbapenem-resistant, hypervirulent Klebsiella pneumoniae between a hospital and urban aquatic environments. Water Res. 2023, 242, 120263. [Google Scholar] [CrossRef] [PubMed]
- Donato, D.; High, M.; Tapia-Arreola, A.K.; Ruiz-Garcia, D.A.; Rodulfo, H.; Sharma, A.; De Donato, M. High frequency of antibiotic resistance genes (ARGs) in the Lerma River Basin, Mexico. Int. J. Environ. Res. Public Health 2022, 19, 13988. [Google Scholar] [CrossRef]
- Lobato, A.; Souza, C.O.; Martins, W.M.B.S.; Barata, R.R.; Camargo, D.S.; Dutra, L.M.G.; Carneiro, I.C.; Costa, C.J.; Brasiliense, D.M. Genomic characterization of BKC-1–producing Klebsiella pneumoniae strain belonging to high-risk clone sequence type 11 isolated from a river in Brazil. Sci. Total Environ. 2022, 850, 157917. [Google Scholar] [CrossRef]
- Hassen, B.; Abbassi, M.S.; Benlabidi, S.; Ruiz-Ripa, L.; Mama, O.M.; Ibrahim, C.; Hassen, A.; Hammami, S.; Torres, C. Genetic characterization of ESBL-producing Escherichia coli and Klebsiella pneumoniae isolated from wastewater and river water in Tunisia: Predominance of CTX-M-15 and high genetic diversity. Environ. Sci. Pollut. Res. 2020, 27, 44368–44377. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Savazzi, E.A.; Stehling, E.G. Genomic insights into multidrug-resistant and hypervirulent Klebsiella pneumoniae co-harboring metal resistance genes in aquatic environments. Ecotoxicol. Environ. Saf. 2020, 201, 110782. [Google Scholar] [CrossRef]
- Jelić, M.; Hrenović, J.; Dekić, S.; Goić-Barišić, I.; Tambić Andrašević, A. First evidence of KPC-producing ST258 Klebsiella pneumoniae in river water. J. Hosp. Infect. 2019, 103, 147–150. [Google Scholar] [CrossRef]
- Lepuschitz, S.; Schill, S.; Stoeger, A.; Pekard-Amenitsch, S.; Huhulescu, S.; Inreiter, N.; Hartl, R.; Kerschner, H.; Sorschag, S.; Springer, B.; et al. Whole genome sequencing reveals resemblance between ESBL-producing and carbapenem resistant Klebsiella pneumoniae isolates from Austrian rivers and clinical isolates from hospitals. Sci. Total Environ. 2019, 662, 227–235. [Google Scholar] [CrossRef]
- Nascimento, T.; Cantamessa, R.; Melo, L.; Fernandes, M.R.; Fraga, E.; Dropa, M.; Sato, M.I.; Cerdeira, L.; Lincopan, N. International high-risk clones of Klebsiella pneumoniae KPC-2/CC258 and Escherichia coli CTX-M-15/CC10 in urban lake waters. Sci. Total Environ. 2017, 598, 910–915. [Google Scholar] [CrossRef]
- Hladicz, A.; Kittinger, C.; Zarfel, G. Tigecycline Resistant Klebsiella pneumoniae Isolated from Austrian River Water. Int. J. Environ. Res. Public Health 2017, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Piedra-Carrasco, N.; Fàbrega, A.; Calero-Cáceres, W.; Cornejo-Sánchez, T.; Brown-Jaque, M.; Mir-Cros, A.; Muniesa, M.; González-López, J.J.; Chang, Y.-F. Carbapenemase-producing enterobacteriaceae recovered from a Spanish river ecosystem. PLoS ONE 2017, 12, e0175246. [Google Scholar] [CrossRef] [PubMed]
- Tafoukt, R.; Touati, A.; Leangapichart, T.; Bakour, S.; Rolain, J.M. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res. 2017, 120, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Kareem, A.A.; Al-Arajy, K.; Jassim, K.A. Prevalence of CTX-M Gene in Klebsiella pneumonia isolated from Surface Water of Tigris River within Baghdad Province. Prevalence 2015, 30, 15–20. [Google Scholar]
- Sotomayor, N.; Villacis, J.E.; Burneo, N.; Reyes, J.; Zapata, S.; de los Ángeles Bayas-Rea, R. Carbapenemase genes in clinical and environmental isolates of Acinetobacter spp. from Quito, Ecuador. PeerJ 2024, 12, e17199. [Google Scholar] [CrossRef]
- Hamidian, M.; Maharjan, R.P.; Farrugia, D.N.; Delgado, N.N.; Dinh, H.; Short, F.L.; Kostoulias, X.; Peleg, A.Y.; Paulsen, I.T.; Cain, A.K. Genomic and phenotypic analyses of diverse non-clinical Acinetobacter baumannii strains reveals strain-specific virulence and resistance capacity. Microb. Genom. 2022, 8, 765. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, R.K.; Gaur, M.; Behera, D.U.; Sahu, A.; Das, A.; Dey, S.; Dixit, S.; Subudhi, E. Environmental carbapenem-resistant Acinetobacter baumannii in wastewater receiving urban river system of eastern India: A public health threat. Int. J. Environ. Sci. Technol. 2022, 20, 9901–9910. [Google Scholar] [CrossRef]
- Havenga, B.; Reyneke, B.; Ndlovu, T.; Khan, W. Genotypic and phenotypic comparison of clinical and environmental Acinetobacter baumannii strains. Microb. Pathog. 2022, 172, 105749. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Liu, L.; Li, L.-Y.; Lin, H.; Wu, X.-Y.; Bi, W.-J.; Wang, L.-T.; Mao, D.-Q.; Luo, Y. The prevalence of ampicillin-resistant opportunistic pathogenic bacteria undergoing selective stress of heavy metal pollutants in the Xiangjiang River, China. Environ. Pollut. 2021, 268, 115362. [Google Scholar] [CrossRef]
- Adewoyin, M.A.; Ebomah, K.E.; Okoh, A.I. Antibiogram profile of Acinetobacter baumannii recovered from selected freshwater resources in the Eastern Cape Province, South Africa. Pathogens 2021, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Barich, D.; Fennessy, M.S.; Slonczewski, J.L. An Ohio State Scenic River Shows Elevated Antibiotic Resistance Genes, Including Acinetobacter Tetracycline and Macrolide Resistance, Downstream of Wastewater Treatment Plant Effluent. Microbiol. Spectr. 2021, 9, e0094121. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-C.; Chou, M.-Y.; Shih, Y.-J.; Huang, T.-Y.; Yang, P.-Y.; Chiu, Y.-C.; Chen, J.-S.; Hsu, B.-M. Distribution and Genotyping of Aquatic Acinetobacter baumannii Strains Isolated from the Puzi River and Its Tributaries Near Areas of Livestock Farming. Water 2018, 10, 1374. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, L.; Zhang, Z.; Huang, H.; Jiang, L. Draft genome sequence of a multidrug-resistant blaOXA-69-producing Acinetobacter baumannii L13 isolated from Tarim River sample in China. J. Glob. Antimicrob. Resist. 2019, 18, 145–147. [Google Scholar] [CrossRef]
- Kittinger, C.; Kirschner, A.; Lipp, M.; Baumert, R.; Mascher, F.; Farnleitner, A.H.; Zarfel, G.E. Antibiotic Resistance of Acinetobacter spp. Isolates from the River Danube: Susceptibility Stays High. Int. J. Environ. Res. Public Health 2018, 15, 52. [Google Scholar] [CrossRef]
- Turano, H.; Gomes, F.; Medeiros, M.; Oliveira, S.; Fontes, L.C.; Sato, M.I.Z.; Lincopan, N. Presence of high-risk clones of OXA-23-producing Acinetobacter baumannii (ST79) and SPM-1-producing Pseudomonas aeruginosa (ST277) in environmental water samples in Brazil. Diagn. Microbiol. Infect. Dis. 2016, 86, 80–82. [Google Scholar] [CrossRef]
- Rojo-Bezares, B.; Casado, C.; Ceniceros, T.; López, M.; Chichón, G.; Lozano, C.; Ruiz-Roldán, L.; Sáenz, Y. Pseudomonas aeruginosa from river water: Antimicrobial resistance, virulence and molecular typing. FEMS Microbiol. Ecol. 2024, 100, 28. [Google Scholar] [CrossRef]
- Sharif, D.I.; Amin, F.; Mehbub, H.; Ratul, R.I. Distribution and antibiotic resistance patterns of Pseudomonas aeruginosa across different point sources of pollution in the Buriganga River, Bangladesh. J. Water Health 2024, 22, 2358–2369. [Google Scholar] [CrossRef]
- Ghosh, D.; Mangar, P.; Choudhury, A.; Saha, A.K.A.; Basu, P.; Saha, D. Characterization of a hemolytic and antibiotic-resistant Pseudomonas aeruginosa strain S3 pathogenic to fish isolated from Mahananda River in India. PLoS ONE 2024, 19, e0300134. [Google Scholar] [CrossRef]
- Hosu, M.C.; Vasaikar, S.D.; Okuthe, G.E.; Apalata, T. Detection of extended spectrum beta-lactamase genes in Pseudomonas aeruginosa isolated from patients in rural Eastern Cape Province, South Africa. Sci. Rep. 2021, 11, 7110. [Google Scholar] [CrossRef] [PubMed]
- Nasreen, M.; Sarker, A.; Malek, M.A.; Ansaruzzaman, M.; Rahman, M. Prevalence and Resistance Pattern of Pseudomonas aeruginosa Isolated from Surface Water. Adv. Microbiol. 2015, 5, 74–81. [Google Scholar] [CrossRef]
- Magalhães, M.J.T.L.; Pontes, G.; Serra, P.T.; Balieiro, A.; Castro, D.; Pieri, F.A.; Crainey, J.L.; Nogueira, P.A.; Orlandi, P.P. Multidrug resistant Pseudomonas aeruginosa survey in a stream receiving effluents from ineffective wastewater hospital plants. BMC Microbiol. 2016, 16, 193. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kajii, S.; Nishiyama, M.; Iguchi, A. Susceptibility of Pseudomonas aeruginosa isolates collected from river water in Japan to antipseudomonal agents. Sci. Total Environ. 2013, 450–451, 148–154. [Google Scholar] [CrossRef]
- Fontes, L.C.; Neves, P.R.; Oliveira, S.; Silva, K.C.; Hachich, E.M.; Sato, M.I.Z.; Lincopan, N. Isolation of Pseudomonas aeruginosa Coproducing Metallo-β-Lactamase SPM-1 and 16S rRNA Methylase RmtD1 in an Urban River. Antimicrob. Agents Chemother. 2011, 55, 3063. [Google Scholar] [CrossRef]
- Jofré Bartholin, M.; Barrera Vega, B.; Berrocal Silva, L. Antibiotic-Resistant Bacteria in Environmental Water Sources from Southern Chile: A Potential Threat to Human Health. Microbiol. Res. 2023, 14, 1764–1773. [Google Scholar] [CrossRef]
- Adachi, F.; Sekizuka, T.; Yamato, M.; Fukuoka, K.; Yamaguchi, N.; Kuroda, M.; Kawahara, R. Characterization of FRI carbapenemase-producing Enterobacter spp. isolated from a hospital and the environment in Osaka, Japan. J. Antimicrob. Chemother. 2021, 76, 3061–3062. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nazareno, P.J.; Nakano, R.; Mondoy, M.; Nakano, A.; Bugayong, M.P.; Bilar, J.; Perez, M.; Medina, E.J.; Saito-Obata, M.; et al. Environmental presence and genetic characteristics of carbapenemase-producing enterobacteriaceae from hospital sewage and river water in the philippines. Appl. Environ. Microbiol. 2020, 86, e01906-19. [Google Scholar] [CrossRef]
- Cohen, R.; Paikin, S.; Rokney, A.; Rubin-Blum, M.; Astrahan, P. Multidrug-resistant enterobacteriaceae in coastal water: An emerging threat. Antimicrob. Resist. Infect. Control 2020, 9, 169. [Google Scholar] [CrossRef]
- Fadare, F.T.; Adefisoye, M.A.; Okoh, A.I. Occurrence, identification, and antibiogram signatures of selected Enterobacteriaceae from Tsomo and Tyhume rivers in the Eastern Cape Province, Republic of South Africa. PLoS ONE 2020, 15, e0238084. [Google Scholar] [CrossRef]
- Guzman-Otazo, J.; Gonzales-Siles, L.; Poma, V.; Bengtsson-Palme, J.; Thorell, K.; Flach, C.F.; Iñiguez, V.; Sjöling, Å. Diarrheal bacterial pathogens and multi-resistant enterobacteria in the Choqueyapu River in La Paz, Bolivia. PLoS ONE 2019, 14, e0210735. [Google Scholar] [CrossRef]
- Kittinger, C.; Lipp, M.; Folli, B.; Kirschner, A.; Baumert, R.; Galler, H.; Grisold, A.J.; Luxner, J.; Weissenbacher, M.; Farnleitner, A.H.; et al. Enterobacteriaceae isolated from the River danube: Antibiotic resistances, with a focus on the presence of ESBL and carbapenemases. PLoS ONE 2016, 11, e0165820. [Google Scholar] [CrossRef]
- Zurfluh, K.; Hächler, H.; Nüesch-Inderbinen, M.; Stephan, R. Characteristics of extended-spectrum β-lactamase- and carbapenemase-producing Enterobacteriaceae isolates from rivers and lakes in Switzerland. Appl. Environ. Microbiol. 2013, 79, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Miao, V.; Kwong, W.; Xia, R.; Davies, J. Identification of a novel fosfomycin resistance gene (fosA2) in Enterobacter cloacae from the Salmon River, Canada. Lett. Appl. Microbiol. 2011, 52, 427–429. [Google Scholar] [CrossRef]
- Vale de Macedo, G.H.R.; Costa, G.D.E.; Oliveira, E.R.; Damasceno, G.V.; Mendonça, J.S.P.; Silva, L.d.S.; Chagas, V.L.; Bazán, J.M.N.; Aliança, A.S.d.S.; Miranda, R.d.C.M.d.; et al. Interplay between ESKAPE Pathogens and Immunity in Skin Infections: An Overview of the Major Determinants of Virulence and Antibiotic Resistance. Pathogens 2021, 10, 148. [Google Scholar] [CrossRef]
- Gotkowska-Płachta, A. The Prevalence of Virulent and Multidrug-Resistant Enterococci in River Water and in Treated and Untreated Municipal and Hospital Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 563. [Google Scholar] [CrossRef]
- Ibekwe, A.M.; Obayiuwana, A.C.; Murinda, S.E. Enterococcus Species and Their Antimicrobial Resistance in an Urban Watershed Affected by Different Anthropogenic Sources. Water 2024, 16, 116. [Google Scholar] [CrossRef]
- Maheux, A.F.; Bissonnette, L.; Boissinot, M.; Bernier, J.L.T.; Huppé, V.; Bérubé, È.; Boudreau, D.K.; Picard, F.J.; Huletsky, A.; Bergeron, M.G. Method for rapid and sensitive detection of Enterococcus sp. and Enterococcus faecalis/faecium cells in potable water samples. Water. Res. 2011, 45, 2342–2354. [Google Scholar] [CrossRef]
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol. Spectr. 2017, 5, 10.1128. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kanda, N.; Furukawa, T. Abundance of Enterococcus species, Enterococcus faecalis and Enterococcus faecium, essential indicators of fecal pollution, in river water. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2012, 47, 1500–1505. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Thomas, V.C.; Narayanan, S.; Olson, S.; Fleming, S.D.; Hancock, L.E. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect. Immun. 2010, 78, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Ekwanzala, M.D.; Abia, A.L.K.; Ubomba-Jaswa, E.; Keshri, J.; Momba, N.B.M. Genetic relatedness of faecal coliforms and enterococci bacteria isolated from water and sediments of the Apies River, Gauteng, South Africa. AMB Express 2017, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg Goldstein, R.E.; Micallef, S.A.; Gibbs, S.G.; Davis, J.A.; He, X.; George, A.; Kleinfelter, L.M.; Schreiber, N.A.; Mukherjee, S.; Joseph, S.W.; et al. Methicillin-resistant Staphylococcus aureus (MRSA) Detected at Four U.S. wastewater treatment plants. Environ. Health Perspect. 2012, 120, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Tolba, O.; Loughrey, A.; Goldsmith, C.E.; Millar, B.C.; Rooney, P.J.; Moore, J.E. Survival of epidemic strains of healthcare (HA-MRSA) and community-associated (CA-MRSA) meticillin-resistant Staphylococcus aureus (MRSA) in river-, sea- and swimming pool water. Int. J. Hyg. Environ. Health 2008, 211, 398–402. [Google Scholar] [CrossRef]
- Carrel, M.; Perencevich, E.N.; David, M.Z. USA300 Methicillin-Resistant Staphylococcus aureus, United States, 2000–2013. Emerg. Infect. Dis. 2015, 21, 1973. [Google Scholar] [CrossRef]
- Azarian, T.; Daum, R.S.; Petty, L.A.; Steinbeck, J.L.; Yin, Z.; Nolan, D.; Boyle-Vavra, S.; Hanage, W.P.; Salemi, M.; David, M.Z. Intrahost Evolution of Methicillin-Resistant Staphylococcus aureus USA300 Among Individuals With Reoccurring Skin and Soft-Tissue Infections. J. Infect. Dis. 2016, 214, 895–905. [Google Scholar] [CrossRef]
- Lepuschitz, S.; Huhulescu, S.; Hyden, P.; Springer, B.; Rattei, T.; Allerberger, F.; Mach, R.L.; Ruppitsch, W. Characterization of a community-acquired-MRSA USA300 isolate from a river sample in Austria and whole genome sequence based comparison to a diverse collection of USA300 isolates. Sci. Rep. 2018, 8, 9467. [Google Scholar] [CrossRef]
- Fergestad, M.E.; Stamsås, G.A.; Morales Angeles, D.; Salehian, Z.; Wasteson, Y.; Kjos, M. Penicillin-binding protein PBP2a provides variable levels of protection toward different β-lactams in Staphylococcus aureus RN4220. Microbiologyopen 2020, 9, e1057. [Google Scholar] [CrossRef]
- Lade, H.; Kim, J.S. Molecular Determinants of β-Lactam Resistance in Methicillin-Resistant Staphylococcus aureus (MRSA): An Updated Review. Antibiotics 2023, 12, 1362. [Google Scholar] [CrossRef]
- Kilani, A.M.; Alabi, E.D.; Adeleke, O.E. Coexistence of the blaZ gene and selected virulence determinants in multidrug-resistant Staphylococcus aureus: Insights from three Nigerian tertiary hospitals. BMC Infect. Dis. 2024, 24, 1269. [Google Scholar] [CrossRef]
- Arêde, P.; Ministro, J.; Oliveira, D.C. Redefining the Role of the β-Lactamase Locus in Methicillin-Resistant Staphylococcus aureus: β-Lactamase Regulators Disrupt the MecI-Mediated Strong Repression on mecA and Optimize the Phenotypic Expression of Resistance in Strains with Constitutive mecA Expression. Antimicrob. Agents Chemother. 2013, 57, 3037. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ballhausen, B.; Kriegeskorte, A.; Schleimer, N.; Peters, G.; Becker, K. The mecA homolog mecC confers resistance against β-lactams in Staphylococcus aureus irrespective of the genetic strain background. Antimicrob. Agents Chemother. 2014, 58, 3791–3798. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Ferreira, E.; Manageiro, V.; Reis, L.; Tejedor-Junco, M.T.; Sampaio, A.; Capelo, J.L.; Caniça, M.; Igrejas, G.; Poeta, P. Distribution and Clonal Diversity of Staphylococcus aureus and Other Staphylococci in Surface Waters: Detection of ST425-t742 and ST130-t843 mecC-Positive MRSA Strains. Antibiotics 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Henriot, C.P.; Martak, D.; Cuenot, Q.; Loup, C.; Masclaux, H.; Gillet, F.; Bertrand, X.; Hocquet, D.; Bornette, G. Occurrence and ecological determinants of the contamination of floodplain wetlands with Klebsiella pneumoniae and pathogenic or antibiotic-resistant Escherichia coli. FEMS Microbiol. Ecol. 2019, 95, fiz097. [Google Scholar] [CrossRef] [PubMed]
- Podschun, R.; Pietsch, S.; Höller, C.; Ullmann, U. Incidence of Klebsiella Species in Surface Waters and Their Expression of Virulence Factors. Appl. Environ. Microbiol. 2001, 67, 3325. [Google Scholar] [CrossRef]
- Barati, A.; Ghaderpour, A.; Chew, L.L.; Bong, C.W.; Thong, K.L.; Chong, V.C.; Chai, L.C. Isolation and Characterization of Aquatic-Borne Klebsiella pneumoniae from Tropical Estuaries in Malaysia. Int. J. Environ. Res. Public Health 2016, 13, 426. [Google Scholar] [CrossRef]
- Liu, W.; Li, M.; Cao, S.; Ishaq, H.M.; Zhao, H.; Yang, F.; Liu, L. The Biological and Regulatory Role of Type VI Secretion System of Klebsiella pneumoniae. Infect. Drug Resist. 2023, 16, 6911–6922. [Google Scholar] [CrossRef]
- Arnold, R.S.; Thom, K.A.; Sharma, S.; Phillips, M.; Kristie Johnson, J.; Morgan, D.J. Emergence of Klebsiella pneumoniae Carbapenemase (KPC)-Producing Bacteria. South. Med. J. 2011, 104, 40. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef]
- Kochan, T.J.; Nozick, S.H.; Medernach, R.L.; Cheung, B.H.; Gatesy, S.W.M.; Lebrun-Corbin, M.; Mitra, S.D.; Khalatyan, N.; Krapp, F.; Qi, C.; et al. Genomic surveillance for multidrug-resistant or hypervirulent Klebsiella pneumoniae among United States bloodstream isolates. BMC Infect. Dis. 2022, 22, 603. [Google Scholar] [CrossRef]
- Korczak, L.; Majewski, P.; Iwaniuk, D.; Sacha, P.; Matulewicz, M.; Wieczorek, P.; Majewska, P.; Wieczorek, A.; Radziwon, P.; Tryniszewska, E. Molecular mechanisms of tigecycline-resistance among Enterobacterales. Front. Cell. Infect. Microbiol. 2024, 14, 1289396. [Google Scholar] [CrossRef] [PubMed]
- Chirabhundhu, N.; Luk-In, S.; Phuadraksa, T.; Wichit, S.; Chatsuwan, T.; Wannigama, D.L.; Yainoy, S. Occurrence and mechanisms of tigecycline resistance in carbapenem- and colistin-resistant Klebsiella pneumoniae in Thailand. Sci. Rep. 2024, 14, 5215. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 249706. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.C.; Zurawski, D.V. Laboratory Maintenance of Acinetobacter baumannii. Curr. Protoc. Microbiol. 2014, 35, 6G.1.1–6G.1.6. [Google Scholar] [CrossRef]
- Ahuatzin-Flores, O.E.; Torres, E.; Chávez-Bravo, E. Acinetobacter baumannii, a Multidrug-Resistant Opportunistic Pathogen in New Habitats: A Systematic Review. Microorganisms 2024, 12, 644. [Google Scholar] [CrossRef]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Adewoyin, M.A.; Ijabadeniyi, O.A.; Igbinosa, E.O.; Okoh, A.I. Machine learning-guided determination of Acinetobacter density in waterbodies receiving municipal and hospital wastewater effluents. Sci. Rep. 2023, 13, 7749. [Google Scholar] [CrossRef]
- Elshafiee, E.A.; Nader, S.M.; Dorgham, S.M.; Hamza, D.A. Carbapenem-resistant Pseudomonas aeruginosa originating from farm animals and people in Egypt. J. Vet. Res. 2019, 63, 333–337. [Google Scholar] [CrossRef]
- Hosu, M.C.; Vasaikar, S.; Okuthe, G.E.; Apalata, T. Molecular Detection of Antibiotic-Resistant Genes in Pseudomonas aeruginosa from Nonclinical Environment: Public Health Implications in Mthatha, Eastern Cape Province, South Africa. Int. J. Microbiol. 2021, 2021, 8861074. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Odjadjare, E.E.; Igbinosa, I.H.; Orhue, P.O.; Omoigberale, M.N.O.; Amhanre, N.I. Antibiotic Synergy Interaction against Multidrug-Resistant Pseudomonas aeruginosa Isolated from an Abattoir Effluent Environment. Sci. World J. 2012, 2012, 308034. [Google Scholar] [CrossRef]
- Aeschlimann, J.R. The Role of Multidrug Efflux Pumps in the Antibiotic Resistance of Pseudomonas aeruginosa and Other Gram-Negative Bacteria. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2003, 23, 916–924. [Google Scholar] [CrossRef]
- Zagui, G.S.; Moreira, N.C.; Santos, D.V.; Paschoalato, C.F.P.R.; Sierra, J.; Nadal, M.; Domingo, J.L.; Darini, A.L.C.; Andrade, L.N.; Segura-Muñoz, S.I. Multidrug-resistant Enterobacter spp. in wastewater and surface water: Molecular characterization of β-lactam resistance and metal tolerance genes. Environ. Res. 2023, 233, 116443. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.B.; Shanker, R.; Gupta, V.K.; Upadhyay, R.S. Q-PCR based culture-independent enumeration and detection of enterobacter: An emerging environmental human pathogen in riverine systems and potable water. Front. Microbiol. 2016, 7, 173935. [Google Scholar] [CrossRef] [PubMed]
- Davin-Regli, A.; Lavigne, J.P.; Pagès, J.M. Enterobacter spp.: Update on Taxonomy, Clinical Aspects, and Emerging Antimicrobial Resistance. Clin. Microbiol. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef]
- Zarfel, G.; Lipp, M.; Gürtl, E.; Folli, B.; Baumert, R.; Kittinger, C. Troubled water under the bridge: Screening of River Mur water reveals dominance of CTX-M harboring Escherichia coli and for the first time an environmental VIM-1 producer in Austria. Sci. Total Environ. 2017, 593–594, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mishra, S.K.; Shrestha, A. Characterisation of ESKAPE Pathogens with Special Reference to Multidrug Resistance and Biofilm Production in a Nepalese Hospital. Infect. Drug. Resist. 2021, 14, 2201. [Google Scholar] [CrossRef]
| ESKAPE Pathogen | Continent | |||||
|---|---|---|---|---|---|---|
| Africa | Americas | Asia | Europe | Oceania | Total no. of Each ESKAPE Pathogen per Continent | |
| Enterococcus faecium/faecalis | 3 | 7 | 4 | 6 | 1 | 21 |
| Staphylococcus aureus | 3 | 4 | 2 | 3 | - | 12 |
| Klebsiella pneumoniae | 2 | 5 | 4 | 4 | - | 15 |
| Acinetobacter baumannii | 2 | 2 | 4 | 3 | - | 11 |
| Pseudomonas aeruginosa | 1 | 2 | 4 | 1 | - | 8 |
| Enterobacter spp. | 2 | 3 | 2 | 2 | - | 9 |
| Total number of used articles | 13 | 23 | 20 | 19 | 1 | 76 |
| Year/Country | Aquatic System | Number of Isolates | ARG | VG | MDR E. faecium /faecalis | ST/CC | Genomic Analysis | Reference |
|---|---|---|---|---|---|---|---|---|
| 2025 South Africa | Palmiet River stream | E. faecium (11) | aac(6′)-Ii ant(6)-Ia dfrG ermB msrC tetL tetM | n. d. | 4 | ST94 ST361 ST2013 ST2042 ST2431 | WGS | [25] |
| 2024 Mexico | River in Veracruz | E. faecalis (2) | n. d. | eda1 ccf | 0 | n. d. | No | [26] |
| 2023 India | Ganga, Yamuna, and Sangam rivers | E. faecium (19) E. faecalis (7) | n. d. | n. d. | n. d. | n. d. | No | [27] |
| 2022 Italy | Salinello Estuary River | E. faecium (2) | aph(3′)-III aac(6′)-Ii ant(6)-Ia aac(6′)-aph(2″) ermB fexB msrC tetM tetL poxtA | n. d. | 1 | n. d. | WGS | [28] |
| 2021 Brazil | Rivers and beaches in the southeastern region | E. faecium (16) | ermB | gelE esp ace | 6 | n. d. | No | [29] |
| 2021 Switzerland | Aare, Rhein, and Rhone rivers Streams | E. faecium (6) | aac(6′)-Ii catA efrA msrC tetM vanA | n. d. | n. d. | ST133 | WGS | [30] |
| 2020 USA | Oconee River | E. faecium (33) E. faecalis (169) | n. d. | n. d. | 7/8 | n. d. | No | [31] |
| 2020 Spain | Alhama River | E. faecium (1) | vanB | n. d. | 1 | n. d. | No | [32] |
| 2020 South Africa | Apies River | E. faecium (13) E. faecalis (5) | aac(6′)-Ii vanA/C/N/L/G isaA tetM | Acm esp hylE | n. d. | ST80, ST203, | WGS, comparative genomic analysis | [33] |
| 2019 South Africa | Rivers and dam in the Northwest Province | E. faecium (11) E. faecalis (9) | vanA vanB | asa1 esp gel hyla | 11/9 | n. d. | No | [34] |
| 2018 Poland | Vistula River | E. faecium (75) E. faecalis (39) | ermC tetA tetO tetW | 43/6 | n. d. | No | [35] | |
| 2016 Brazil | Tiete ˆand Pinheiros rivers | E. faecium (5) E. faecalis (1) | vanA | n. d. | 5/1 | ST17, ST18, ST78, ST117, ST192, ST280, ST412 and ST478 | Comparative genomic analysis | [36] |
| 2016 India | River Gomti | E. faecalis (33) | vanB | gelE ace efaA esp | 33 | n. d. | No | [37] |
| 2015 Japan | Yae River | E. faecium (38) E. faecalis (20) | vanC1 vanC2/3 | n. d. | 2/3 | n. d. | No | [38] |
| 2014 Portugal | Ave River | E. faecium (18) E. faecalis (15) | vanA | n. d. | 16/10 | n. d. | No | [39] |
| 2014 Iran | Babolrud River | E. faecium (7) E. faecalis (20) | n. d. | n. d. | n. d. | n. d. | No | [40] |
| 2013 Italy | Monte Cotugno Lake | E. faecium (132) E. faecalis (21) | n. d. | Agg efaA gelE | n. d. | n. d. | No | [41] |
| 2013 USA | Passaic River | E. faecium (103) E. faecalis (396) | n. d. | gelE β hemolysis cylA/B/M bacteriocin | 33/10 | n. d. | No | [42] |
| 2012 Australia | Coomera River | E. faecium (47) E. faecalis (55) | vanA vanB | gelE | 0/0 | n. d. | No | [43] |
| 2011 Canada | South Nation River | E. faecium (145) E. faecalis (567) | n. d. | ccf cpd esp enlA gelE | n. d. | n. d. | No | [44] |
| 2011 Mexico | Mololoa River | E. faecalis (38) | n. d. | n. d. | n. d. | n. d. | No | [45] |
| Country | Aquatic System | Number of Isolates | ARG | VG | MDR | Molecular Typing | Genomic Analysis | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| spa-Type | ST/CC | ||||||||
| 2023 Brazil | Extrema River Spring | MRSA (20) | n.d. | n. d. | 14 | n. d. | n. d. | No | [46] |
| 2021 USA | Lehia, Richardson’s, Honoliʻi, Pohoiki, Kealoha, Onekahakaha beach parks, Wailuku River Estuary | mecA-MRSA (9) | aph(3′)-III blaZ ermC mecA, mphC msrA | hlgA hlgB hlgC lukD lukE lukF-PV lukS-PV sek seq | 9 | n. d. | ST8 (CC8). | WGS assembly and analysis | [47] |
| MSSA (27) | blaZ ermT aph(3′)-III ant(6)-Ia mphC tetK | hlgA/B/C hemolysin genes lukE seg | 4 | n. d. | ST5 ST398 ST72 ST15 (CC15) ST508 (CC45) ST97 (CC97) ST518 ST6 ST1155 ST3269 ST1181 | ||||
| 2021 Portugal | Rivers from the Douro River Basin | mecC-MRSA (3) | blaZ-SCCmecIX | Hld | 0 | t742 | ST425 | No | [48] |
| MSSA (9) | blaZ ermT vgaA | hla hlb hld | 0 | t008 t571 t742 t208 t098 t4735 t8083 | ST398 ST8 (CC8) ST49 (CC49) ST3223 ST49 (CC49) ST6835 ST133 ST6836 | ||||
| 2021 South Africa | Rivers from Bon Accord Dam | mecA-MRSA (6) | blaZ ermA | sec and seq. | 0 | n. d. | ST80 (CC80) ST728 | No | [49] |
| 2019 South Africa | Durban area Rivers | mecA-MRSA (80) | aac(6′) aph(2) blaZ ermC msrA tetK | hlgA hlgD lukS/FPV sea | 80 | n. d. | n. d. | No | [50] |
| 2017 Austria | River Mur | mecA-MRSA (1) | n. d. | hlgA/B/C agr type arcA/B/C aur cap5H | 1 | t008 | ST8 (CC8) | WGS | [51] |
| 2017 South Africa | Beaches in Eastern Cape Province | mecA-MRSA (5) MSSA (25) | blaZ mecA rpoB ermB tetM | n. d. | 30 | n. d. | n. d. | No | [52] |
| 2015 India | River Cauvery | MRSA (42) | n. d. | n. d. | 42 | n. d. | n. d. | No | [53] |
| 2014 Spain | Game estate Rivers | mecC-MRSA (3) | blaZLGA251 | n. d. | n. d. | t11212 | ST425 | WGS | [54] |
| 2014 India | Ganga River | MRSA (16) | n. d. | n. d. | n. d. | n. d. | n. d. | No | [55] |
| MSSA (72) | n. d. | n. d. | n. d. | n. d. | n. d. | ||||
| 2012 USA | Beaches in the Marine, Freshwater, and Lake Washington | mecA-MRSA (31), | tetK ermC msrA aadD | n. d. | 26 | n. d. | ST8, ST30, ST45, ST88, ST15, ST88, ST97 ST1875 ST133 ST1956 ST2049 | No | [56] |
| MSSA (14) | n. d. | n. d. | n. d. | n. d. | n. d. | ||||
| 2009 USA | Washington State beaches | MRSA (6) | ccrB ermA tetK tetM | n. d. | 5 | n. d. | ST145 ST45 ST59 ST30 | No | [57] |
| MSSA (4) | None | n. d. | 0 | n. d. | ST15 ST59 ST30 | ||||
| Country | Aquatic System | Number of Isolates | ARG | VG | MDR | ST/CC | Genomic Analysis | Reference |
|---|---|---|---|---|---|---|---|---|
| 2024 Portugal | Rivers and dam along the Douro River Basin | 9 | aadA1 blaAmpC blaCTX-U blaCTX-M9 blaSHV strA sul2 | bfp papC, | 4 | n. d. | No | [58] |
| 2024 China | Qinhuai River | 5 | AcrB aph(3′)-Ib aph(6)-Id bacA mdtB oqxB rsmA blaSHV-1 ugd | n. d. | n. d. | n. d. | No | [59] |
| 2023 India | Kathajodi River | 1 | blaCTX-M blaNDM-5 blaSHV blaTEM | FimH entB irp-1 mrkD ybtS | 1 | ST437 | No | [60] |
| 2023 China | Dongluo, Quanfu, and Shunhe rivers | 4 | aadA blaCTX-M blaKPC-2 blaNDM-1 blaTEM fosA rmtB | entA/B/C/D/E/F entD fepA/B/C/D/G iutA | 4 | ST17 ST11 ST730 | WGS | [61] |
| 2022 Mexico | Rivers and dam along the Lerma River basin | 7 | aac(3)-IIa aac(6′)Ib-cr blaCTX-M-15 blaOXA-1 blaOXA-232 blaSHV-11 blaTEM-1B catB4 dfrA14 fosA oqxA/B QnrS1 QnrB66 strA/B sul2 tetA | Dam, fimH ppdD | 6 | n. d. | WGS | [62] |
| 2022 Brazil | Guajará Bay River | 1 | blaBKC-1 blaCTX-M-15 blaSHV-11 aph(3′)-VIa aac(6′)-Ib-cr aac(3)-Iia qnrE1 qnrB19 rsmA emrR crp fosA6 catB3 rsmA | iutA acrA/B mrkA/C/D/F/H/I fimA/B/C/D/E/F/G/H/I/K | 1 | ST11 CC258 | WGS | [63] |
| 2020 Tunisia | Rouriche River | 2 | aadA2 blaCTX-M-15 dfrA12 blaSHV qacE∆1 sul1 sul2 tetA | 2 | ST1540 ST661. | No | [64] | |
| 2020 Brazil | Sertãozinho Stream, Euclides Moreli Lake, and Monjolinho River | 3 | blaSHV-26 blaSHV-27 blaSHV-81 fosA sul1 tetA | iutA entB ybtPQ irp1 fimA/B/C/D/E/F/G/H/I/K, mrkA/B/C/D/F ecpA/B/C/D/E7R, pulB/C/D/E/F/G/H/I/J/K/L/M/ fepCG iroCN mceG kfuBC, | 3 | ST661 (CC661) ST4415 (CC515) ST4416 (CC2654) | WGS, genomic islands, phage-related sequences | [65] |
| 2019 Croatia | Krapina River | 4 | blaKPC-2 blaSHV-1 aac (3′)-II aac(6′)-Ib aph(3′)-Ia | n. d. | 4 | ST258. | No | [66] |
| 2019 Austria | Inn, Drau, Glan, Traun, and Danube Rivers | 5 | aac(3)-Iic aac(6′)-Ib aph(3′)-Ia aadA baeR blaCTX-M-15 blaOXA-1 blaSHV-1 blaVIM-1 ermB/R fosA6 QnrB1 mphA | iutA mrkA/mrkB/C/D/F/H/I/J ybtS/tX | 5 | ST11 ST985 ST405 ST3400 ST323 | WGS | [67] |
| 2017 Brazil | Ibirapuera Lake | 2 | blaKPC-2 blaSHV-11 oqxA/B dfrA30 tetA fosA | n. d. | 2 | ST11/CC258 | WGS | [68] |
| 2017 Austria | River Mur | 2 | RamR | n. d. | 0 | ST2392 ST2394 | No | [69] |
| 2017 Spain | Llobregat River | 1 | blaKPC-2 | fimH mrkD wabG uge magA rmpA ureA allS kfuBC | 1 | ST634 | No | [70] |
| 2017 Algeria | Soummam River | 3 | blaOXA 48 blaSHV | n. d. | 3 | ST133 ST2192 ST2055 | No | [71] |
| 2015 Iraq | Tigris River | 40 | blaCTX-M | n. d. | n. d. | n. d. | No | [72] |
| Country | Aquatic System | Number of Isolates | ARG | VG | MDR/ XDR | ST/CC | Genomic Analysis | Reference |
|---|---|---|---|---|---|---|---|---|
| 2024 Ecuador | Machángara and Monjas rivers | 10 | blaOXA-51 | n. d. | 3 | n. d. | No | [73] |
| 2024 China | Qinhuai River | 2 | msrE mphE | n. d. | n. d. | n. d. | No | [59] |
| 2022 France | Seine River | 1 | blaAmpC blaOXA-23 parC strAB tetA | blc ompA smpA csuA, csuC pgaA bmfR/S gigA gacA/S | 1 | ST2 | WGS | [74] |
| 2022 India | Kathajodi River | 42 | blaNDM blaOXA-48 blaTEM | Type 1 fimbriae Biofilm, Hemolytic activity, Gelatinase | 23/19 | n. d. | No | [75] |
| 2022 South Africa | Plankenbrug River | 7 | 0 | n. d. | 3/1 | ST945, ST2520. | No | [76] |
| 2021 China | Xiangjiang River | 1 | blaOXA-2 | n. d. | 0 | n. d. | No | [77] |
| 2021 South Africa | Great Fish, Keiskamma, and Tyhume rivers | 410 | apHA1 apHA2 blaCTX-M blaCTX-M1/2 blaCTX-M-9 blaVEB blaGES blaIMP blaKPC blaPER, blaOXA-48-like blaOXA-51 blaSHV blaTEM blaVIM qnrB qnrD tetA tetB tetL tetC tetM | n. d. | n. d. | n. d. | No | [78] |
| 2021 USA | Kokosing River | 5 | aadA4 cfxA6 msrE mphE tet (39) | n. d. | 5 | n. d. | Shotgun sequencing | [79] |
| 2018 Taiwan | Puzi River | 11 | n. d. | n. d. | 0 | n. d. | No | [80] |
| 2019 China | Tarim River | 1 | blaOXA-69 blaADC-2 | n. d. | 1 | n. d. | WGS | [81] |
| 2017 Germany to Romania | Danube River | 135 | blaOXA-23 blaOXA-24 blaOXA-51 blaTEM-1 blaVIM-2 | n. d. | 16 | n. d. | No | [82] |
| 2016 Brazil | Tietê Pinheiros Rivers | 3 | blaOXA-23 blaOXA-51 | n. d. | 3 | ST79/CC79 | No | [83] |
| Country | Aquatic System | Number of Isolates | ARG | VG | MDR | ST/CC | Genomic Analysis | Reference |
|---|---|---|---|---|---|---|---|---|
| 2024 China | Qinhuai River | 4 | blaLCR-1 mphF blaOXA-101 blaOXA-827 blaTEM-1 | n. d. | n. d. | n. d. | Metagenomic sequencing | [59] |
| 2024 Spain | Iregua River | 52 | n. d. | exoU/S/Y/T/A lasI/R aprA rhlAB/I/R exlA | 0 | ST136 ST274 ST679 ST782 ST2540 | No | [84] |
| 2024 Bangladesh | Buriganga River | 70 | n. d. | n. d. | 3 | n. d. | No | [85] |
| 2024 India | Mahananda River | 1 | aph (3′)-IIb catB/B7 fosA emrE gyrA mexA/B/C/D/E/F/L/G/H oprD/M/N opmD/E/H blaOXA-50 pmrA | algW exoT/Y flhB hfq modA mucA nuoD PA0082 pilD phzS purD/H pyrF relA | 1 | n. d. | NGS, Comparative genomic analysis | [86] |
| 2021 South Africa | Mthatha Dam Mthatha River | 36 | blaSHV blaCTX-M blaTEM blaVIM | n. d. | 20 | n. d. | No | [87] |
| 2016 Brazil | Tietê and Pinheiros Rivers | 3 | blaSPM-1 | n. d. | 3 | ST277 | No | [83] |
| 2015 Bangladesh. | Lake and River in Dhaka City | 28 | n. d. | n. d. | 28 | n. d. | No | [88] |
| 2016 Brazil | Mindu stream | 8 | n. d. | n. d. | 0 | n. d. | No | [89] |
| 2013 Japan | Kiyotake River Yae River | 516 | n. d. | n. d. | n. d. | n. d. | No | [90] |
| 2011 Brazil | Tietê River | 1 | blaSPM-1 blaOXA-56 rmtD1 aacA4 aadA7 sul1 dhfr | n. d. | 1 | n. d. | No | [91] |
| Country | Aquatic System | Number of Isolates | ARG | VG | MDR | ST/CC | Genomic Analysis | Reference |
|---|---|---|---|---|---|---|---|---|
| 2024 China | Qinhuai River | E. cloacae (2) E. hormaechei (1) | DHA-7 mexB ugd | n. d. | n. d. | n. d. | Metagenomic sequencing | [59] |
| 2023 Chile | Cachapoal River and Villarrica Lake | E. cloacae (2) | blaTEM blaCTX-M | n. d | 2 | n. d. | No. | [92] |
| 2021 Japan | River in Osaka | E. asburiae (1), E. spp. (1) | blaFRI-4 blaFRI-8 | n. d. | 2 | n. d. | WGS | [93] |
| 2020 Philippines | Metro Manila River | E. cloacae (1) E. hormaechei (1) E. kobei (1) E. tabaci (1) E. xiangfangensis (1) | blaGES-20 blaIMI18 | n. d. | n. d. | n. d. | WGS | [94] |
| 2020 Israel | Alexander River estuary | E. asburiae (1) E. bugandensis (1) | blaIMI, NmcR fosA4 marA ramA | n. d. | 2 | n. d. | Metagenomic sequencing | [95] |
| 2020 South Africa | Tsomo and Tyhume rivers | E. aerogenes (7) E. amnigenus (1) E. asburiae (2) E. cloacae (4) | blaCTX-M blaSHV blaFOX, catII sul1 tetA tetB tetD | n. d. | 14 | n. d. | No | [96] |
| 2019 Bolivia | Choqueyapu River | E. cloacae (1) | AAC(3)-Iic AAC(6′)-Ib-cr aadA APH(3′)-Ib APH(6)-Id blaCTX-M-3 blaOXA-1 Sul2 blaTEM-1 Tet(A) | n. d. | n. d. | n. d. | WGS | [97] |
| 2017 Spain | Llobregat River | E. cloacae (2) | blaIMI-2 blaKPC-2 blaOXA-1 qnrB6 | n. d. | 2 | ST822 ST823 | No | [70] |
| 2016 Germany | Denube River | E. cloacae (3) E. cancerogenus (1) E. asburiae (1) | blaSHV-2 blaSHV-12 blaTEM-3 blaCTX-M-1 | n. d. | 5 | ST145 ST159 ST505 | No | [98] |
| 2013 Switzerland | Landquart and Lorze rivers | E. cloacae (1) E. amnigenus (1) | blaTEM blaCTXM-15 | n. d. | 2 | n. d. | No | [99] |
| 2011 Canada | Salmon River | E. cloacae (1) | fosA2 | n. d- | 1 | n. d. | No | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaniyan, T.O.; Martínez-Vázquez, A.V.; Escobedo-Bonilla, C.M.; López-Rodríguez, C.; Huerta-Luévano, P.; Castrejón-Sánchez, O.; de la Cruz-Flores, W.L.; Cedeño-Castillo, M.J.; de Luna-Santillana, E.d.J.; Cruz-Hernández, M.A.; et al. The Prevalence of ESKAPE Pathogens and Their Drug Resistance Profiles in Aquatic Environments Around the World. Microbiol. Res. 2025, 16, 201. https://doi.org/10.3390/microbiolres16090201
Olaniyan TO, Martínez-Vázquez AV, Escobedo-Bonilla CM, López-Rodríguez C, Huerta-Luévano P, Castrejón-Sánchez O, de la Cruz-Flores WL, Cedeño-Castillo MJ, de Luna-Santillana EdJ, Cruz-Hernández MA, et al. The Prevalence of ESKAPE Pathogens and Their Drug Resistance Profiles in Aquatic Environments Around the World. Microbiology Research. 2025; 16(9):201. https://doi.org/10.3390/microbiolres16090201
Chicago/Turabian StyleOlaniyan, Tunde Olarinde, Ana Verónica Martínez-Vázquez, Cesar Marcial Escobedo-Bonilla, Cristina López-Rodríguez, Patricia Huerta-Luévano, Oziel Castrejón-Sánchez, Wendy Lizeth de la Cruz-Flores, Manuel J. Cedeño-Castillo, Erick de Jesús de Luna-Santillana, Maria Antonia Cruz-Hernández, and et al. 2025. "The Prevalence of ESKAPE Pathogens and Their Drug Resistance Profiles in Aquatic Environments Around the World" Microbiology Research 16, no. 9: 201. https://doi.org/10.3390/microbiolres16090201
APA StyleOlaniyan, T. O., Martínez-Vázquez, A. V., Escobedo-Bonilla, C. M., López-Rodríguez, C., Huerta-Luévano, P., Castrejón-Sánchez, O., de la Cruz-Flores, W. L., Cedeño-Castillo, M. J., de Luna-Santillana, E. d. J., Cruz-Hernández, M. A., Rivera, G., & Bocanegra-García, V. (2025). The Prevalence of ESKAPE Pathogens and Their Drug Resistance Profiles in Aquatic Environments Around the World. Microbiology Research, 16(9), 201. https://doi.org/10.3390/microbiolres16090201








