1. Introduction
Endometriosis is a chronic inflammatory condition affecting roughly 10% of women of reproductive age, causing debilitating symptoms such as chronic pelvic pain, dysmenorrhea and infertility [
1,
2]. Despite its prevalence, the exact causes of endometriosis remain unclear, with research suggesting a combination of hormonal, genetic, and immune dysregulation factors [
3,
4].
Recent studies have highlighted a potential role of the human microbiota—the complex ecosystem of bacteria, fungi, and viruses in the body—in endometriosis development. Microbial imbalance in the gut and reproductive tracts may contribute to chronic inflammation, estrogen metabolism disruption, and immune dysfunction, all of which are implicated in endometriosis progression. For instance, research has found differences in the vaginal and intestinal microbiomes of women with endometriosis compared to healthy controls, suggesting microbial involvement in disease pathogenesis [
5].
The gut microbiota (GM) plays a pivotal role in regulating endogenous hormone metabolism through enzymatic activities collectively termed the “estrobolome” [
6]. This microbial–endocrine axis facilitates the reactivation of estrogen metabolites via β-glucuronidase activity, with significant implications for conditions ranging from endometriosis to breast cancer [
7]. Recent metagenomic analyses reveal that exogenous hormone administration can induce substantial shifts in microbial diversity, particularly reducing beneficial Bacteroides species while promoting potentially pathogenic Proteobacteria [
8].
These microbiome alterations may explain the gastrointestinal symptoms frequently reported by patients undergoing hormonal therapy, while also raising important questions about long-term metabolic consequences. Current research suggests that adjunctive microbiome-modulating strategies, including targeted probiotic supplementation and dietary fiber interventions, may help mitigate these effects.
Hormonal therapies, including oral contraceptives, selective estrogen receptor modulators, and gonadotropin-releasing hormone analogs, represent cornerstone treatments for numerous endocrine-related conditions. However, emerging evidence suggests these interventions may exert profound effects on the gut microbiome, potentially mediating both therapeutic benefits and adverse effects [
9].
Currently, emerging research suggests that the modulation of the microbiome may influence immune responses involved in the pathogenesis of endometriosis. For example, certain microbial metabolites, such as short-chain fatty acids (SCFAs), possess anti-inflammatory properties and can modulate immune cell activity, potentially reducing the development and progression of endometrial lesions [
10]. Additionally, dysbiosis in the reproductive tract microbiota may impair local immune tolerance, thereby facilitating the implantation of ectopic endometrial tissue [
11]. Interventions targeting the microbiome, including prebiotics and synbiotics, are being investigated for their potential to restore microbial balance and improve clinical outcomes in patients with endometriosis [
12]. Future studies should aim to identify specific microbial signatures associated with disease severity and response to therapy, paving the way for personalized microbiome-based treatments.
Understanding the microbiota–endometriosis connection could revolutionize treatment approaches, including probiotics, dietary interventions, and microbiome-targeted therapies. Since it is well known that progesterone affects the reproductive tract microbiome [
13], the present study concentrated on the effects of dienogest (DNG), a progestin medication, on the gut microbiome. We demonstrated that DNG affects the gut microbiome, exerting novel dual hormonal and microbiomial effects and revealing novel mechanisms by which progestin therapy may achieve therapeutic benefits in endometriosis.
2. Materials and Methods
A prospective single-center study was conducted at the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of the Ministry of Health of the Russian Federation from December 2021 to December 2023. Microbiological examination was performed on 17 women with endometriosis (mean age 30.9 (7.8) years, mean BMI 20.2 (2.0) kg/m2), with diagnosis confirmed by ultrasound, magnetic resonance imaging and presence of characteristic endometriosis-associated pain complaints (dysmenorrhea, dyspareunia, chronic pelvic pain, etc.). Microbiological examination of fecal samples was performed at two points: at baseline before therapy and after daily oral ingestion of DNG 2 mg for at least 6 months (mean duration of therapy was 6.7 (1.3) months).
Inclusion criteria:
Presence of external genital endometriosis signs according to pelvic MRI;
Age between 18 and 45 years;
Ability to collect fecal samples for microbiological examination in the morning on the day of the study;
Signed informed consent for participation in the study approved by the Center’s Ethics Committee.
Exclusion criteria:
Presence of severe somatic pathology;
Presence of oncological diseases of the female reproductive system at the time of inclusion or in medical history;
Use of hormonal, antibacterial or probiotic medications at least 3 months prior to study inclusion;
Contraindications for hormone therapy;
Indications for surgical treatment;
Failure to perform repeat pelvic MRI after at least 6 months of suppressive hormone therapy;
Medication use for less than 6 months;
Acute and chronic organic gastrointestinal diseases at the time of inclusion or in medical history;
The presence of gastrointestinal symptoms that could interfere with sample collection, such as diarrhea, constipation, rectal bleeding associated with organic pathology (hemorrhoids, anal fissures, etc.) or of unspecified etiology;
Low compliance;
Pregnancy and lactation.
Patient inclusion in the study was strictly in accordance with the above criteria. Study participants were informed about the study objectives, scope of planned examinations and, if necessary, further treatment options, possibility of alternative therapy and withdrawal from the study.
All women enrolled in the study signed informed consent for participation. The study was approved by the Ethics Committee for Biomedical Research of the V.I. Kulakov National Medical Research Center for Obstetrics, Gynecology and Perinatology of the Ministry of Health of the Russian Federation (Protocol No. 12 dated 25 November 2021).
2.1. Microbiological Examination of Fecal Samples
Collection of biological samples was carried out in the morning into a sterile container on any day except during menstruation. Subsequently, the feces were transferred to the laboratory; the time between sample collection and inoculation onto nutrient media did not exceed 6 h. A serial tenfold dilution of the substrate in saline solution was carried out 9 times. Nutrient media for cultivating obligate anaerobic microorganisms were prepared under oxygen-free conditions in a three-component gas mixture (N2—80%, H2—10%, CO2—10%). The prepared nutrient media were then stored in GasPak gas-generating pouches, which were kept in a refrigerator at +4 °C.
Iron-sulfite agar and Perfringens agar, after being inoculated with the material, were overlaid with the same agar, melted and cooled to 46 °C. Additionally, inoculation was performed on Petri dishes with the following nutrient media:
After inoculation, the seeded Petri dishes were placed in a thermostat at 37 °C. Petri dishes with Sabouraud agar were inoculated and placed in a thermostat at 30 °C and 37 °C. Petri dishes with Lactobacilli agar and Campylobacter-selective medium were inoculated and placed in a CO2 incubator with 5% carbon dioxide content.
Strict anaerobes were cultured in an anaerobic chamber under oxygen-free conditions in a three-component gas mixture (N2—80%, H2—10%, CO2—10%). The following nutrient media were placed inside the chamber in Petri dishes:
After incubation (3–4 days), quantitative determination and species identification of all isolated microorganisms were performed. Species identification of the isolated microorganisms was carried out using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) on a MicroFlex mass spectrometer with MALDI BioTyper software (Bruker Daltonics, Bremen, Germany), version 5.0.
2.2. Statistical Processing of the Material
For statistical data processing, we used IBM SPSS Statistics (version 26) and MedCalc statistical software (version 20.104—64-bit). For graph construction, we used OriginPro 2021 (version 9.8.0.200), Microsoft office (Excel) and MedCalc program.
For quantitative indicators, the following were calculated: mean value (M), standard deviation (SD), median (Me), interquartile range (Q1; Q3). For qualitative and ordinal indicators, frequencies (%) were calculated. All obtained quantitative parameters were tested for normal distribution using the Shapiro–Wilk test. Numerical parameters with normal distribution are presented in the format M (SD). Parameters with non-normal distribution are presented as Me (Q1; Q3). To determine differences between patient groups before and during therapy: for normally distributed parameters, we used paired Student’s
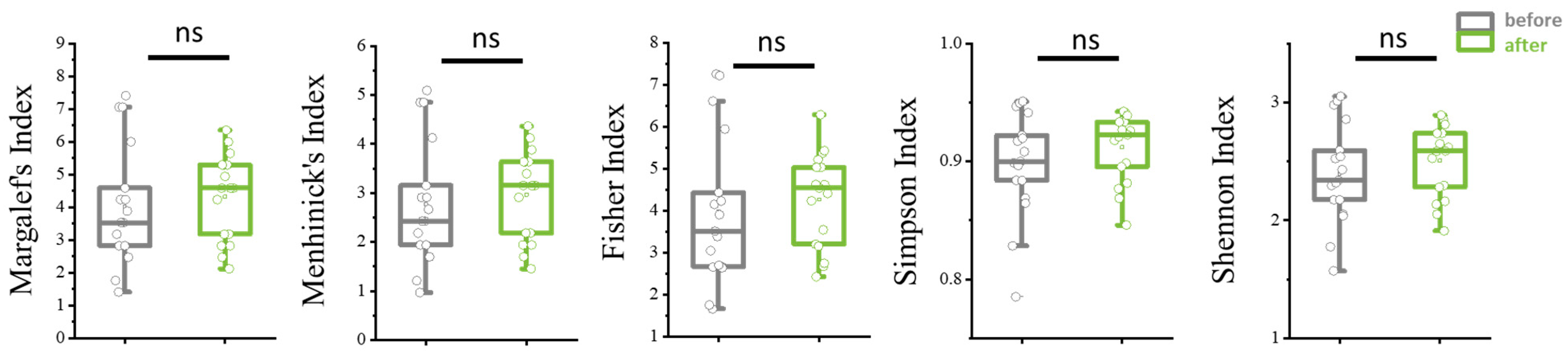
t-test, for non-normally distributed parameters, we used the Wilcoxon test. Species richness was calculated using Margalef’s index and the Menhinick index; alpha diversity was assessed using Simpson’s index and Shannon’s index (
Table 1). The critical significance level for testing statistical hypotheses was set at 0.05.
3. Results
General data on microbial diversity are presented in
Table 2. There was a certain tendency toward increased species diversity, higher numbers of isolated microorganisms, and increased microbial counts per patient. More detailed information about the gut microbiota composition in patients at baseline and during DNG therapy is provided in
Supporting Information.
Species richness and diversity indices are essential tools in ecological studies to assess the complexity of biological communities. Species richness, such as the Menhinick index (dMn), measures the total number of different species present in a sample, providing a straightforward estimate of biodiversity. The Menhinick index is calculated by dividing the number of species by the square root of the total number of individuals, allowing for comparison across samples with varying sizes.
Diversity indices like Margalef’s (dMg), Shannon’s (Sh), and Simpson’s (Si) indices offer more nuanced insights into community structure. Margalef’s index emphasizes species richness relative to the total number of individuals, highlighting how many different species are present in relation to sample size. The Shannon index accounts for both abundance and evenness of species, giving more weight to rare species and providing a measure of entropy within the community. Higher Shannon values indicate greater diversity and more equitable distribution among species.
The Simpson index focuses on dominance and the probability that two randomly selected individuals belong to the same species. A lower Simpson index value signifies higher diversity, as no single species dominates the community. Together, these indices provide a comprehensive picture of biodiversity, capturing aspects of richness, evenness, and dominance that are crucial for understanding ecological dynamics and assessing environmental impacts on ecosystems.
Additionally, there was a tendency toward increased species and alpha diversity of the microbiota during therapy, though the results did not reach statistical significance (
Figure 1).
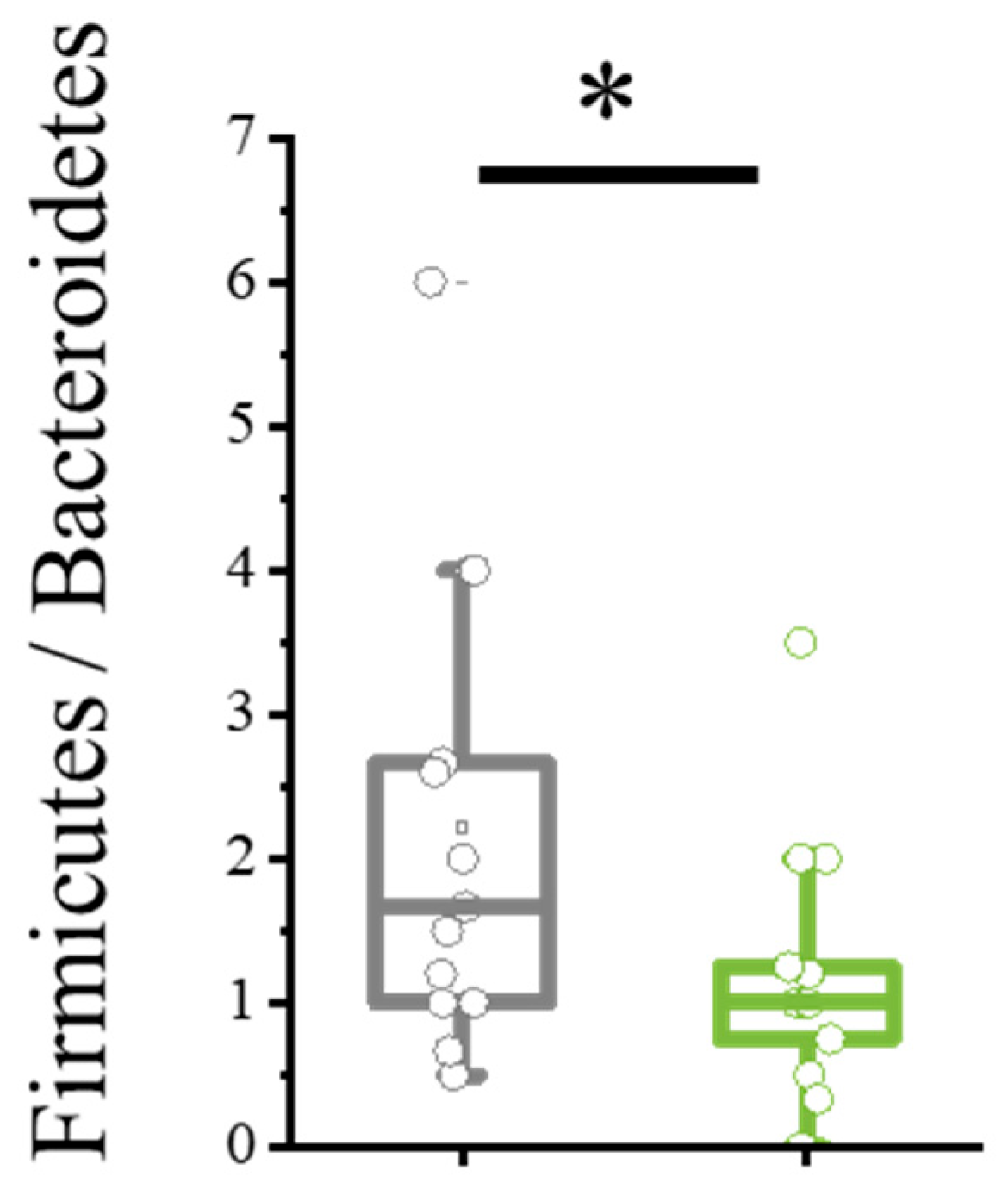
Bacillota/Bacteroidota ratio is an important metric used to assess the balance of gut microbiota, which plays a crucial role in human health. Bacillota, formerly known as Firmicutes, includes bacteria involved in energy absorption and storage, while Bacteroidota (Bacteroidetes) encompasses bacteria that help break down complex carbohydrates and support immune function. A higher Bacillota/Bacteroidota ratio has been associated with obesity and metabolic disorders, whereas a lower ratio is often linked to leanness and better metabolic health. Monitoring this ratio provides insights into gut microbial composition and its impact on overall well-being, making it a valuable indicator in microbiome research and clinical diagnostics. Maintaining a balanced ratio is essential for optimal digestion, immune response, and metabolic regulation.
During dienogest therapy, a significant decrease in the Bacillota/Bacteroidota ratio was observed (
p = 0.0421), achieved through a reduction in Bacillota bacteria—40.69% at baseline vs. 36.0% at follow-up, primarily due to decreases in
Staphylococcus spp. (
Figure 2).
In addition to the statistically significant decrease in frequency of Gram-positive facultative anaerobic flora (
Staphylococcus spp.) and reduced titers of certain microorganisms like
S. aureus and
S. epidermidis, significant changes in gut microbiota composition affected other genera as well. Specifically, during DNG therapy there was a decrease in absolute numbers of
Enterococcus spp. and an increase in commensal microorganisms—
Lactobacillus spp. (
Figure 3).
In addition, there was a tendency towards a decrease in the frequency of occurrence of a number of opportunistic microorganisms. Specifically, a reduction was observed in bacteria of the Staphylococcaceae family: species S. epidermidis (5/17 (29.41%) at baseline and 1/17 (5.89%) during therapy), S. hominis (2/17 (11.76%) and 1/17 (5.89%) respectively), S. capitis (2/17 (11.76%) and 0 respectively); of the Enterococcaceae family: species Enterococcus faecium (9/17 (52.94%) and 7/17 (41.18%) respectively), E. durans (3/17 (17.65%) and 0 respectively), E. avium (3/17 (17.65%) and 1/17 (5.89%) respectively); of the Clostridiaceae family: species Clostridium perfringens (3/17 (17.65%) and 1/17 (5.89%)).
Furthermore, a less significant decrease was noted in the number of other opportunistic microorganisms, such as bacteria of the Enterobacteriaceae family (Enterobacter bugandensis, lactose-negative hemolysis-negative Escherichia coli, Klebsiella pneumoniae, Klebsiella aerogenes) and the Streptococcaceae family (Streptococcus mutans, Streptococcus mitis, Streptococcus gallolyticus, Streptococcaceae oralis), the Clostridiaceae family (Clostridium paraputrificum, Clostridium bifermentans); fungi of the Saccharomycetaceae family (Saccharomyces Cerevisiae, Candida parapsilosis), the Arthrodermataceae family (Nannizzia incurvata). However, these results had limited significance as they concerned only single cases of microorganism detection.
According to the results of culture-based examination, there was a tendency towards an increase in the frequency of occurrence of symbiotic bacteria of the Bacteroidales order: species Bacteroides ovatus (2/17 (11.76%) at baseline and 5/17 (29.41%) during therapy), Parabacteroides merdae (1/17 (5.89%) and 3/17 (17.65%) respectively), Parabacteroides distasonis (4/17 (23.53%) and 7/17 (41.18%) respectively); of the Bifidobacteriales order: species Bifidobacterium bifidum (3/17 (17.65%) and 7/17 (41.18%)).
DNG therapy was also associated with increased titers of the symbiotic obligate anaerobic bacterium Collinsella aerofaciens (baseline 8.0 CFU/g, during therapy 8.0 (8.0; 10.0) CFU/g, p = 0.049). The detection frequency of this microorganism showed a tendency toward twofold increase during therapy (3/17 (17.65%) at baseline vs. 6/17 (35.29%) during therapy, p = 0.224). A similar trend was observed in previously presented results, where C. aerofaciens was detected twice as frequently in the control group compared to NGE patients and was present in high titers of 1010 CFU/g.
4. Discussion
Recent advances in microbiome research have revealed that gut microbiota dysbiosis may play a critical role in disease progression through immune modulation, hormonal regulation, and systemic inflammation. The gut–endometriosis axis operates via three core mechanisms:
Microbial β-glucuronidase reactivates estrogens, fostering a hyperestrogenic state [
14];
Intestinal barrier disruption permits bacterial translocation, triggering TLR4-mediated inflammation [
15];
SCFA depletion impairs regulatory T cells function, promoting pro-inflammatory Th17 responses [
16].
Clinically, endometriosis patients exhibit depleted
Faecalibacterium prausnitzii (an anti-inflammatory commensal) and enriched
Escherichia coli (a pathobiont linked to endotoxemia) [
17,
18]. Germ-free mice colonized with patient-derived microbiota show increased lesion growth, confirming a causal role for dysbiosis. In the few human studies presented today, the hypothesis of a possible connection between the presence of endometriosis and changes in microbiota composition is being investigated. For example, one study showed a decrease in alpha diversity in endometriosis, as well as differences in the abundance of 12 genera belonging to the classes
Bacilli,
Bacteroidia,
Clostridia,
Coriobacteriia, and
Gammaproteobacter between patients with endometriosis and the control group. Patients with isolated ovarian endometrioid cysts had a higher abundance of
Lachnobacterium, belonging to the class Clostridia, and
Adlercreutzia, belonging to the class
Coriobacteriia, compared to patients with a combination of several forms of endometriosis. Women with endometriosis and gastrointestinal symptoms had lower levels of SMB53 in the class Clostridia, lower levels of
Odoribacter, and higher levels of bacteria from the genus
Prevotella compared to patients without gastrointestinal symptoms. A correlation was found between an increase in the number of bacteria from the genus
Prevotella and symptoms such as constipation (
p = 0.014), bloating and flatulence (
p = 0.016), and nausea and vomiting (
p = 0.017). Thus, the connection between different intestinal symptoms of the disease and the presence of endometriosis may serve as one of the confirmations of the bacterial infection hypothesis [
19]. In another study, Shan J et al. also showed a decrease in alpha diversity and a higher
Bacillota/
Bacteroidota ratio in patients with endometriosis. Differences were also observed in the abundance of taxa such as
Actinomycetota,
Tenericutes,
Blautia, Bifidobacterium, Dorea, and
Streptococcus between the two groups. At the same time, the predominant taxon in women with endometriosis was Prevotella_7, while in the control group it was Coprococcus_2 [
16]. An increase in the number of bacteria from the genus
Prevotella was also detected in the study by Svensson et al., where a significant correlation with intestinal symptoms in patients with endometriosis was found [
19]. Ata B. showed that in women with endometriosis, fecal samples exhibited a significant decrease in the number of bacteria from the genera
Sneathia,
Barnesella, and
Gardnerella (
p < 0.01); vaginal samples showed a complete absence of Gemella and
Atopobium; and endocervical samples showed a complete absence of
Atopobium and
Sneathia and a significant increase in the number of
Alloprevotella (
p < 0.01) [
20]. Thus, the study of microbiota composition in patients with endometriosis is of scientific interest; however, the available literature data are contradictory and share only some commonalities—a decrease in microbiological diversity, an increase in the abundance of opportunistic pathogens, and a decrease in the number of commensal bacteria.
Given these findings, microbiota-targeted interventions, such as probiotics, prebiotics, and fecal microbiota transplantation (FMT), are emerging as potential therapeutic strategies. However, mechanistic studies and randomized controlled trials (RCTs) are still lacking, underscoring the need for further research into this novel pathophysiological pathway.
Current literature addresses the effects of estrogens, estrogen-containing medications (including combined oral contraceptives and hormone replacement therapy), as well as certain progestogens such as depot medroxyprogesterone acetate and the levonorgestrel-releasing intrauterine system on microbiome composition [
21,
22,
23,
24,
25]. However, available research data remain limited and inconsistent, justifying further investigations into the influence of sex steroid hormones on gut microbiota and female reproductive tract microbiota. This is particularly relevant in the context of hormone-dependent conditions such as endometriosis.
This study, for the first time, obtained data for the influence of DNG on the composition of gut microbiota in patients with endometriosis. To date, data on the effects of dienogest on the composition of the intestinal microbiota are limited, and the mechanisms of action of dienogest associated with microbial composition are also unclear. Based on the results of the conducted study, a tendency of microbiological parameters to improve in patients with endometriosis upon therapy with DNG was noted. This, on the one hand, may be associated with the direct influence of preparations on GM, which is connected with its anti-inflammatory activity through the inhibitory action on the growth and production of pro-inflammatory cytokines in endometrioid cells. Moreover, the influence of progesterone, as exogenous and endogenous, on composition microbiota is covered in studies conducted on pregnant women. For example, in their experimental study, Zhou Z. and co-authors demonstrated that exogenous introduction progesterone increased transepithelial electrical resistance in large intestine tissues due to strengthening the expression of the tight contacts of the integral protein occludin, causing a dose-dependent effect. We have grounds to believe that progesterone may promote the reduction in permeability mucous membrane intestine, preventing systemic microbial translocation and the development chronic inflammation [
26].
On other side, DNG may influence GM indirectly by reducing the degree of prevalence and activity of endometrioid foci due to its antiproliferative activity, antiangiogenic activity and other effects [
20]. In this context, DNG had a tendency to increase species richness and demonstrated a statistically significant reduction in Bacillota/Bacteroidota ratio (
p = 0.0421). The Bacillota/Bacteroidota ratio is a significant indicator for assessing the gut microbial composition: according to Shan J et al., both a decrease in alpha diversity and an increase in the Bacillota/Bacteroidota ratio are observed in patients with endometriosis compared to conventionally healthy women [
16]. Upon reduction, the index Bacillota/Bacteroidota on one side may influence the absolute reduction in the quantity of facultative–anaerobic opportunistic pathogens, such as
Staphylococcus spp. (type Bacillota). On the other hand, there was some tendency for the number of bacteroids to increase (type: Bacteroidota). Besides this, an increase in the colonization of commensal bacteria,
Lactobacillus spp. and
Collinsella aerofaciens, was noted.
C. aerofaciens is representative of the normoflora in the human large intestine, known its ability to ferment a series of carbohydrates of both plant and animal origin and produce H2, ethanol, short-chain fatty acids (SCFA) and lactate [
27]. In particular,
C. aerofaciens possesses the ability to produce butyrate, also referred to as ‘useful’ SCFA, supporting the integrity of the epithelial barrier [
28,
29]. A reduction in
C. aerofaciens in patients with irritable bowel syndrome was also shown, as was their role as predictors of positive responses in patients on probiotics as therapy [
30].
The obtained results of the conducted study indicate that taking DNG through direct and indirect action promotes a change in the composition of microbiota toward increased bacterial diversity due to the bacteria–symbionts interaction, producing ‘useful’ SCFA. On the other hand, it reduces the number and titer of opportunistic pathogens, capable of producing endotoxins and participating in the metabolism of estrogen.
5. Conclusions
Endometriosis is associated with distinct microbial signatures across gut and reproductive niches, driven by inflammation, estrogen metabolism, and barrier dysfunction. Targeting these microbial alterations may offer novel diagnostic and therapeutic avenues.
Thus, DNG therapy for 6 months is effective not only in reducing the severity of clinical manifestations in patients with endometriosis, which is common knowledge, but also in improving the composition of the intestinal microbiota. There was a trend towards an increase in species and taxonomic diversity, a decrease in the Bacillota/Bacteroidota index and Staphylococcus spp. colonization, and an increase in the number of symbiont bacteria Lactobacillus spp. and Collinsella aerofaciens.
6. Strengths and Limitations
This is the first study known to date examining the effects of dienogest on the composition of the gut microbiota in patients with endometriosis.
The strength of the study is the comprehensive microbiological examination of this group of patients using culture methods with a broad assessment of the composition of the intestinal microbiota and verification of the isolated microorganisms down to the species level. The data obtained by these methods have advantages in clinical practice, in contrast to sequencing, which is an expensive and limited method. Diagnosis of the disease was based on the results of expert ultrasound and MRI scans of the pelvic organs, performed in the National Centre of the Russian Federation.
The limitations of this study are the small sample of patients, the absence of histological verification in most patients due to their conservative investigation (only three women had previously undergone laparoscopy with subsequent histology), and the absence of sequencing, which is planned to be carried out in future studies. In addition, despite the similarity of the data obtained, there are some limitations in comparison and interpretation, since different microbiological research methods were used.