Bacteremia Outbreak Due to Achromobacter xylosoxidans in Hospitalized COVID-19 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Definitions
2.2. Blood Cultures
2.3. DNA Extraction
2.4. Next-Generation Sequencing and Bioinformatic Analysis
2.5. Outbreak Investigation
3. Results
3.1. Patient Demographics and Clinical Presentation
3.2. Microbial Identification and Antibiotic Resistance Profile
3.3. Molecular Characterization of A. xylosoxidans
3.4. Investigation of the Source of the Outbreak
3.5. Control Measures and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef]
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide; Patient Safety; A World Aliance for Safer Health Care; WHO Press: Geneva, Switzerland, 2011; Available online: http://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.pdf?sequence=1 (accessed on 1 May 2025).
- Tao, C.; Gan, Y.; Su, W.; Li, Z.; Tang, X. Effectiveness of hospital disinfection and experience learnt from 11 years of surveillance. J. Biomed. Res. 2019, 33, 408–413. [Google Scholar] [CrossRef]
- Amini Tapouk, F.; Nabizadeh, R.; Mirzaei, N.; Hosseini Jazani, N.; Yousefi, M.; Valizade Hasanloei, M.A. Comparative efficacy of hospital disinfectants against nosocomial infection pathogens. Antimicrob. Resist. Infect. Control 2020, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, E.; Daloglu, A.E.; Inanoglu, K.; Ozturk, N.K.; Ozmen, S.B. Bacterial and fungal secondary infections occurring in COVID-19 patients followed in intensive care: A retrospective study. J. Infect. Dev. Ctries. 2024, 18, 350–354. [Google Scholar] [CrossRef]
- Haque, O.I.; Shameem, M.; Hashim, W. Secondary infections in critically ill patients with COVID-19: A retrospective single-center study. Lung India 2023, 40, 210–214. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Markovskaya, Y.; Gavioli, E.M.; Cusumano, J.A.; Glatt, A.E. Coronavirus disease 2019 (COVID-19): Secondary bacterial infections and the impact on antimicrobial resistance during the COVID-19 pandemic. Antimicrob. Steward Healthc. Epidemiol. 2022, 2, e114. [Google Scholar] [CrossRef]
- Matijasic, N.; Tripalo Batos, A.; Lenicek Krleza, J.; Rogulj, M.; Pavic, I. Achromobacter xylosoxidans Purulent Bronchitis in a Previously Healthy Child: An Unexpected Consequence of COVID-19 Infection. Cureus 2022, 14, e21711. [Google Scholar] [CrossRef]
- Guchhait, P.; Chaudhuri, B.N.; Das, S. Bloodstream Infections with Opportunistic Pathogens in the COVID-19 Era: A Real Challenge Necessitating Stringent Infection Control. J. Lab. Physicians 2023, 15, 131–138. [Google Scholar] [CrossRef]
- Devi, P.; Maurya, R.; Mehta, P.; Shamim, U.; Yadav, A.; Chattopadhyay, P.; Kanakan, A.; Khare, K.; Vasudevan, J.S.; Sahni, S.; et al. Increased Abundance of Achromobacter xylosoxidans and Bacillus cereus in Upper Airway Transcriptionally Active Microbiome of COVID-19 Mortality Patients Indicates Role of Co-Infections in Disease Severity and Outcome. Microbiol. Spectr. 2022, 10, e0231121. [Google Scholar] [CrossRef]
- Sinha, S.; Raj, N.; Dobhal, S.; Das, A.; Agarwal, J. Achromobacter spp.: A retrospective review of rare and emerging pathogen. MGM J. Med. Sci. 2023, 10, 106–110. [Google Scholar] [CrossRef]
- Schoch, P.E.; Cunha, B.A. Nosocomial Achromobacter xylosoxidans Infections. Infect. Control Hosp. Epidemiol. 1988, 9, 84–87. [Google Scholar] [CrossRef]
- Yabuuchi, E.; Kawamura, Y.; Kosako, Y.; Ezaki, T. Emendation of Genus Achromobacter and Achromobacter xylosoxidans (Yabuuchi and Yano) and Proposal of Achromobacter ruhlandii (Packer and Vishniac) Comb. Nov., Achromobacter piechaudii (Kiredjian et al.) Comb. Nov., and Achromobacter xylosoxidans Subsp. denitrificans (Rüger and Tan) Comb. Nov. Microbiol. Immunol. 1998, 42, 429–438. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, H.; Lim, S.M.; Kang, J.M. Nosocomial outbreak of Achromobacter spp. bacteremia due to germicide contamination: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6374–6381. [Google Scholar] [CrossRef]
- Hugon, E.; Marchandin, H.; Poirée, M.; Fosse, T.; Sirvent, N. Achromobacter bacteraemia outbreak in a paediatric onco-haematology department related to strain with high surviving ability in contaminated disinfectant atomizers. J. Hosp. Infect. 2015, 89, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Amoureux, L.; Bador, J.; Fardeheb, S.; Mabille, C.; Couchot, C.; Massip, C.; Salignon, A.L.; Berlie, G.; Varin, V.; Neuwirth, C. Detection of Achromobacter xylosoxidans in Hospital, Domestic, and Outdoor Environmental Samples and Comparison with Human Clinical Isolates. Appl. Environ. Microbiol. 2013, 79, 7142–7149. [Google Scholar] [CrossRef]
- Arjun, R.; John, K.E.; Niyas, V.K.M.; Nair, S.R.; Mohan, V.; Ratheesh, R.S. Achromobacter spp. bacteremia outbreak related to contaminated furosemide ampoules. Infez. Med. 2021, 29, 427–433. [Google Scholar] [CrossRef]
- Gomez-Cerezo, J.; Suarez, I.; Rios, J.J.; Pena, P.; Garcia de Miguel, M.J.; de Jose, M.; Monteagudo, O.; Linares, P.; Barbado-Cano, A.; Vazquez, J.J. Achromobacter xylosoxidans Bacteremia: A 10-Year Analysis of 54 Cases. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 360–363. [Google Scholar] [CrossRef]
- Marion-Sanchez, K.; Pailla, K.; Olive, C.; Le Coutour, X.; Derancourt, C. Achromobacter spp. healthcare associated infections in the French West Indies: A longitudinal study from 2006 to 2016. BMC Infect. Dis. 2019, 19, 795. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Guo, J.; Yan, W.; Jin, Y.; Pan, F.; Fang, X.; Qin, L.; Liu, C. Hospital-acquired pneumonia due to Achromobacter xylosoxidans in the elderly: A single-center retrospective study in Beijing. J. Infect. Dev. Ctries. 2017, 11, 10–18. [Google Scholar] [CrossRef]
- Liu, T.; Coenye, J.L.; Burns, P.W.; Whitby, T.L.; Stull, J.J.; LiPuma, J.J. Ribosomal DNA-Directed PCR for Identification of Achromobacter (Alcaligenes) xylosoxidans Recovered from Sputum Samples from Cystic Fibrosis Patients. J. Clin. Microbiol. 2002, 40, 1210–1213. [Google Scholar] [CrossRef]
- Turton, J.F.; Mustafa, N.; Shah, J.; Hampton, C.V.; Pike, R.; Kenna, D.T. Identification of Achromobacter xylosoxidans by detection of the blaOXA-114-like gene intrinsic in this species. Diagn. Microbiol. Infect. Dis. 2011, 70, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Bonis, M.; Hunter, J.M.M. Profile: Achromobacter xylosoxidans: The cloak-and-dagger opportunist. J. Med. Microbiol. 2022, 71, 001505. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, Y.; Ma, Y.; Liu, F.; Lu, N.; Yang, X.; Luan, C.; Yi, Y.; Zhu, B. Genomic Insights into Intrinsic and Acquired Drug Resistance Mechanisms in Achromobacter xylosoxidans. Antimicrob. Agents Chemother. 2015, 59, 1152–1161. [Google Scholar] [CrossRef]
- Mahoney, M.V.; Hirsch, E.B.; Wright, W.F. AMRrounds: Achromobacter xylosoxidans susceptibility—So It Goes. JAC-Antimicrob. Resist. 2024, 6, dlae134. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Principles and Procedures for Blood Cultures, 2nd ed.; Approved Guideline M47; CLSI: Wayne, PA, USA, 2022; Available online: https://cdn.bfldr.com/YLD4EVFU/at/kp4fxwnckcv9zqs6ts93xh/m47ed2e_sample.pdf (accessed on 1 May 2025).
- National Library of Medicine. National Center of Biotechnology Information. BLAST®. Available online: https://blast.ncbi.nlm.nih.gov (accessed on 25 June 2025).
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018, 19, 307. [Google Scholar] [CrossRef]
- Clausen, P.T.; Zankari, E.; Aarestrup, F.M.; Lund, O. Benchmarking of methods for identification of antimicrobial resistance genes in bacterial whole genome data. J. Antimicrob. Chemother. 2016, 71, 2484–2488. [Google Scholar] [CrossRef]
- Alcock, B.P.; Huynh, W.; Chalil, R.; Smith, K.W.; Raphenya, A.R.; Wlodarski, M.A.; Edalatmand, A.; Petkau, A.; Syed, S.A.; Tsang, K.K.; et al. CARD 2023: Expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2023, 51, D690–D699. [Google Scholar] [CrossRef]
- Spilker, T.; Vandamme, P.; Lipuma, J.J. A multilocus sequence typing scheme implies population structure and reveals several putative novel Achromobacter species. J. Clin. Microbiol. 2012, 50, 3010–3015. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-Access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Bryan, C.S. Clinical implications of positive blood cultures. Clin. Microbiol. Rev. 1989, 2, 329–353. [Google Scholar] [CrossRef]
- Nielsen, S.L. The incidence and prognosis of patients with bacteremia. Dan. Med. J. 2015, 62, B5128. Available online: https://www.researchgate.net/publication/280118814 (accessed on 4 July 2025). [PubMed]
- Doern, G.V.; Carroll, K.C.; Diekema, D.J.; Garey, K.W.; Rupp, M.E.; Weinstein, M.P.; Sexton, D.J. Practical Guidance for Clinical Microbiology Laboratories: A Comprehensive Update on the Problem of Blood Culture Contamination and a Discussion of Methods for Addressing the Problem. Clin. Microbiol. Rev. 2019, 33, e00009-19. [Google Scholar] [CrossRef] [PubMed]
- Protano, C.; Cammalleri, V.; Romano Spica, V.; Valeriani, F.; Vitali, M. Hospital environment as a reservoir for cross transmission: Cleaning and disinfection procedures. Ann. Ig. 2019, 31, 436–448. [Google Scholar] [CrossRef]
- Chaaban, T.; Ezzeddine, Z.; Ghssein, G. Antibiotic Misuse during the COVID-19 Pandemic in Lebanon: A Cross-Sectional Study. COVID 2024, 4, 921–929. [Google Scholar] [CrossRef]
- Adebisi, Y.A.; Alaran, A.J.; Okereke, M.; Oke, G.I.; Amos, O.A.; Olaoye, O.C.; Oladunjoye, I.; Olanrewaju, A.Y.; Ukor, N.A.; Lucero-Prisno, D.E., 3rd. COVID-19 and Antimicrobial Resistance: A Review. Infect. Dis. 2021, 14, 11786337211033870. [Google Scholar] [CrossRef]
- Tena, D.; Carranza, R.; Barberá, J.R.; Valdezate, S.; Garrancho, J.M.; Arranz, M.; Sáez-Nieto, J.A. Outbreak of long-term intravascular catheter-related bacteremia due to A. xylosoxidans in a hemodialysis unit. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 727–732. [Google Scholar] [CrossRef]
- Said, M.; Mitton, B.; Skosana, L.B.; Kopotsa, K.; Naidoo, R.; Amutenya, V. Outbreak and control of Achromobacter denitrificans at an academic hospital in Pretoria, South Africa. Infect. Control Hosp. Epidemiol. 2023, 44, 24–30. [Google Scholar] [CrossRef]
- Vázquez Castellanos, J.L.; Copado Villagrana, E.D.; Torres Mendoza, B.M.G.; Gallegos Durazo, D.L.; González Plascencia, J.; Mejía-Zárate, A.K. First bacteremia outbreak due Achromobacter spp. in hemodialysis patients in Mexico. Nefrologia (Engl. Ed.) 2022, 42, 101–103. [Google Scholar] [CrossRef]
- Trancassini, M.; Iebba, V.; Citerà, N.; Tuccio, V.; Magni, A.; Varesi, P.; De Biase, R.V.; Totino, V.; Santangelo, F.; Gagliardi, A.; et al. Outbreak of Achromobacter xylosoxidans in an Italian Cystic fibrosis center: Genome variability, biofilm production, antibiotic resistance, and motility in isolated strains. Front. Microbiol. 2014, 5, 138. [Google Scholar] [CrossRef]
- Begum, H.A.; Tanni, T.R.; Shahid, M.A. Analysis of Water Absorption of Different Natural Fibers. J. Textile Sci. Technol. 2021, 7, 152–160. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Khoshkharam, M.; Shen, H.; Cheng, Q. Cultivation of Cotton in China and Iran with Considering Biological Activities and Its Health Benefits. Cercet. Agronomice in Moldova 2020, 53, 105–120. [Google Scholar] [CrossRef]
- Oie, S.; Kamiya, A. Microbial Contamination of Antiseptic-Soaked Cotton Balls. Biol. Pharm. Bull. 1997, 20, 667–669. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wißmann, J.E.; Kirchhoff, L.; Brüggemann, Y.; Todt, D.; Steinmann, J.; Steinmann, E. Persistence of Pathogens on Inanimate Surfaces: A Narrative Review. Microorganisms 2021, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Mandell, W.F.; Garvey, G.J.; Neu, H.C. Achromobacter xylosoxidans Bacteremia. Rev. Infect. Dis. 1987, 9, 1001–1005. [Google Scholar] [CrossRef]
- Gorska, A.; Sloderbach, A.; Marszałł, M.P. Siderophore-drug complexes: Potential medicinal applications of the ‘Trojan horse’ strategy. Trends Pharmacol. Sci. 2014, 35, 442–449. [Google Scholar] [CrossRef]
- Ezzeddine, Z.; Ghssein, G. Towards new antibiotics classes targeting bacterial metallophores. Microb. Pathog. 2023, 182, 106221. [Google Scholar] [CrossRef]
- Schalk, I.J. A Trojan-horse strategy including a bacterial suicide action for the efficient use of a specific Gram-positive antibiotic on Gram-negative bacteria. J. Med. Chem. 2018, 61, 3842–3844. [Google Scholar] [CrossRef]
- Fluit, A.C.; Bayjanov, J.R.; Aguilar, M.D.; Benaissa-Trouw, B.; Tunney, M.M.; Westreenen, M.V.; Meis, J.F.; Elborn, J.S.; Cantón, R.; Ekkelenkamp, M.B. Taxonomic position, antibiotic resistance, and virulence factors of clinical Achromobacter isolates. Front. Biosci. 2022, 14, 9. [Google Scholar] [CrossRef]
- Pouch, S.M.; Patel, G.; AST Infectious Diseases Community of Practice. Multidrug-resistant Gram-negative bacterial infections in solid organ transplant recipients—Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13594. [Google Scholar] [CrossRef]
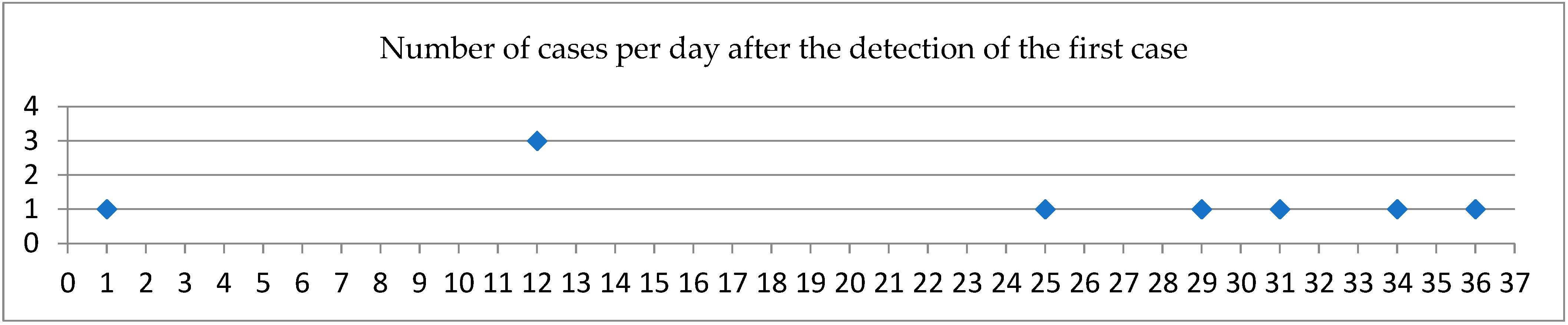
| Age | Sex | COVID-19 Vaccine Doses | Days Between Admission and Detection of A. xylosoxidans | Underlying Diseases |
|---|---|---|---|---|
| 55 | Male | 0 | 4 | - |
| 61 | Female | 0 | 11 | Hypothyroidism |
| 59 | Male | 0 | 10 | - |
| 61 | Female | 1 | 17 | Asthma |
| 70 | Male | 3 | 11 | Myocardial infarction, dyslipidemia |
| 87 | Female | 3 | 19 | Hypertension, gastroesophageal reflux disease |
| 76 | Male | 2 | 12 | Hypertension, dyslipidemia |
| 71 | Male | 0 | 11 | Hypertension, dyslipidemia, diabetes |
| 83 | Female | 2 | 12 | Breast cancer, Alzheimer’s |
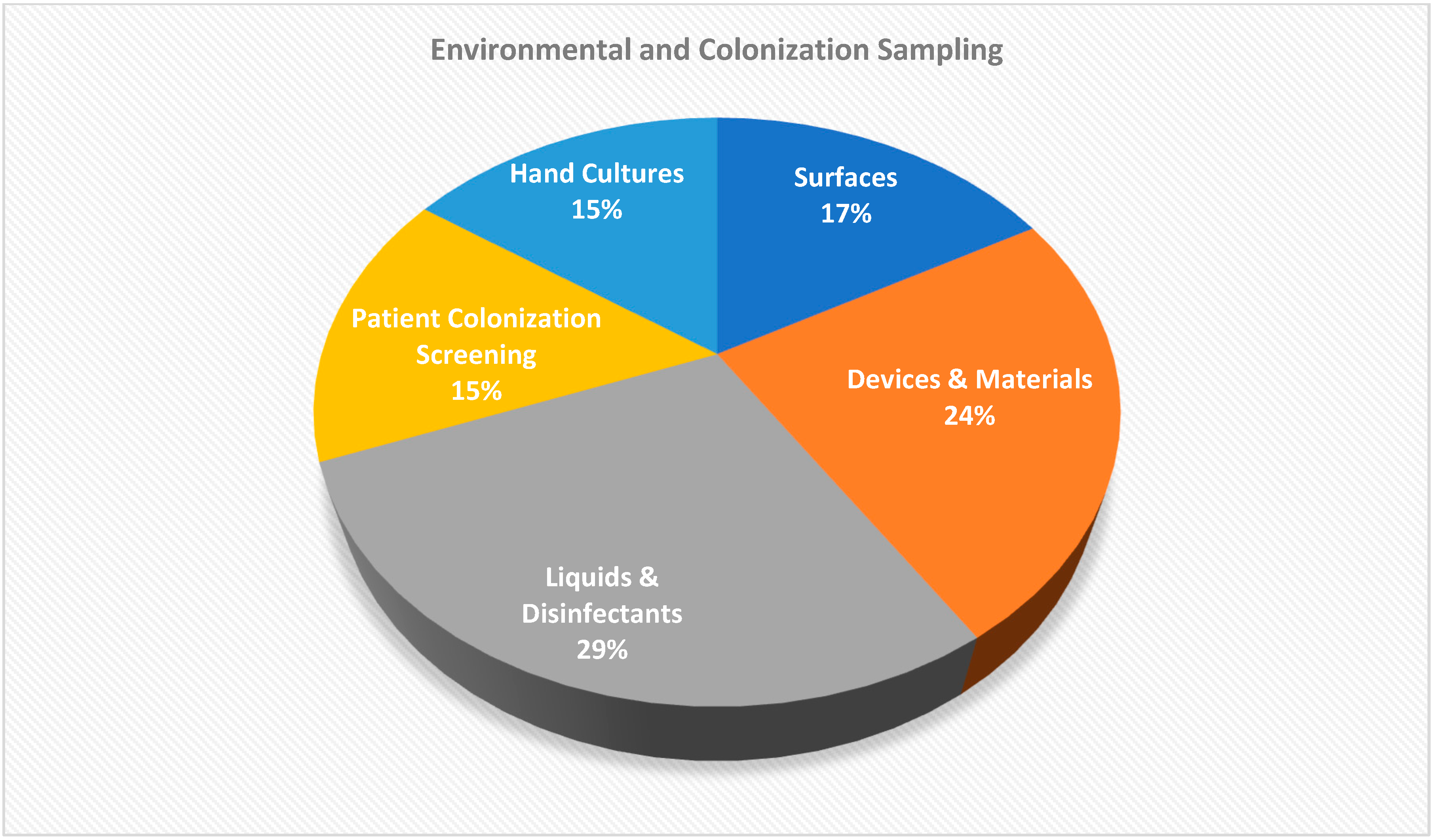
| Sample Type | Number (n = 140) | Culture Result | Organism Detected |
|---|---|---|---|
| Environmental surfaces | 22 | No growth | — |
| Liquid and disinfectant solutions | 37 | No growth | — |
| Materials and devices | 31 | No growth | — |
| Colonization screening samples | 20 | No growth | — |
| Hand cultures | 20 | No growth | — |
| Cotton | 1 | Positive | Achromobacter xylosoxidans |
| Blood cultures | 9 | Positive | Achromobacter xylosoxidans |
| Antimicrobial Agent | Number of Isolates (n = 9) | ||
|---|---|---|---|
| Number of Resistant Isolates | Resistance Rate (%) | Interpretation | |
| Amikacin | 9 | 100 | Resistant |
| Gentamicin | 9 | 100 | Resistant |
| Tobramycin | 9 | 100 | Resistant |
| Aztreonam | 9 | 100 | Resistant |
| Cefotaxime | 9 | 100 | Resistant |
| Cefoxitin | 9 | 100 | Resistant |
| Ceftazidime | 9 | 100 | Resistant |
| Ciprofloxacin | 9 | 100 | Resistant |
| Colistin | 0 | 0 | Susceptible |
| Imipenem | 0 | 0 | Susceptible |
| Meropenem | 0 | 0 | Susceptible |
| Trimethoprim/Sulfamethoxazole | 0 | 0 | Susceptible |
| Piperacillin/Tazobactam | 0 | 0 | Susceptible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsekoura, M.; Petridis, G.; Koutsouflianiotis, K.; Pappa, S.; Papa, A.; Kontopoulou, K. Bacteremia Outbreak Due to Achromobacter xylosoxidans in Hospitalized COVID-19 Patients. Microbiol. Res. 2025, 16, 156. https://doi.org/10.3390/microbiolres16070156
Tsekoura M, Petridis G, Koutsouflianiotis K, Pappa S, Papa A, Kontopoulou K. Bacteremia Outbreak Due to Achromobacter xylosoxidans in Hospitalized COVID-19 Patients. Microbiology Research. 2025; 16(7):156. https://doi.org/10.3390/microbiolres16070156
Chicago/Turabian StyleTsekoura, Magdalini, Georgios Petridis, Konstantinos Koutsouflianiotis, Styliani Pappa, Anna Papa, and Konstantina Kontopoulou. 2025. "Bacteremia Outbreak Due to Achromobacter xylosoxidans in Hospitalized COVID-19 Patients" Microbiology Research 16, no. 7: 156. https://doi.org/10.3390/microbiolres16070156
APA StyleTsekoura, M., Petridis, G., Koutsouflianiotis, K., Pappa, S., Papa, A., & Kontopoulou, K. (2025). Bacteremia Outbreak Due to Achromobacter xylosoxidans in Hospitalized COVID-19 Patients. Microbiology Research, 16(7), 156. https://doi.org/10.3390/microbiolres16070156






