Abstract
Helicobacter pylori, which colonizes the human gastric mucosa, uses a cluster of polar, sheathed flagella to swim across the mucous layer of the stomach. The function and biogenesis of the H. pylori flagellar sheath are poorly understood. Cardiolipin is a phospholipid that accumulates in regions of the membrane that have negative curvature, such as the cell pole, cell septum, and flagellar sheath. The final step in cardiolipin biosynthesis is catalyzed by cardiolipin synthase. H. pylori has at least two cardiolipin synthases, one of which is cardiolipin synthase C (ClsC). Bioinformatic analysis revealed that homologs of H. pylori ClsC are restricted to Helicobacter species that have sheathed flagella and the ClsC homologs are predicted lipoproteins. Fluorescence microscopy revealed that a ClsC super-folder green fluorescent protein localized to the cell pole and cell septum in H. pylori G27. Comparing the proteomes of isolated sheathed flagella from the H. pylori B128 wild type and a clsC::cat mutant, we identified five proteins that were absent in the mutant flagellum preparations. One of the proteins was FaaA, an autotransporter that localizes to the flagellar sheath. These findings suggest that the localization of FaaA and possibly other proteins to the flagellar sheath is dependent on ClsC.
1. Introduction
Helicobacter pylori is a Gram-negative, helical-shaped bacterium that belongs to the phylum Campylobacterota. H. pylori maintains a stable presence in the highly specific ecological niche of the human gastric mucosa, and it is estimated that approximately half of the world’s population is infected with the bacterium [1]. While the majority of individuals infected with H. pylori are asymptomatic, H. pylori infections can lead to a variety of gastric pathologies, including chronic gastritis, peptic ulcer disease, and gastric cancer [2,3,4]. Gastric cancer accounts for one-third of cancer-related deaths, and the majority of these gastric cancer-related deaths are attributed to H. pylori infection [2,5]. Due to the linkage between H. pylori infection and gastric cancer, H. pylori was labeled as a class I carcinogen in 1994 by the International Agency for Research on Cancer and the World Health Organization [6].
H. pylori cells typically possess four to six sheathed, polar flagella that are used for motility, which is required for the colonization of the gastric mucosa in animal models [7,8]. The flagellar sheath surrounds the filament and is contiguous with the outer membrane [9]. The flagellar sheath is a structure that is found in a number of other bacteria, including most Helicobacter and Vibrio species [10]. Although the role of the flagellar sheath in H. pylori is not known, proposed functions include the protection of the flagellar filament from depolymerization by gastric acid and the promotion of adherence to the gastric epithelium [9,11]. The flagellar sheaths of some bacterial species have been suggested to be important in avoiding the detection of the flagellins (i.e., flagellar filament proteins) by the host innate immune system [10]. Bacterial flagellins activate Toll-like receptor 5 (TLR5) in the host to induce interleukin-8 (IL-8) secretion as part of the innate immune response [12]. In support of this idea, Yoon and Mekalanos showed that, even though the flagellins of Vibrio cholerae and Salmonella enterica serovar Typhimurium (S. Typhimurium) elicit similar TLR5-mediated innate immune responses, the sheathed flagella of V. cholerae display a significant reduction in triggering a host innate immune response compared to the unsheathed S. Typhimurium flagella [13]. The H. pylori flagellar sheath likely does not play the primary role in avoiding surveillance by the innate immune system, however, as H. pylori flagellins are significantly less potent than S. Typhimurium flagellins in activating TLR5-mediated IL-8 secretion [14,15].
Another potential function of the H. pylori flagellar sheath is the generation of outer membrane vesicles (OMVs). OMVs are released as part of normal bacterial growth and function in a variety of processes, including the delivery of lipopolysaccharide (LPS), proteins, and toxins to target cells; the secretion of enzymes involved in nutrient acquisition; horizontal gene transfer; functioning as decoys for bacteriophages and antibiotics; and the stress response [16,17]. In various Vibrio species, the flagellar sheath has been demonstrated to be a major source of OMVs [18,19]. OMVs derived from the flagellar sheath in Vibrio species have been proposed to originate from membrane blebs along the sheath, which are released as the flagella rotate [18].
For a given bacterial species, knowledge of the proteins that localize to the flagellar sheath is important in understanding the function of the sheath. To this end, several early studies sought to identify the proteins that are present in the sheath [9,11,20,21,22,23,24]. One early study reported that the flagellar sheath of Bdellovibrio bacteriovorus has significantly less protein (23–28% dry weight) than the typical bacterial outer membrane, which generally contains 40–70% protein by dry weight [24]. The reported protein content of the B. bacteriovorus sheath suggests that proteins do not exchange freely between the sheath and outer membrane, which implies that mechanisms are in place that control the protein content of the sheath. Consistent with this hypothesis, Doig and Trust identified six H. pylori protein antigens in the outer membrane that were not detected in the flagellar sheath [20]. Moreover, some proteins have been reported to localize preferentially to the H. pylori flagellar sheath. One such protein is H. pylori adhesion A (HpaA), although there are conflicting reports on the localization of this protein. HpaA was initially observed on the cell surface by immunogold labeling but was not observed on the sheath [25]. A subsequent immunogold labeling study reported that HpaA localized specifically to the sheath [11,26], while another study reported that HpaA was present on both the sheath and cell surface [27]. Another protein that localizes to the H. pylori flagellar sheath is flagellar-associated autotransporter A (FaaA), which was shown by both immunogold labeling and fluorescence microscopy to localize to the sheath [28]. Although the function of FaaA is not known, disrupting faaA resulted in various flagellum-related defects, including decreased motility, reduced flagellation, an increased frequency of broken flagella, and a higher proportion of flagella that localized to nonpolar sites [28].
In an effort to identify proteins with potential roles in the function or biogenesis of the H. pylori flagellar sheath, Gibson and co-workers identified homologs of 42 H. pylori proteins that were widespread in Helicobacter species that possess flagellar sheaths (FS+ species) but were underrepresented in Helicobacter species with sheath-less flagella (FS− species) [29]. One of the proteins that was found preferentially in FS+ Helicobacter species was cardiolipin synthase C (ClsC), which catalyzes the final step in the synthesis of the phospholipid cardiolipin [30]. Cardiolipin consists of four acyl chains attached to a glycerol backbone, which results in the cardiolipin molecule being cone-shaped with a small polar head and a large hydrophobic tail. Cardiolipin molecules form clusters or microdomains that have intrinsic curvature and thus lower energy when localized to regions of the membrane that have negative curvature [31,32]. Consequently, cardiolipin tends to accumulate in membrane regions with negative curvature, such as the cell pole and septum in rod-shaped bacteria [33,34,35,36]. Given that the flagellar sheath is a long, tube-like structure, as well as the proclivity of cardiolipin to accumulate in membranes with negative curvature, one would expect the sheath to contain significant amounts of cardiolipin. Consistent with this hypothesis, the H. pylori flagellar sheath does indeed have significant amounts of cardiolipin [37]. Additional supporting evidence for this hypothesis is the observation that the most abundant fatty acyl chains in the H. pylori flagellar sheath are myristic acid (C14:0) and cyclo-nonadecanoic acid (cycC19:0) [9], which are also the most common fatty acyl chains in H. pylori cardiolipin species [30,38,39].
The disruption of clsC in H. pylori strains G27 and B128 resulted in decreased amounts of cardiolipin, although some cardiolipin was still synthesized in the clsC mutants, indicating that H. pylori strains have at least one more cardiolipin synthase [30]. Escherichia coli has three different cardiolipin synthases, ClsA, ClsB, and ClsC [40,41,42]. Interestingly, the vast majority of the cardiolipin in the H. pylori clsC mutants was in the form of monolysocardiolipin [30], which has three instead of four acyl chains.
We report here that homologs of H. pylori ClsC are restricted to Helicobacter species that have sheathed flagella, and these ClsC homologs are predicted lipoproteins. We further show that a ClsC super-folder green fluorescent protein (ClsC-sfGFP) fusion localized to the cell pole and cell septum in H. pylori G27. Finally, we investigated whether cardiolipin might be required for the localization of some proteins to the H. pylori flagellar sheath, since cardiolipin is required for the polar localization of some proteins in E. coli, including ProP, MscS, and ClsA [36,43]. Comparing the proteomes of flagella preparations from a H. pylori B128 clsC::cat mutant and the wild-type H. pylori B128 parental strain, we identified five proteins in the wild-type flagellum preparations that were not found in the flagella isolated from the clsC::cat mutant. All five proteins are predicted to have signal peptides recognized by the Sec translocon and cleaved by signal peptidase I (Sec/SPI signal peptide), indicating that the proteins are likely secreted across the inner membrane. One of the proteins, FaaA, is an autotransporter that is reported to localize to the flagellar sheath [28]. Taken together, these data suggest that the localization of FaaA and possibly other proteins to the H. pylori flagellar sheath is dependent on ClsC.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
E. coli Turbo (New England Biolabs, Ipswitch, MA, USA) was used for cloning procedures and was cultured in lysogeny broth (LB) and LB agar. Growth media for E. coli strains were supplemented with ampicillin (100 µg/mL) or kanamycin (30 µg/mL) when appropriate. H. pylori G27 (kindly provided by D. Scott Merrell), H. pylori B128 (kindly provided by Richard M. Peck, Jr.), and clsC::cat mutants derived from G27 and B128 [30] were grown on tryptic soy agar supplemented with 5% heat-inactivated horse serum (TSA-HS) and 30 μL/mL kanamycin when appropriate. H. pylori strains on agar medium were grown at 37 °C under atmospheric conditions consisting of 10% CO2, 8% O2, and 82% N2. For liquid cultures, H. pylori strains were grown at 37 °C with shaking in Mueller–Hinton broth (MHB) supplemented with 5% heat-inactivated horse serum under an atmosphere consisting of 5% CO2, 10% H2, 10% O2, and 75% N2.
2.2. Construction of ClsC-sfGFP Fusion
The ClsC-sfGFP was expressed from plasmid pDE43, which is a derivative of the shuttle vector pHel3 [44] and was constructed as follows. Plasmid pHel3-Myc [45] is a derivative of pHel3 that carries the H. pylori fliF promoter upstream of tandem BspQ1 sites used for Golden Gate cloning, which is followed by a sequence encoding a flexible linker, c-Myc epitope, and DDDDK epitope. Plasmid pHel3-GFP was generated by replacing the sequences encoding the c-Myc and DDDDK epitopes in pHel3-Myc with a sequence encoding a sfGFP [46] that was optimized for H. pylori codon usage and synthesized by Azenta Life Sciences (South Plainfield, NJ, USA). Primers sgfp1 (5′-TCGATATCTAGATCTCGAGTTTATTTATACAATTCATCCATGCC-3′) and sgfp2 (5′-TCAGGTGAATTTGCGGCCGCATGAGCAAAGGCGAAGAATTG-3′) were used to amplify the codon-optimized sfGFP gene sequence, and primers phel3-myc1 (5′-ACTCGAGATCTAGATATCGATG-3′) and phel3-myc2 (5′-GCGGCCGCAAATTCACCT-3′) were used to amplify most of plasmid pHel3-Myc. PrimeSTAR Max polymerase (Takara Bio USA Inc, San Jose, CA, USA) was used to amplify the pHel3-Myc and sfGFP DNA sequences as described by the supplier, with the exception of using annealing temperatures of 63 °C and 57 °C, respectively. Following the PCR, the samples were treated with the restriction enzyme DpnI (Promega, Madison, WI, USA) to digest the template DNA, and the amplicons were then transformed into E. coli Turbo cells for in vivo assembly as described in [47] to generate plasmid pHel3-GFP. Primers clsC-BspQ1F (5′-TGGCTCTTCTATGAAAATCTTTTTAGTCCTTTTAAGCGTC-3′) and clsC-BspQ1R (5′-ATGCTCTTCTACCAAGCTCTCTTTCAGGAAGGACT-3′) were used to amplify clsC from H. pylori B128 genomic DNA, and we introduced BspQ1 sites at the ends of the resulting amplicon. PrimeSTAR Max polymerase was used to amplify clsC, following the supplier’s protocol, using an annealing temperature of 64 °C. The clsC amplicon and plasmid pHel3-GFP were digested with pBspQ1 and ligated as described previously [45] to create plasmid pClsC-GFP, which was introduced by natural transformation into H. pylori G27, and the H. pylori G27 clsC::cat mutant and transformants were selected on TSA-HS supplemented with kanamycin.
2.3. Fluorescence Microscopy
One mL overnight cultures of H. pylori strains bearing the plasmid pClsC-GFP were centrifuged at 8700× g for 1 min to pellet the cells. The resulting supernatant liquids were discarded, and the cells were then resuspended in 300 µL of phosphate-buffered saline (PBS). For cells that were stained with the FM4-64 dye, 1 µL of a 10 mM FM4-64 dye stock solution was added to the 1 mL samples of the overnight cultures, and the samples were incubated for 30 min prior to the centrifugation step. Glass microscopy slides were prepared by applying 5 µL of a 10% (w/v) poly-lysine solution to each slide and allowing the slides to air-dry. Seven µL of the resuspended cells was applied to the slide, which was then covered with a 1.5 mm coverslip. Cells were visualized by fluorescence microscopy using a Nikon Ti-U fluorescence microscope equipped with a Lumenocor SOLA SM II light engine. The microscope was fitted with a 100× oil immersion objective (NA 1.45) and the GFP HISM Zero Shift and Texas Red Longpass filter sets. Images were captured using a CooolSNAP My camera (Teledyne Photometrics, Tuscon, AZ, USA) and controlled via the Nikon NIS-Elements BR software package (v. 4.20.1). The images were taken on DIC channel 1 with an exposure time of ~20 ms, GFP channel 3 with an exposure time of ~2 s, and FM464 channel 4 with an exposure time of ~200 ms. The resultant images were processed and analyzed using the ImageJ Fuji software package version 2.14.0/1.5f.
2.4. Mass Spectrometry Analysis of Isolated H. pylori Flagella
H. pylori flagella were isolated and analyzed as described [48]. Briefly, wild-type H. pylori B128 and the H. pylori B128 clsC::cat mutant grown on TSA-HS were resuspended in 3 to 4 mL phosphate-buffered saline (PBS). The cell suspensions were vortexed for 60 s to shear the flagella from the cells, and the cells were subsequently pelleted by centrifugation at 7300× g for 10 min. The resulting supernatants were further clarified by passage through 0.4 µm filters, and the filtrates were centrifuged at 106,000× g for 30 min to pellet the flagella. The flagellar pellets were then resuspended in 200 to 300 μL PBS, and the amounts of protein in the samples were quantified using the Pierce BCA Protein Assay (ThermoFisher Scientific, Waltham, MA, USA), as per the supplier’s instructions. Portions of H. pylori flagella preparations (5 μg total protein) were loaded onto an SDS–polyacrylamide gel and electrophoresed into the top of the resolving gel. Gel slices containing the proteins were sent to the University of Georgia Proteomics and Mass Spectrometry facility for proteomic analysis. Proteins in the gel slices were digested with trypsin by staff at the mass spectrometry facility, and the resulting peptides were analyzed by LC-MS/MS with a ThermoScientific Orbitrap Velo Elite mass spectrometer coupled with nano-HPLC using a 90 min elution gradient. Mass spectrometry data were searched against a protein database using the Mascot software search engine (Matrix Science, Boston, MA, USA) for protein identification.
3. Results
3.1. Homologs of H. pylori ClsC Are Predicted Lipoproteins and Are Restricted to Helicobacter Species That Have Flagellar Sheaths
As indicated above, a survey of 35 FS+ Helicobacter species and 9 FS− Helicobacter species revealed previously that ClsC homologs are prevalent in FS+ species but are absent in FS− species [29]. Given that genome sequences for more Helicobacter species have become available since this earlier survey, we used blastp to search the NCBI non-redundant protein database (accessed on 20 February 2025) and JGI Integrated Microbial Genomes and Microbiomes (IMG/M) database (accessed on 20 February 2025) for homologs of H. pylori G27 ClsC in an additional nine FS+ Helicobacter species and four FS− Helicobacter species. A list of the Helicobacter species examined is indicated in Table S1 along with references for the Helicobacter species [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]. For the blastp analysis, an E-value of 1 × 10−20 was used as the cutoff. Of the 44 Helicobacter FS+ species surveyed, 35 had a predicted ClsC homolog (Table S2). All of the ClsC homologs identified in the survey had E-values for the blastp analysis that were <1 × 10−121, which was well below the cutoff value (Table S2). In contrast, ClsC homologs were not identified in any of the 13 FS− Helicobacter species (Table S2). A Fisher’s exact test indicated that the difference in the distribution of ClsC homologs between the FS+ and FS− Helicobacter species was statistically significant (p-value < 0.00001).
The SignalP 6.0 server (https://services.healthtech.dtu.dk/services/SignalP-6.0/; accessed on 14 October 2024) predicts signal peptides in protein sequences from all domains of life. SignalP 6.0 predicted, with a moderately strong likelihood (0.684), that H. pylori B128 ClsC possessed a lipoprotein signal peptide (Table S2). SignalP 6.0 similarly predicted, with a strong likelihood (>0.98), lipoprotein signal peptides for most of the ClsC homologs from the other Helicobacter species identified in our survey (Table S2; accessed on 27 February 2025). A few of the ClsC homologs were predicted to lack a lipoprotein signal peptide, but using an alternative start codon for these proteins identified predicted lipoprotein signal peptides with high likelihood scores (>0.93; Table S2). Importantly, the predicted lipoprotein signal peptides for all of the ClsC homologs had the invariant cysteine residue that is at the cleavage site and is acylated (Figure 1). Taken together, these findings suggest that H. pylori ClsC and the ClsC homologs in other Helicobacter species are lipoproteins.

Figure 1.
N-terminal sequences of H. pylori ClsC and select ClsC homologs from other Helicobacter species. The backslash indicates the predicted cleavage site at the invariant cysteine residue of the lipoprotein signal peptide. The invariant cysteine together with the three proceeding amino acid residues constitute a motif referred to as the lipobox (indicated in red), which is important for the recognition and processing of the apolipoprotein. The consensus sequence for the lipobox is [LVI][ASTVI][GAS][C] [97]. For the Helicobacter bilis ClsC homolog, using the methionine that is underlined and in boldface as the N-terminal residue of the protein changed the likelihood score for a lipoprotein signal peptide from 0.0026 to 1.
3.2. H. pylori and Other Helicobacter Species Have a Potential Eukaryotic-Type Cardiolipin Synthase
In addition to ClsC, H. pylori has at least one more cardiolipin synthase, as H. pylori clsC mutants are still able to synthesize cardiolipin [30]. Cardiolipin synthases catalyze the final step in the cardiolipin biosynthetic pathway, and there are two general types of cardiolipin synthases. Bacterial-type cardiolipin synthases have two phospholipase D domains and synthesize cardiolipin by transferring a phosphatidic acid from either phosphatidylglycerol or phosphatidylethanolamine to a phosphatidylglycerol molecule [42,98,99]. Eukaryotic-type cardiolipin synthases possess a cytidine diphosphate (CDP)-alcohol phosphatidyltransferase domain that generates cardiolipin by transferring phosphatidic acid from CDP-diacylglycerol to phosphatidylglycerol [100,101]. H. pylori ClsC is typical of bacterial-type cardiolipin synthases in that it possesses two phospholipase D domains. A search of the H. pylori 26695 genome for candidates for other cardiolipin synthases identified two additional phospholipase D (PLD) family members, HP0323 (NucT) and HP1499. Both NucT and HP1499 have a single phospholipase D domain and belong to the endonuclease PLD subfamily, suggesting that these proteins do not have cardiolipin synthase activity. Moreover, H. pylori NucT is a nuclease associated with the outer membrane and is proposed to be involved in both purine recycling and competence [102,103], while the function of HP1499 is unknown.
Interestingly, a search of the H. pylori 26695 genome identified HP1016 as a CDP-alcohol phosphatidyltransferase domain protein, indicating that this protein may be a cardiolipin synthase. A blastp search of the NCBI non-redundant protein database (accessed on 27 February 2025) and JGI IMG/M database (accessed on 27 February 2025) for homologs of HP1016 in the Helicobacter species that had been surveyed for predicted ClsC homologs identified HP1016 homologs in all but three of the fifty-seven Helicobacter species surveyed (Table S2). In contrast to ClsC, the HP1016 homologs were equally represented in FS+ and FS− Helicobacter species. The HP1016 homologs from the various Helicobacter species were predicted by the Phobius server (https://phobius.sbc.su.se/; accessed on 15 April 2025) to have three to six transmembrane (TM) helices (Table S2), indicating that they are likely integral inner membrane proteins.
To determine if HP1016 is indeed a cardiolipin synthase, we attempted to delete the hp1016 homolog in both a H. pylori B128 clsC::cat mutant and its wild-type parental strain with the intention of examining the phospholipid content of the resulting mutants. Despite several attempts, we were unable to knock out the hp1016 homolog in either of the two H. pylori B128 strains and were therefore unable to determine if HP1016 has cardiolipin synthase activity using our planned approach. The failure to disrupt the hp1016 homolog in H. pylori B128 suggests that the gene is essential.
3.3. ClsC Localizes to the Cell Pole and Cell Septum in H. pylori
As a predicted lipoprotein, H. pylori ClsC could localize to either the inner membrane or outer membrane. Zavan and co-workers reported that ClsC was one of the top thirty proteins that were significantly more abundant in H. pylori OMVs compared to the global proteome [104], which strongly suggests that ClsC localizes to the outer membrane. As indicated previously, ClsA co-localizes with cardiolipin to the cell pole in E. coli [43]. To determine if H. pylori ClsC similarly localizes to specific regions of the cell, we examined the localization of a ClsC-sfGFP fusion in H. pylori G27 using fluorescence microscopy. For the initial analysis, we examined the localization of the ClsC-sfGFP fusion in a clsC::cat mutant to avoid possible competition between the ClsC-sfGFP fusion and native ClsC for binding sites within the cell. Fluorescent foci attributed to the ClsC-sfGFP fusion were observed near the cell poles in virtually all cells, as well as near the midcell in many of the cells (Figure 2). Scanning fluorescence microscopy images of several of the cells confirmed the presence of distinct fluorescent foci within the cells (Figure 2D–I).
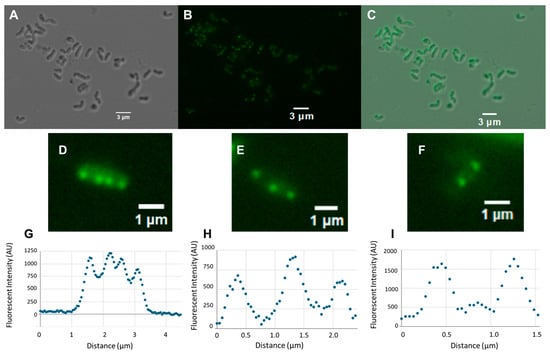
Figure 2.
Differential interference contrast (DIC) and fluorescence microscopy images of H. pylori cells expressing a ClsC-sfGFP fusion protein. (A) DIC microscopy image of a field of cells in the H. pylori G27 clsC::cat mutant expressing the ClsC-sfGFP fusion. (B) Fluorescence microscopy image of the field of H. pylori cells shown in panel (A). (C) Merger of the DIC microscopy and fluorescence microscopy images shown in panels (A,B), respectively. (D–F) Fluorescence microscopy images of individual H. pylori cells expressing the ClsC-sfGFP fusion protein. Fluorescence microscopy images of H. pylori cells displaying four (D), three (E), or two (F) fluorescent foci were scanned and analyzed using the ImageJ Fuji software package version 2.14.0/1.5f. (G–I) Graphic displays from each scan are presented below the corresponding fluorescence microscopy image. In the graphs shown here, the scans extend beyond the lengths of the cells.
The localization of the ClsC-sfGFP fusion near the midcell in many of the cells suggested that the fusion protein was recruited to the cell septum. To examine the validity of this hypothesis, we compared the lengths of cells that had fluorescent foci only at the cell poles versus cells that had fluorescent foci at both the cell poles and the midcell. The average length of cells that had midcell fluorescent foci was 2.72 ± 0.15 µm (n = 50), while average length of cells that had fluorescent foci only at the cell poles was 1.52 ± 0.034 µm (n = 50). The difference in cell length between the two cell types was significant, as determined using a Mann–Whitney U test (p = 0.00008). We inferred from these data that the longer cells were undergoing cell division and the ClsC-sfGFP fusion localized to the cell septum in these cells. To address further the hypothesis that the ClsC-sfGFP fusion localizes to the cell septum, we stained H. pylori cells with the lipophilic fluorescent dye FM4-64 to visualize the membranes. The staining of H. pylori cells with FM4-64 revealed that fluorescent foci due to the ClsC-sfGFP fusion that localized near the midcell were closely associated with membranes that, in some cases, appeared to bisect the cell (Figure 3C,D). In cells that had two distinct fluorescent foci at the midcell, the foci appeared to be separated by the membrane that bisected the cell. We postulate that the membrane separating the two fluorescent foci is part of the cell septum.
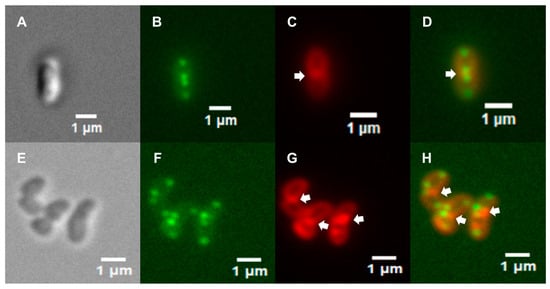
Figure 3.
Fluorescence microscopy of H. pylori cells stained with FM4-64 dye. (A) DIC microscopy image of a H. pylori G27 clsC::cat mutant cell stained with FM4-64 dye. (B) Fluorescence microscopy of H. pylori cell shown in panel (A) viewed in the green (GFP) channel. Fluorescent foci are present near each cell pole, and two fluorescent foci are present near the midcell. (C) Fluorescence microscopy image of H. pylori cell shown in panel (A) viewed in the red (FM4-64) channel. (D) Merger of the fluorescence microscopy images shown in panels (B,C). (E) DIC microscopy image of wild-type H. pylori G27 cells expressing the ClsC-sfGFP fusion that were stained with FM4-64 dye. (F) Fluorescence microscopy image of H. pylori cells shown in panel (E) viewed in the green channel. (G) Fluorescence microscopy image of H. pylori cells shown in panel (E) viewed in the red channel. (H) Merger of the fluorescence microscopy images shown in panels (F,G). The arrows in panels (C,D,G,H) indicate putative cell septa.
The examination of wild-type H. pylori G27 cells expressing the ClsC-sfGFP fusion revealed that they similarly displayed distinct fluorescent foci near the cell poles and midcell (Figure 3F,H), indicating that the presence of native ClsC does not interfere with the localization of the ClsC-sfGFP fusion to these sites. As observed with the clsC::cat mutant, fluorescent foci near the midcell in the wild-type cells were associated with membranes that appeared to bisect the cell (Figure 3H).
3.4. The Presence of Some Proteins Within the H. pylori Flagellar Sheath Appears to Be Dependent on ClsC
Given that ProP, MscS, and ClsA co-localize with cardiolipin to the cell pole in E. coli [36,43], we wished to determine if the localization of some proteins to the H. pylori flagellar sheath was dependent on cardiolipin. For this study, we used a H. pylori B128 clsC::cat mutant, since the H. pylori G27 clsC mutant is aflagellated, while the H. pylori B128 clsC mutant displays wild-type flagellation and motility in soft agar medium [30]. The molecular basis for the difference in flagellation between the H. pylori G27 clsC mutant and H. pylori B128 clsC mutant is not known, but replacing the flgI (which encodes the flagellar P-ring protein) allele in the H. pylori G27 clsC mutant with the H. pylori B128 flgI allele rescues flagellum biogenesis [30]. Although the H. pylori B128 clsC::cat mutant still synthesizes small amounts of cardiolipin, most of the cardiolipin in the mutant is in the form of monolysocardiolipin [30], and we reasoned that the depletion of cardiolipin in the clsC::cat mutant may be sufficient to alter the partitioning of some proteins to the sheath. To identify flagellar sheath proteins that may be dependent on cardiolipin for their localization, we compared the proteomes of flagella prepared from wild-type H. pylori B128 and the H. pylori B128 clsC::cat mutant. Flagella that were sheared from the H. pylori cells were isolated with their accompanying sheaths and analyzed by mass spectroscopy in two biological replicates for both the wild type and the clsC mutant.
A combined total of 190 non-ribosomal proteins were identified in at least two of the samples (Table S3). The proteins listed in Table S3 are ordered by their protein scores for the first biological replicate of the clsC::cat mutant. The Mascot software package from Matrix Science (www.matrixscience.com; accessed on 13 September 2023) was used to identify the proteins from the mass spectral data. Mascot calculates a protein score from the combined scores of all observed mass spectra, and the score indicates the confidence for the protein identification. Although the protein scores are not quantitative for protein levels, the scores often correlate with the relative abundance of proteins. In all four samples, the major flagellin (FlaA), minor flagellin (FlaB), hook protein (FlgE), and hook-associated proteins (FlgL and FlgK) had some of the highest protein scores (Table S3). Sixty-four percent (121 of 190) of the identified proteins were known or predicted to be associated with the flagellum, flagellar sheath, outer membrane, or periplasmic space (Table S3). The remaining proteins were either predicted inner membrane or cytoplasmic proteins. With the exception of a few highly expressed proteins, such as the urease and GroEL/GroES chaperone, the Mascot protein scores were relatively low for most of the cytoplasmic proteins. Thus, the proportion of proteins known or predicted to be associated with the flagellum, flagellar sheath, outer membrane, or periplasmic space increased to 84% when considering the 100 proteins with the highest protein scores. Taken together, these data indicate that the flagellar preparations were highly enriched for flagellar, sheath, outer membrane, and periplasmic proteins.
Five proteins were present in both replicates of the wild-type flagellar preparation but were not detected in the flagellar preparations from the clsC::cat mutant (Table 1). Two of the proteins, HofH and FaaA, are known outer membrane proteins and are therefore promising candidates for proteins that are dependent on ClsC for their localization to the flagellar sheath. HofH is an outer membrane β-barrel protein, while FaaA is an autotransporter that shares homology with the pore-forming VacA toxin and localizes to the flagellar sheath [28].

Table 1.
Proteins identified in the wild-type H. pylori B128 flagellar preparations but not the flagellar preparations from the H. pylori B128 clsC::cat mutant.
The other three proteins present in both replicates of the wild-type flagellar preparation but not detected in the isolated flagella of the clsC::cat mutant (HP0519, HP0408, and HP0709) were predicted by SignalP 6.0 to have Sec/SPI signal peptides—the likelihood scores for HP0519, HP0408, and HP0709 were 0.9988, 0.9989, and 0.9279, respectively (accessed on 1 April 2025). Thus, it is very likely that these proteins are secreted across the inner membrane. The function of HP0519 is unknown, but it belongs to a family of Helicobacter proteins that contain two or more copies of a degenerate 34–36-amino-acid repeat motif that is characteristic of eukaryotic Sel1 proteins [105]. The function of HP0408 is also unknown. The AlphaFold database feature in the Dali server (http://ekhidna2.biocenter.helsinki.fi/dali/ accessed on 15 April 2025) indicated that the predicted tertiary structure of HP0408 shared significant homology with E. coli ModA (Z-score = 10.6 accessed on 15 April 2025). ModA is a periplasmic molybdate-binding protein, and the structural homology of the proteins indicates that HP0408 may be a periplasmic binding protein. The function of HP0709 is also unknown, but it belongs to a family of proteins that includes enzymes that catalyze nucleophilic reactions of fluoride or chloride ions to the C-5′ carbon of S-adenosyl-L-methionine (SAM) [106]. If HP0709 is a periplasmic protein, it is doubtful that it is a SAM-utilizing enzyme, since SAM would not be present in the periplasm.
4. Discussion
While the sheathed flagellum of H. pylori is a distinctive feature of this bacterial pathogen, the function and biogenesis of the bacterium’s flagellar sheath remains enigmatic. The biogenesis of the flagellar sheath requires the mobilization of both lipids and proteins to the flagellated cell pole for their incorporation into the nascent sheath. One phospholipid required for sheath biogenesis is cardiolipin, which accumulates in membrane regions with negative curvature, such as the sheath, due to the capacity of cardiolipin molecules to form microdomains that have intrinsic curvature [31,32]. H. pylori ClsC and homologs of the enzyme present in other Helicobacter species are predicted to be lipoproteins (Table S2), and this feature is likely important in determining the subcellular localization of ClsC within the bacterial cell.
Although we did not detect ClsC in the preparations of isolated flagella from wild-type H. pylori B128 (Table S3), ClsC was reported to be highly enriched in H. pylori OMVs compared to the global proteome [104], which strongly suggests that ClsC localizes to the outer membrane. Our failure to detect ClsC in the proteomes of the isolated flagella may be due to the enzyme being in low abundance. Flagellar sheath biogenesis exhibits a significant demand for cardiolipin in the outer membrane, and a potential advantage of the localization of ClsC to the outer membrane is that it circumvents the need for the anterograde transport of cardiolipin (i.e., transport from the inner membrane to the outer membrane). The observation that ClsC homologs appear to be restricted to Helicobacter species that have sheathed flagella (Table S2) supports the notion that synthesizing cardiolipin within the outer membrane imparts a physiological advantage in sheath biogenesis. The biosynthesis of cardiolipin within the outer membrane would allow cardiolipin to be rapidly incorporated into the nascent flagellar sheath. More importantly, however, synthesizing cardiolipin in the outer membrane would avoid competition between cardiolipin and other phospholipids in anterograde transport. AsmA-like proteins are proposed to be responsible for the majority of phospholipid anterograde transport [107]. AsmA-like proteins have an N-terminal α-helix that anchors the protein in the inner membrane and a large periplasmic domain composed of repeating β-taco domains that form a long β-groove with a hydrophobic interior [107]. The hydrophobic groove of AsmA-like proteins likely shields the fatty acids of phospholipids as they diffuse across the periplasm. E. coli possesses six AsmA-like proteins (AsmA, TamB, YhdP, YdbH, YhjG, and YicH) [107], while H. pylori has only two AsmA-like proteins (HP0586 and HP0358). It would be interesting to determine if replacing ClsC with a cardiolipin synthase that resides in the inner membrane [e.g., E. coli ClsA] impacts the kinetics of flagellar sheath biogenesis or perhaps affects cell fitness by placing an added burden on the anterograde transport of phospholipids.
The ClsC-sfGFP fusion localized to the cell pole and cell septum (Figure 2 and Figure 3), which may have been consequential, since the membrane regions in these areas of the cell are where were cardiolipin accumulates. Cardiolipin is required for the polar localization of some proteins in E. coli [36,43], and it is possible that the localization of ClsC to the cell pole and septum is similarly dependent on cardiolipin. Alternatively, ClsC may recognize a landmark protein or engage another protein that localizes to the cell pole and septum. Future experiments designed to identify ClsS interaction partners may shed light on the molecular basis for the localization of ClsC to the cell pole and septum, as well as identifying proteins that may regulate the activity of ClsC.
Finally, a significant gap in our knowledge of sheath biogenesis and function is understanding how outer membrane proteins are partitioned between the sheath and outer membrane. Comparing the proteomes of isolated flagella from the wild type and the clsC mutant, we identified five proteins (FaaA, HofH, HP0519, HP0408, and HP0709) whose presence in the flagella preparations appeared to be dependent on ClsC (Table 1 and Table S3). It seems unlikely that the failure to detect these proteins in the isolated flagella of the clsC mutant was due to the reduced expression of the proteins, as a previous transcriptome analysis indicated the transcript levels of the genes encoding the proteins were comparable in the wild type and the H. pylori G27 clsC mutant [30]. The failure to identify FaaA in the isolated flagella of the clsC mutant is of particular interest since FaaA localizes predominantly in the flagellar sheath [28]. The Sel1 family protein HP0519 is also of interest since it appeared to be relatively abundant in the wild-type flagellar samples (based on the Mascot score, number of unique peptides, and percent coverage for the protein) but was undetected in the isolated flagella of the clsC::cat mutant (Table 1 and Table S3). We are unaware of any reports on the subcellular localization of HP0519, but we are working currently to address this issue. We are also working to verify that the localization of FaaA is dependent on ClsC by using fluorescently labeled antibodies to the protein and examining the H. pylori cells by fluorescence microscopy. While it is possible that ClsC plays a direct role in affecting the localization of FaaA and HofH to the sheath, it seems more likely that the failure of these proteins to localize to the sheath is due to the depletion of cardiolipin in the sheath. If the localization of FaaA and/or HofH to the sheath is dependent on cardiolipin, it is likely that these proteins have an affinity for cardiolipin microdomains within the sheath.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres16070155/s1, Table S1: Helicobacter species examined for homologs of ClsC and HP1016; Table S2: Homologs of ClsC and HP1016 in Helicobacter species; Table S3: Proteins identified in the flagellar preparations of wild-type H. pylori B128 and H. pylori B128 clsC mutant.
Author Contributions
Conceptualization, T.R.H. and D.N.; investigation, D.N. and N.E.; writing—original draft preparation, D.N.; writing—review and editing, T.R.H. and V.J.S.; visualization, D.N. and N.E.; supervision, T.R.H. and V.J.S.; project administration, T.R.H.; funding acquisition, T.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institutes of Health, grant number AI140444. The proteomics data generated in the study were supported by instrument grant NIH S10 OD025118 to the UGA PAMS facility.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials; further inquiries can be directed to the corresponding author.
Acknowledgments
We thank Robert Maier and Stéphane Benoit for the use of the equipment and laboratory space and thank Lindsay Berardi for the technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| sfGFP | Super-folder green fluorescent protein |
| TLR5 | Toll-like receptor 5 |
| IL-8 | Interleukin-8 |
| OMV | Outer membrane vesicle |
| LPS | Lipopolysaccharide |
| FS+ | Flagellar sheath positive |
| FS− | Flagellar sheath negative |
| SPI | Signal peptidase I |
| PLD | Phospholipase D |
| TM | Transmembrane |
| SAM | S-adenosyl-L-methionine |
| TSA-HS | Tryptic soy agar supplemented with horse serum |
| MHB | Mueller–Hinton broth |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| PBS | Phosphate-buffered saline |
| SDS | Sodium dodecyl sulfate |
| CDP | Cytidine diphosphate |
| LC | Liquid chromatography |
| HPLC | High-performance liquid chromatography |
| MS | Mass spectrometry |
| NCBI | National Center for Biotechnology Information |
| JGI | Joint Genome Institute |
| IMG/M | Integrated Microbial Genomes and Microbiomes |
References
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Atherton, J.C.; Blaser, M.J. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J. Clin. Investig. 2009, 119, 2475–2487. [Google Scholar] [CrossRef]
- Cover, T.L.; Blaser, M.J. Helicobacter pylori and gastroduodenal disease. Annu. Rev. Med. 1992, 43, 135–145. [Google Scholar] [CrossRef]
- Kuipers, E.J. Helicobacter pylori and the risk and management of associated diseases: Gastritis, ulcer disease, atrophic gastritis and gastric cancer. Aliment. Pharmacol. Ther. 1997, 11 (Suppl. S1), 71–88. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Lee, D.S. Helicobacter pylori in gastric carcinogenesis. World J. Gastrointest. Oncol. 2015, 7, 455–465. [Google Scholar] [CrossRef]
- Anonymous. Schistosomes, liver flukes and Helicobacter pylori. IARC Monogr. Eval. Carcinog. Risks Hum. 1994, 61, 1–241. [Google Scholar]
- Eaton, K.A.; Morgan, D.R.; Krakowka, S. Motility as a factor in the colonisation of gnotobiotic piglets by Helicobacter pylori. J. Med. Microbiol. 1992, 37, 123–127. [Google Scholar] [CrossRef]
- Ottemann, K.M.; Lowenthal, A.C. Helicobacter pylori uses motility for initial colonization and to attain robust infection. Infect. Immun. 2002, 70, 1984–1990. [Google Scholar] [CrossRef]
- Geis, G.; Suerbaum, S.; Forsthoff, B.; Leying, H.; Opferkuch, W. Ultrastructure and biochemical studies of the flagellar sheath of Helicobacter pylori. J. Med. Microbiol. 1993, 38, 371–377. [Google Scholar] [CrossRef]
- Chu, J.; Liu, J.; Hoover, T.R. Phylogenetic distribution, ultrastructure, and function of bacterial flagellar sheaths. Biomolecules 2020, 10, 363. [Google Scholar] [CrossRef]
- Luke, C.J.; Penn, C.W. Identification of a 29 kDa flagellar sheath protein in Helicobacter pylori using a murine monoclonal antibody. Microbiology 1995, 141 Pt 3, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, F.; Smith, K.D.; Ozinsky, A.; Hawn, T.R.; Yi, E.C.; Goodlett, D.R.; Eng, J.K.; Akira, S.; Underhill, D.M.; Aderem, A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Mekalanos, J.J. Decreased potency of the Vibrio cholerae sheathed flagellum to trigger host innate immunity. Infect. Immun. 2008, 76, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, A.T.; Yu, Y.; Krishna, U.S.; Israel, D.A.; Lyons, S.L.; Peek, R.M., Jr. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J. Infect. Dis. 2004, 189, 1914–1920. [Google Scholar] [CrossRef]
- Lee, S.K.; Stack, A.; Katzowitsch, E.; Aizawa, S.I.; Suerbaum, S.; Josenhans, C. Helicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5. Microbes Infect. 2003, 5, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial outer membrane vesicles: From discovery to applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef]
- Aschtgen, M.S.; Lynch, J.B.; Koch, E.; Schwartzman, J.; McFall-Ngai, M.; Ruby, E. Rotation of Vibrio fischeri flagella produces outer membrane vesicles that induce host development. J. Bacteriol. 2016, 198, 2156–2165. [Google Scholar] [CrossRef]
- Brennan, C.A.; Hunt, J.R.; Kremer, N.; Krasity, B.C.; Apicella, M.A.; McFall-Ngai, M.J.; Ruby, E.G. A model symbiosis reveals a role for sheathed-flagellum rotation in the release of immunogenic lipopolysaccharide. Elife 2014, 3, e01579. [Google Scholar] [CrossRef][Green Version]
- Doig, P.; Trust, T.J. Identification of surface-exposed outer membrane antigens of Helicobacter pylori. Infect. Immun. 1994, 62, 4526–4533. [Google Scholar] [CrossRef]
- Furuno, M.; Sato, K.; Kawagishi, I.; Homma, M. Characterization of a flagellar sheath component, PF60, and its structural gene in marine Vibrio. J. Biochem. 2000, 127, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Hranitzky, K.W.; Mulholland, A.; Larson, A.D.; Eubanks, E.R.; Hart, L.T. Characterization of a flagellar sheath protein of Vibrio cholerae. Infect. Immun. 1980, 27, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Luke, C.J.; Kubiak, E.; Cockayne, A.; Elliott, T.S.; Penn, C.W. Identification of flagellar and associated polypeptides of Helicobacter (formerly Campylobacter) pylori. FEMS Microbiol. Lett. 1990, 59, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, L.S.; Rittenberg, S.C. Isolation and composition of sheathed flagella from Bdellovibrio bacteriovorus 109J. J. Bacteriol. 1985, 163, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Evans, D.J., Jr.; Moulds, J.J.; Graham, D.Y. N-acetylneuraminyllactose-binding fibrillar hemagglutinin of Campylobacter pylori: A putative colonization factor antigen. Infect. Immun. 1988, 56, 2896–2906. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Logan, R.P.; Foynes, S.; Cockayne, A.; Wren, B.W.; Penn, C.W. A flagellar sheath protein of Helicobacter pylori is identical to HpaA, a putative N-acetylneuraminyllactose-binding hemagglutinin, but is not an adhesin for AGS cells. J. Bacteriol. 1997, 179, 5643–5647. [Google Scholar] [CrossRef]
- Lundstrom, A.M.; Blom, K.; Sundaeus, V.; Bolin, I. HpaA shows variable surface localization but the gene expression is similar in different Helicobacter pylori strains. Microb. Pathog. 2001, 31, 243–253. [Google Scholar] [CrossRef]
- Radin, J.N.; Gaddy, J.A.; Gonzalez-Rivera, C.; Loh, J.T.; Algood, H.M.; Cover, T.L. Flagellar localization of a Helicobacter pylori autotransporter protein. mBio 2013, 4, e00613-00612. [Google Scholar] [CrossRef]
- Gibson, K.; Chu, J.K.; Zhu, S.; Nguyen, D.; Mrazek, J.; Liu, J.; Hoover, T.R. A tripartite efflux system affects flagellum stability in Helicobacter pylori. Int. J. Mol. Sci. 2022, 23, 11609. [Google Scholar] [CrossRef]
- Chu, J.K.; Zhu, S.; Herrera, C.M.; Henderson, J.C.; Liu, J.; Trent, M.S.; Hoover, T.R. Loss of a cardiolipin synthase in Helicobacter pylori G27 blocks flagellum assembly. J. Bacteriol. 2019, 201, e00372-00319. [Google Scholar] [CrossRef]
- Huang, K.C.; Mukhopadhyay, R.; Wingreen, N.S. A curvature-mediated mechanism for localization of lipids to bacterial poles. PLoS Comput. Biol. 2006, 2, e151. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. USA 2011, 108, 6264–6269. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Munoz-Rojas, J.; Hurtado, A.; Ramos, J.L.; Segura, A. A Pseudomonas putida cardiolipin synthesis mutant exhibits increased sensitivity to drugs related to transport functionality. Environ. Microbiol. 2007, 9, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F.; Shoda, M.; Harashima, R.; Sadaie, Y.; Hara, H.; Matsumoto, K. Cardiolipin domains in Bacillus subtilis marburg membranes. J. Bacteriol. 2004, 186, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Mileykovskaya, E.; Dowhan, W. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J. Bacteriol. 2000, 182, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Romantsov, T.; Helbig, S.; Culham, D.E.; Gill, C.; Stalker, L.; Wood, J.M. Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol. Microbiol. 2007, 64, 1455–1465. [Google Scholar] [CrossRef]
- Chu, J. Understanding the Role of Cardiolipin in Helicobacter pylori Flagellar Synthesis. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2019. [Google Scholar]
- Hirai, Y.; Haque, M.; Yoshida, T.; Yokota, K.; Yasuda, T.; Oguma, K. Unique cholesteryl glucosides in Helicobacter pylori: Composition and structural analysis. J. Bacteriol. 1995, 177, 5327–5333. [Google Scholar] [CrossRef]
- Zhou, P.; Hu, R.; Chandan, V.; Kuolee, R.; Liu, X.; Chen, W.; Liu, B.; Altman, E.; Li, J. Simultaneous analysis of cardiolipin and lipid A from Helicobacter pylori by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Mol. Biosyst. 2012, 8, 720–725. [Google Scholar] [CrossRef]
- Guo, D.; Tropp, B.E. A second Escherichia coli protein with CL synthase activity. Biochim. Biophys. Acta 2000, 1483, 263–274. [Google Scholar] [CrossRef]
- Pluschke, G.; Hirota, Y.; Overath, P. Function of phospholipids in Escherichia coli. Characterization of a mutant deficient in cardiolipin synthesis. J. Biol. Chem. 1978, 253, 5048–5055. [Google Scholar] [CrossRef]
- Tan, B.K.; Bogdanov, M.; Zhao, J.; Dowhan, W.; Raetz, C.R.; Guan, Z. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc. Natl. Acad. Sci. USA 2012, 109, 16504–16509. [Google Scholar] [CrossRef] [PubMed]
- Romantsov, T.; Gonzalez, K.; Sahtout, N.; Culham, D.E.; Coumoundouros, C.; Garner, J.; Kerr, C.H.; Chang, L.; Turner, R.J.; Wood, J.M. Cardiolipin synthase A colocalizes with cardiolipin and osmosensing transporter ProP at the poles of Escherichia coli cells. Mol. Microbiol. 2018, 107, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Heuermann, D.; Haas, R. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 1998, 257, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Rosinke, K.; Tachiyama, S.; Mrasek, J.; Liu, J.; Hoover, T.R. A Helicobacter pylori flagellar motor accessory is needed to maintain the barrier function of the outer membrane during flagellar rotation. PLoS Pathog. 2025, 21, e1012860. [Google Scholar] [CrossRef]
- Dinh, T.; Bernhardt, T.G. Using superfolder green fluorescent protein for periplasmic protein localization studies. J. Bacteriol. 2011, 193, 4984–4987. [Google Scholar] [CrossRef] [PubMed]
- Kostylev, M.; Otwell, A.E.; Richardson, R.E.; Suzuki, Y. Cloning should be simple: Escherichia coli DH5a-mediated assembly of multiple DNA fragments with short end homologies. PLoS ONE 2015, 10, e0137466. [Google Scholar] [CrossRef]
- Nguyen, D.; Ivester, R.G.; Rosinke, K.; Hoover, T.R. Helicobacter pylori HP0135 is a small lipoprotein that has a role in outer membrane stability. Molecules 2025, 30, 204. [Google Scholar] [CrossRef]
- Arnold, I.C.; Zigova, Z.; Holden, M.; Lawley, T.D.; Rad, R.; Dougan, G.; Falkow, S.; Bentley, S.D.; Muller, A. Comparative whole genome sequence analysis of the carcinogenic bacterial model pathogen Helicobacter felis. Genome Biol. Evol. 2011, 3, 302–308. [Google Scholar] [CrossRef]
- Aydin, F.; Karakaya, E.; Kayman, T.; Abay, S.; Saticioglu, I.B. Helicobacter turcicus sp. nov., a catalase-negative new member of the Helicobacter genus, isolated from Anatolian ground squirrel (Spermophilus xanthoprymnus) in Turkey. Int. J. Syst. Evol. Microbiol. 2022, 72, 005338. [Google Scholar] [CrossRef]
- Aydin, F.; Saticioglu, I.B.; Ay, H.; Kayman, T.; Karakaya, E.; Abay, S. Description of the two novel species of the genus Helicobacter: Helicobacter anatolicus sp. nov., and Helicobacter kayseriensis sp. nov., isolated from feces of urban wild birds. Syst. Appl. Microbiol. 2022, 45, 126326. [Google Scholar] [CrossRef]
- Baele, M.; Decostere, A.; Vandamme, P.; Ceelen, L.; Hellemans, A.; Mast, J.; Chiers, K.; Ducatelle, R.; Haesebrouck, F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int. J. Syst. Evol. Microbiol. 2008, 58, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Baele, M.; Decostere, A.; Vandamme, P.; Van den Bulck, K.; Gruntar, I.; Mehle, J.; Mast, J.; Ducatelle, R.; Haesebrouck, F. Helicobacter baculiformis sp. nov., isolated from feline stomach mucosa. Int. J. Syst. Evol. Microbiol. 2008, 58, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Berthenet, E.; Benejat, L.; Menard, A.; Varon, C.; Lacomme, S.; Gontier, E.; Raymond, J.; Boussaba, O.; Toulza, O.; Ducournau, A.; et al. Whole-genome sequencing and bioinformatics as pertinent tools to support Helicobacteracae taxonomy, based on three strains suspected to belong to novel Helicobacter species. Front. Microbiol. 2019, 10, 2820. [Google Scholar] [CrossRef]
- Collado, L.; Jara, R.; Gonzalez, S. Description of Helicobacter valdiviensis sp. nov., an Epsilonproteobacteria isolated from wild bird faecal samples. Int. J. Syst. Evol. Microbiol. 2014, 64, 1913–1919. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Fox, J.G.; Mendes, E.N.; Paster, B.J.; Gates, C.E.; Kirkbride, C.A.; Eaton, K.A. ‘Flexispira rappini’ strains represent at least 10 Helicobacter taxa. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 5, 1781–1787. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Seymour, C.; Fraser, G.J.; Paster, B.J.; Fox, J.G. Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. Int. J. Syst. Bacteriol. 1994, 44, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Eppinger, M.; Baar, C.; Linz, B.; Raddatz, G.; Lanz, C.; Keller, H.; Morelli, G.; Gressmann, H.; Achtman, M.; Schuster, S.C. Who ate whom? Adaptive Helicobacter genomic changes that accompanied a host jump from early humans to large felines. PLoS Genet. 2006, 2, e120. [Google Scholar] [CrossRef]
- Fox, J.G.; Boutin, S.R.; Handt, L.K.; Taylor, N.S.; Xu, S.; Rickman, B.; Marini, R.P.; Dewhirst, F.E.; Paster, B.J.; Motzel, S.; et al. Isolation and characterization of a novel Helicobacter species, “Helicobacter macacae,” from rhesus monkeys with and without chronic idiopathic colitis. J. Clin. Microbiol. 2007, 45, 4061–4063. [Google Scholar] [CrossRef]
- Fox, J.G.; Cabot, E.B.; Taylor, N.S.; Laraway, R. Gastric colonization by Campylobacter pylori subsp. mustelae in ferrets. Infect. Immun. 1988, 56, 2994–2996. [Google Scholar] [CrossRef]
- Fox, J.G.; Shen, Z.; Xu, S.; Feng, Y.; Dangler, C.A.; Dewhirst, F.E.; Paster, B.J.; Cullen, J.M. Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J. Clin. Microbiol. 2002, 40, 2513–2519. [Google Scholar] [CrossRef]
- Fox, J.G.; Taylor, N.S.; Howe, S.; Tidd, M.; Xu, S.; Paster, B.J.; Dewhirst, F.E. Helicobacter anseris sp. nov. and Helicobacter brantae sp. nov., isolated from feces of resident Canada geese in the greater Boston area. Appl. Environ. Microbiol. 2006, 72, 4633–4637. [Google Scholar] [CrossRef]
- Fox, J.G.; Yan, L.L.; Dewhirst, F.E.; Paster, B.J.; Shames, B.; Murphy, J.C.; Hayward, A.; Belcher, J.C.; Mendes, E.N. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J. Clin. Microbiol. 1995, 33, 445–454. [Google Scholar] [CrossRef]
- Frank, J.; Dingemanse, C.; Schmitz, A.M.; Vossen, R.H.; van Ommen, G.J.; den Dunnen, J.T.; Robanus-Maandag, E.C.; Anvar, S.Y. The complete genome sequence of the murine pathobiont Helicobacter typhlonius. Front. Microbiol. 2015, 6, 1549. [Google Scholar] [CrossRef] [PubMed]
- Franklin, C.L.; Beckwith, C.S.; Livingston, R.S.; Riley, L.K.; Gibson, S.V.; Besch-Williford, C.L.; Hook, R.R., Jr. Isolation of a novel Helicobacter species, Helicobacter cholecystus sp. nov., from the gallbladders of Syrian hamsters with cholangiofibrosis and centrilobular pancreatitis. J. Clin. Microbiol. 1996, 34, 2952–2958. [Google Scholar] [CrossRef] [PubMed]
- Gruntar, I.; Kostanjsek, R.; Pirs, T.; Papic, B. Helicobacter colisuis sp. nov., isolated from caecal contents of domestic pigs (Sus scrofa domesticus). Int. J. Syst. Evol. Microbiol. 2022, 72, 005600. [Google Scholar] [CrossRef] [PubMed]
- Gruntar, I.; Papic, B.; Pate, M.; Zajc, U.; Ocepek, M.; Kusar, D. Helicobacter labacensis sp. nov., Helicobacter mehlei sp. nov., and Helicobacter vulpis sp. nov., isolated from gastric mucosa of red foxes (Vulpes vulpes). Int. J. Syst. Evol. Microbiol. 2020, 70, 2395–2404. [Google Scholar] [CrossRef]
- Harper, C.G.; Feng, Y.; Xu, S.; Taylor, N.S.; Kinsel, M.; Dewhirst, F.E.; Paster, B.J.; Greenwell, M.; Levine, G.; Rogers, A.; et al. Helicobacter cetorum sp. nov., a urease-positive Helicobacter species isolated from dolphins and whales. J. Clin. Microbiol. 2002, 40, 4536–4543. [Google Scholar] [CrossRef]
- Hu, S.; Jin, D.; Lu, S.; Liu, S.; Zhang, J.; Wang, Y.; Bai, X.; Xiong, Y.; Huang, Y.; Xu, H.; et al. Helicobacter himalayensis sp. nov. isolated from gastric mucosa of Marmota himalayana. Int. J. Syst. Evol. Microbiol. 2015, 65, 1719–1725. [Google Scholar] [CrossRef]
- Jalava, K.; Kaartinen, M.; Utriainen, M.; Happonen, I.; Hanninen, M.L. Helicobacter salomonis sp. nov., a canine gastric Helicobacter sp. related to Helicobacter felis and Helicobacter bizzozeronii. Int. J. Syst. Bacteriol. 1997, 47, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.J.; Dong, H.J.; Shin, J.H.; Kim, I.Y.; Ho, H.; Oh, S.H.; Yoon, Y.M.; Choi, Y.K.; Suh, J.G.; Nam, K.H.; et al. Helicobacter apodemus sp. nov., a new Helicobacter species identified from the gastrointestinal tract of striped field mice in Korea. J. Vet. Sci. 2015, 16, 475–481. [Google Scholar] [CrossRef]
- Joosten, M.; Linden, S.; Rossi, M.; Tay, A.C.; Skoog, E.; Padra, M.; Peters, F.; Perkins, T.; Vandamme, P.; Van Nieuwerburgh, F.; et al. Divergence between the highly virulent zoonotic pathogen Helicobacter heilmannii and its closest relative, the low-virulence “Helicobacter ailurogastricus” sp. nov. Infect. Immun. 2016, 84, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.; Fujimoto, Y.; Kutsuna, R.; Tomida, J.; Yamamoto, K.I.; Miyoshi-Akiyama, T.; Okuno, M.; Ogura, Y.; Matsuoka, M.; Kawaguchi, T.; et al. Helicobacter kumamotonensis sp. nov., isolated from human clinical specimens. Int. J. Syst. Evol. Microbiol. 2023, 73, 005732. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Phillips, M.W.; O’Rourke, J.L.; Paster, B.J.; Dewhirst, F.E.; Fraser, G.J.; Fox, J.G.; Sly, L.I.; Romaniuk, P.J.; Trust, T.J.; et al. Helicobacter muridarum sp. nov., a microaerophilic helical bacterium with a novel ultrastructure isolated from the intestinal mucosa of rodents. Int. J. Syst. Bacteriol. 1992, 42, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Loman, N.J.; Snyder, L.A.; Linton, J.D.; Langdon, R.; Lawson, A.J.; Weinstock, G.M.; Wren, B.W.; Pallen, M.J. Genome sequence of the emerging pathogen Helicobacter canadensis. J. Bacteriol. 2009, 191, 5566–5567. [Google Scholar] [CrossRef]
- Lopez-Cantillo, M.; Vidal-Veuthey, B.; Mella, A.; de la Haba, R.R.; Collado, L. Helicobacter ibis sp. nov., isolated from faecal droppings of black-faced ibis (Theristicus melanopis). Int. J. Syst. Evol. Microbiol. 2023, 73, 005983. [Google Scholar] [CrossRef] [PubMed]
- Melito, P.L.; Munro, C.; Chipman, P.R.; Woodward, D.L.; Booth, T.F.; Rodgers, F.G. Helicobacter winghamensis sp. nov., a novel Helicobacter sp. isolated from patients with gastroenteritis. J. Clin. Microbiol. 2001, 39, 2412–2417. [Google Scholar] [CrossRef]
- Mendes, E.N.; Queiroz, D.M.; Dewhirst, F.E.; Paster, B.J.; Moura, S.B.; Fox, J.G. Helicobacter trogontum sp. nov., isolated from the rat intestine. Int. J. Syst. Bacteriol. 1996, 46, 916–921. [Google Scholar] [CrossRef]
- Moyaert, H.; Decostere, A.; Vandamme, P.; Debruyne, L.; Mast, J.; Baele, M.; Ceelen, L.; Ducatelle, R.; Haesebrouck, F. Helicobacter equorum sp. nov., a urease-negative Helicobacter species isolated from horse faeces. Int. J. Syst. Evol. Microbiol. 2007, 57, 213–218. [Google Scholar] [CrossRef]
- Patterson, M.M.; Schrenzel, M.D.; Feng, Y.; Xu, S.; Dewhirst, F.E.; Paster, B.J.; Thibodeau, S.A.; Versalovic, J.; Fox, J.G. Helicobacter aurati sp. nov., a urease-positive Helicobacter species cultured from gastrointestinal tissues of Syrian hamsters. J. Clin. Microbiol. 2000, 38, 3722–3728. [Google Scholar] [CrossRef]
- Schott, T.; Rossi, M.; Hanninen, M.L. Genome sequence of Helicobacter bizzozeronii strain CIII-1, an isolate from human gastric mucosa. J. Bacteriol. 2011, 193, 4565–4566. [Google Scholar] [CrossRef] [PubMed]
- Segawa, T.; Ohno, Y.; Tsuchida, S.; Ushida, K.; Yoshioka, M. Helicobacter delphinicola sp. nov., isolated from common bottlenose dolphins Tursiops truncatus with gastric diseases. Dis. Aquat. Organ. 2020, 141, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Feng, Y.; Muthupalani, S.; Sheh, A.; Cheaney, L.E.; Kaufman, C.A.; Gong, G.; Paster, B.J.; Fox, J.G. Novel Helicobacter species H. japonicum isolated from laboratory mice from Japan induces typhlocolitis and lower bowel carcinoma in C57BL/129 IL10-/- mice. Carcinogenesis 2016, 37, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Feng, Y.; Sheh, A.; Everitt, J.; Bertram, F.; Paster, B.J.; Fox, J.G. Isolation and characterization of a novel Helicobacter species, Helicobacter jaachi sp. nov., from common marmosets (Callithrix jaachus). J. Med. Microbiol. 2015, 64, 1063–1073. [Google Scholar] [CrossRef]
- Shen, Z.; Fox, J.G.; Dewhirst, F.E.; Paster, B.J.; Foltz, C.J.; Yan, L.; Shames, B.; Perry, L. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int. J. Syst. Bacteriol. 1997, 47, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Mannion, A.; Lin, M.; Esmail, M.; Bakthavatchalu, V.; Yang, S.; Ho, C.; Feng, Y.; Smith, B.; Elliott, J.; et al. Helicobacter monodelphidis sp. nov. and Helicobacter didelphidarum sp. nov., isolated from grey short-tailed opossums (Monodelphis domestica) with endemic cloacal prolapses. Int. J. Syst. Evol. Microbiol. 2020, 70, 6032–6043. [Google Scholar] [CrossRef]
- Shen, Z.; Mannion, A.; Whary, M.T.; Muthupalani, S.; Sheh, A.; Feng, Y.; Gong, G.; Vandamme, P.; Holcombe, H.R.; Paster, B.J.; et al. Helicobacter saguini, a novel Helicobacter isolated from cotton-top tamarins with ulcerative colitis, has proinflammatory properties and induces typhlocolitis and dysplasia in gnotobiotic IL-10-/- mice. Infect. Immun. 2016, 84, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xu, S.; Dewhirst, F.E.; Paster, B.J.; Pena, J.A.; Modlin, I.M.; Kidd, M.; Fox, J.G. A novel enterohepatic Helicobacter species ’Helicobacter mastomyrinus’ isolated from the liver and intestine of rodents. Helicobacter 2005, 10, 59–70. [Google Scholar] [CrossRef]
- Simmons, J.H.; Riley, L.K.; Besch-Williford, C.L.; Franklin, C.L. Helicobacter mesocricetorum sp. nov., A novel Helicobacter isolated from the feces of Syrian hamsters. J. Clin. Microbiol. 2000, 38, 1811–1817. [Google Scholar] [CrossRef]
- Smet, A.; Flahou, B.; D’Herde, K.; Vandamme, P.; Cleenwerck, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F. Helicobacter heilmannii sp. nov., isolated from feline gastric mucosa. Int. J. Syst. Evol. Microbiol. 2012, 62, 299–306. [Google Scholar] [CrossRef]
- Stanley, J.; Linton, D.; Burnens, A.P.; Dewhirst, F.E.; On, S.L.; Porter, A.; Owen, R.J.; Costas, M. Helicobacter pullorum sp. nov.-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology 1994, 140 Pt 12, 3441–3449. [Google Scholar] [CrossRef]
- Stanley, J.; Linton, D.; Burnens, A.P.; Dewhirst, F.E.; Owen, R.J.; Porter, A.; On, S.L.; Costas, M. Helicobacter canis sp. nov., a new species from dogs: An integrated study of phenotype and genotype. J. Gen. Microbiol. 1993, 139, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Suerbaum, S.; Josenhans, C.; Sterzenbach, T.; Drescher, B.; Brandt, P.; Bell, M.; Droge, M.; Fartmann, B.; Fischer, H.P.; Ge, Z.; et al. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 2003, 100, 7901–7906. [Google Scholar] [CrossRef] [PubMed]
- Totten, P.A.; Fennell, C.L.; Tenover, F.C.; Wezenberg, J.M.; Perine, P.L.; Stamm, W.E.; Holmes, K.K. Campylobacter cinaedi (sp. nov.) and Campylobacter fennelliae (sp. nov.): Two new Campylobacter species associated with enteric disease in homosexual men. J. Infect. Dis. 1985, 151, 131–139. [Google Scholar] [CrossRef]
- Traverso, F.R.; Bohr, U.R.; Oyarzabal, O.A.; Rohde, M.; Clarici, A.; Wex, T.; Kuester, D.; Malfertheiner, P.; Fox, J.G.; Backert, S. Morphologic, genetic, and biochemical characterization of Helicobacter magdeburgensis, a novel species isolated from the intestine of laboratory mice. Helicobacter 2010, 15, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Van den Bulck, K.; Decostere, A.; Baele, M.; Vandamme, P.; Mast, J.; Ducatelle, R.; Haesebrouck, F. Helicobacter cynogastricus sp. nov., isolated from the canine gastric mucosa. Int. J. Syst. Evol. Microbiol. 2006, 56, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.M.; Priya, M.L.; Selvan, A.T.; Madera, M.; Gough, J.; Aravind, L.; Sankaran, K. A database of bacterial lipoproteins (DOLOP) with functional assignments to predicted lipoproteins. J. Bacteriol. 2006, 188, 2761–2773. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tan, B.K.; Zhao, J.; Guan, Z. In vivo and in vitro synthesis of phosphatidylglycerol by an Escherichia coli cardiolipin synthase. J. Biol. Chem. 2016, 291, 25144–25153. [Google Scholar] [CrossRef]
- Tropp, B.E. Cardiolipin synthase from Escherichia coli. Biochim. Biophys. Acta 1997, 1348, 192–200. [Google Scholar] [CrossRef]
- Schlame, M.; Brody, S.; Hostetler, K.Y. Mitochondrial cardiolipin in diverse eukaryotes. Comparison of biosynthetic reactions and molecular acyl species. Eur. J. Biochem. 1993, 212, 727–735. [Google Scholar] [CrossRef]
- Tamai, K.T.; Greenberg, M.L. Biochemical characterization and regulation of cardiolipin synthase in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1990, 1046, 214–222. [Google Scholar] [CrossRef]
- Liechti, G.W.; Goldberg, J.B. Helicobacter pylori salvages purines from extracellular host cell DNA utilizing the outer membrane-associated nuclease NucT. J. Bacteriol. 2013, 195, 4387–4398. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, E.J.; Pinto, A.V.; Petroni, E.A.; Tolmasky, M.E.; Ielpi, L. Evidence for the active role of a novel nuclease from Helicobacter pylori in the horizontal transfer of genetic information. J. Bacteriol. 2004, 186, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Zavan, L.; Bitto, N.J.; Johnston, E.L.; Greening, D.W.; Kaparakis-Liaskos, M. Helicobacter pylori growth stage determines the size, protein composition, and preferential cargo packaging of outer membrane vesicles. Proteomics 2019, 19, e1800209. [Google Scholar] [CrossRef] [PubMed]
- Ponting, C.P.; Aravind, L.; Schultz, J.; Bork, P.; Koonin, E.V. Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J. Mol. Biol. 1999, 289, 729–745. [Google Scholar] [CrossRef]
- Deng, H.; O’Hagan, D. The fluorinase, the chlorinase and the duf-62 enzymes. Curr. Opin. Chem. Biol. 2008, 12, 582–592. [Google Scholar] [CrossRef]
- Kumar, S.; Ruiz, N. Bacterial AsmA-like proteins: Bridging the gap in intermembrane phospholipid transport. Contact 2023, 6, 25152564231185931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).