Distribution of Treponema Species in Active Digital Dermatitis Lesions and Non-Lesional Skin of Dairy Cattle
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Bovine Digital Dermatitis Lesions and Sample Collection
2.2. DNA Extraction and Standard PCR Assay
2.3. Qualitative Real-Time PCR
Sequence Data Collection
2.4. Statistical Analysis
3. Results
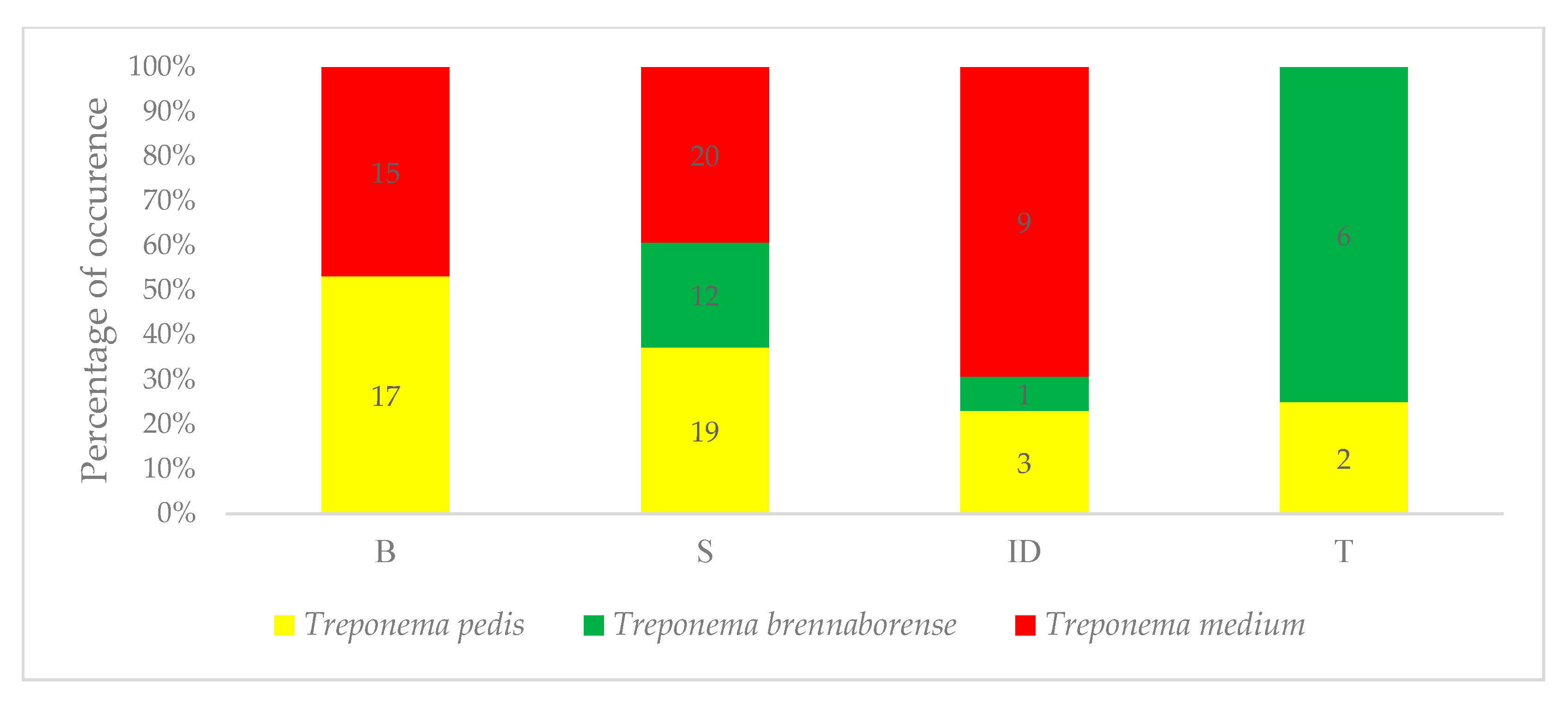
3.1. Prevalence of Treponemes
3.2. Evaluation of Treponemal Distribution
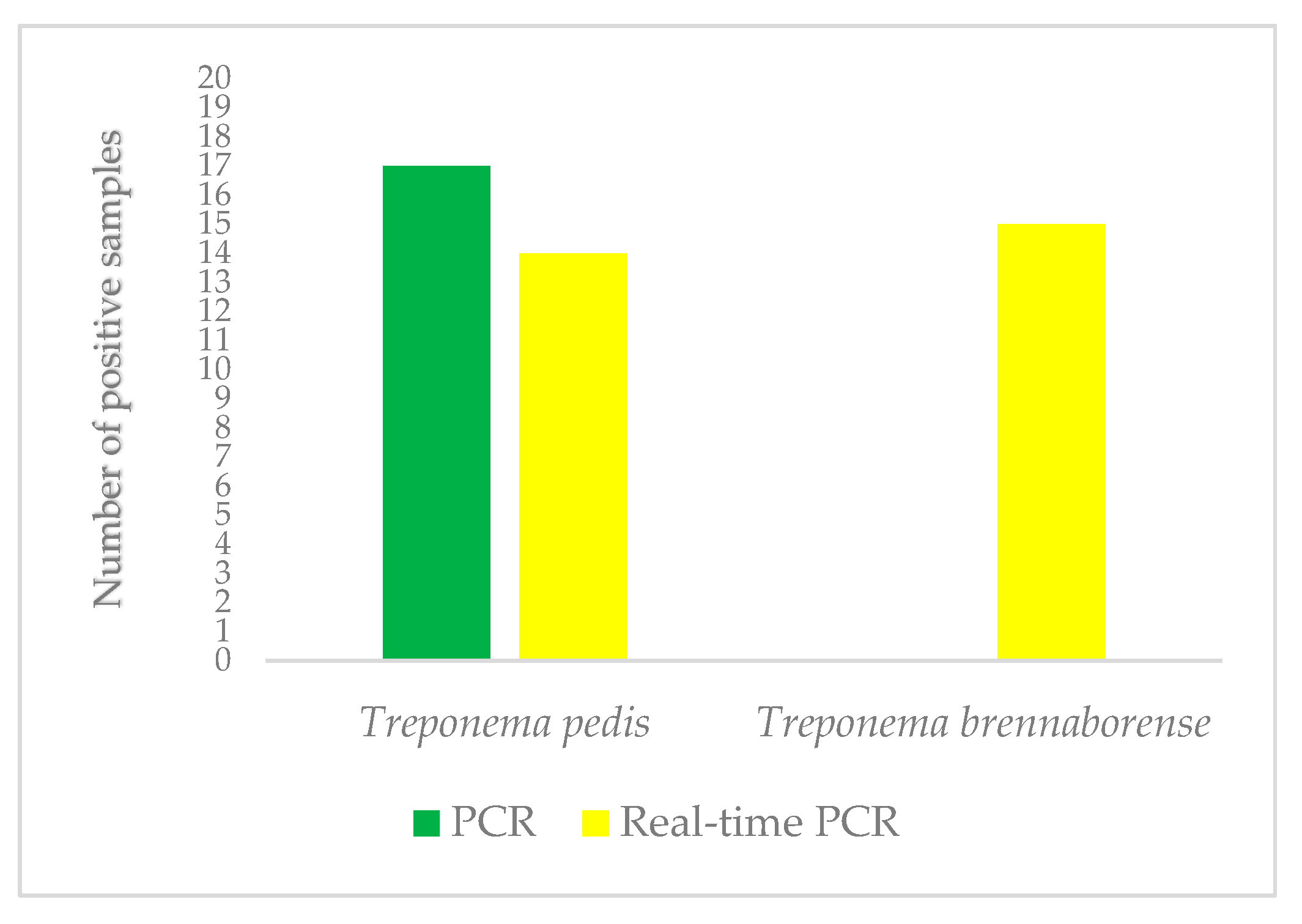
3.3. Comparison of Standard and Real-Time PCR in the Detection of Treponema Pedis and Treponema Brennaborense
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheli, R.; Mortellaro, C. La dermatite digitale del bovino. In Proceedings of the 8th International Conference on Diseases of Cattle, Piacenza, Milan, Italy, 9–13 September 1974; pp. 208–213. [Google Scholar] [CrossRef]
- Caddey, B.; De Buck, J. Meta-Analysis of Bovine Digital Dermatitis Microbiota Reveals Distinct Microbial Community Structures Associated With Lesions. Front. Cell. Infect. Microbiol. 2021, 11, 685861. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.E.; Monir, A.; Abdallah, H.M.; Mahmoud, A.F.; Elsheikh, H.A.; Refaai, W.; Ahmed, A.B.; Elsheikh, H.E.M.; El-Sheikh, M.; Mesalam, A. Digital dermatitis in dairy cattle in Egypt: Herd-level risk factors and Treponema spp. prevalence across lesion M-scores. Vet. J. 2025, 309, 1062289. [Google Scholar] [CrossRef] [PubMed]
- Read, D.H.; Walker, R.L. Papillomatous digital dermatitis (Footwarts) in California dairy cattle: Clinical and gross pathologic findings. J. Vet. Diagn. Investig. 1998, 10, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Murray, R.D.; Carter, S.D. Bovine digital dermatitis: Current concepts from laboratory to farm. Vet. J. 2016, 211, 3–13. [Google Scholar] [CrossRef]
- Döpfer, D.; Holzhauer, M.; Boven, M. The dynamics of digital dermatitis in populations of dairy cattle: Model-based estimates of transition rates and implications for control. Vet. J. 2012, 193, 648–653. [Google Scholar] [CrossRef]
- Biemans, F.; Bijma, P.; Boots, N.M.; de Jong, M.C.M. Digital dermatitis in dairy cattle: The contribution of different disease classes to transmission. Epidemics 2018, 23, 76–84. [Google Scholar] [CrossRef]
- Stokes, J.E.; Leach, K.A.; Main, D.C.; Whay, H.R. The reliability of detecting digital dermatitis in the milking parlour. Vet. J. 2012, 193, 679–684. [Google Scholar] [CrossRef]
- Gomez, A.; Cook, N.B.; Socha, M.T.; Döpfer, D. First-lactation performance in cows affected by digital dermatitis during the rearing period. J. Dairy Sci. 2015, 98, 4487–4498. [Google Scholar] [CrossRef]
- Afonso, J.S.; Oikonomou, G.; Carter, S.; Clough, H.E.; Griffiths, B.E.; Rushton, J. Diagnosis of bovine digital dermatitis: Exploring the usefulness of indirect ELISA. Front. Vet. Sci. 2021, 8, 728691. [Google Scholar] [CrossRef]
- Cortes, J.A.; Hendrick, S.; Janzen, E.; Pajor, E.A.; Orsel, K. Economic impact of digital dermatitis, foot rot, and bovine respiratory disease in feedlot cattle. Transl. Anim. Sci. 2021, 5, txab076. [Google Scholar] [CrossRef]
- Schulz, T.; Gundelach, Y.; Feldmann, M.; Hoedemaker, M. Early detection and treatment of lame cows. Tierärztliche Prax. Ausg. G Großtiere/Nutztiere 2016, 44, 5–11. [Google Scholar] [CrossRef]
- Wilson-Welder, J.H.; Alt, D.P.; Nally, J.E. The etiology of digital dermatitis in ruminants: Recent perspectives. Vet. Med. 2015, 6, 155–164. [Google Scholar] [CrossRef]
- Nally, J.E.; Hornsby, R.L.; Alt, D.P.; Whitelegge, J.P. Phenotypic and proteomic characterization of treponemes associated with bovine digital dermatitis. Vet. Microbiol. 2019, 235, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, H.M.; Mamuad, L.L.; Jin, S.J.; Kim, S.H.; Kwon, S.W.; Lee, S.S.; Lee, S.M.; Cho, Y.I. Genotypic and Phenotypic Characterization of Treponema phagedenis from Bovine Digital Dermatitis. Microorganisms 2020, 8, 1520. [Google Scholar] [CrossRef] [PubMed]
- Beninger, C.; Naqvi, S.A.; Naushad, S.; Orsel, K.; Luby, C.; Derakhshani, H.; Khafipour, E.; De Buck, J. Associations between digital dermatitis lesion grades in dairy cattle and the quantities of four Treponema species. Vet. Res. 2018, 49, 111. [Google Scholar] [CrossRef]
- Bay, V.; Gillespie, A.; Ganda, E.; Evans, N.J.; Carter, S.D.; Lenzi, L.; Lucaci, A.; Haldenby, S.; Barden, M.; Griffiths, B.E.; et al. The bovine foot skin microbiota is associated with host genotype and the development of infectious digital dermatitis lesions. Microbiome 2023, 11, 4. [Google Scholar] [CrossRef]
- Zojaji, V.; Mokhtari, A.; Mohamadnia, A. Molecular Isolation of Treponema spp. from Ovine Footrot Lesions, Finding Evidence for Contagious Ovine Digital Dermatitis. Iran. J. Vet. Surg. 2024, 19, 118–124. [Google Scholar] [CrossRef]
- Anklam, K.; Kulow, M.; Yamazaki, W.; Döpfer, D. Development of real-time PCR and loop-mediated isothermal amplification (LAMP) assays for the differential detection of digital dermatitis associated treponemes. PLoS ONE 2017, 12, e0178349. [Google Scholar] [CrossRef]
- Caddey, B.; Orsel, K.; Naushad, S.; Derakhshani, H.; De Buck, J. Identification and Quantification of Bovine Digital Dermatitis-Associated Microbiota across Lesion Stages in Feedlot Beef Cattle. mSystems 2021, 6, e0070821. [Google Scholar] [CrossRef]
- Döpfer, D.; Koopmans, A.; Meijer, F.A.; Szakáll, I.; Schukken, Y.H.; Klee, W.; Bosma, R.B.; Cornelisse, J.L.; van Asten, A.J.; ter Huurne, A.A. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 1997, 140, 620–623. [Google Scholar] [CrossRef]
- Brandt, S.; Apprich, V.; Hackl, V.; Tober, R.; Danzer, M.; Kainzbauer, C.; Gabriel, C.; Stanek, C.; Kofler, J. Prevalence of bovine papillomavirus and Treponema DNA in bovine digital dermatitis lesions. Vet. Microbiol. 2011, 148, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Karaffová, V.; Marcinková, E.; Bobíková, K.; Herich, R.; Revajová, V.; Stašová, D.; Kavuľová, A.; Levkutová, M.; Levkut, M.; Lauková, A.; et al. TLR4 and TLR21 expression, MIF, IFN-β, MD-2, CD14 activation, and sIgA production in chickens administered with EFAL41 strain challenged with Campylobacter jejuni. Folia Microbiol. 2017, 62, 89–97. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Sullivan, L.E.; Evans, N.J.; Blowey, R.W.; Grove-White, D.H.; Clegg, S.R.; Duncan, J.S.; Carter, S.D. A molecular epidemiology of treponemes in beef cattle digital dermatitis lesions and comparative analyses with sheep contagious ovine digital dermatitis and dairy cattle digital dermatitis lesions. Vet. Microbiol. 2015, 178, 77–87. [Google Scholar] [CrossRef]
- Cook, N. LifeStep—A Lesion Oriented, Life Cycle Approach to Preventing Lameness in Dairy Herds. In Proceedings of the KvaegKongress, Herning, Denmark, 1 February 2016; pp. 1–11. Available online: https://www.landbrugsinfo.dk/kvaeg/dansk-kvaeg-kongres/sider/bilag-kk-nigel-b-cook-02.pdf (accessed on 20 January 2022).
- Hesseling, J.; Legione, A.R.; Stevenson, M.A.; McCowan, C.I.; Pyman, M.F.; Finochio, C.; Nguyen, D.; Roic, C.L.; Thiris, O.L.; Zhang, A.J.; et al. Bovine digital dermatitis in Victoria, Australia. Aust. Vet. J. 2019, 97, 404–413. [Google Scholar] [CrossRef]
- Mamuad, L.L.; Seo, B.J.; Faruk, M.S.A.; Espiritu, H.M.; Jin, S.J.; Kim, W.I.; Lee, S.S.; Cho, Y.I. Treponema spp., the dominant pathogen in the lesion of bovine digital dermatitis and its characterization in dairy cattle. Vet. Microbiol. 2020, 245, 108696. [Google Scholar] [CrossRef]
- Moreira, T.F.; Facury Filho, E.J.; Carvalho, A.U.; Strube, M.L.; Nielsen, M.W.; Klitgaard, K.; Jensen, T.K. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. PLoS ONE 2018, 13, e0193870. [Google Scholar] [CrossRef]
- Canales, N.; Bustamante, H.; Wilson-Welder, J.; Thomas, C.; Ramirez, E.; Salgado, M. First Molecular Confirmation of Treponema spp. in Lesions Consistent with Digital Dermatitis in Chilean Dairy Cattle. Pathogens 2022, 11, 510. [Google Scholar] [CrossRef]
- Dias, A.P.; De Buck, J. Detection and quantification of bacterial species DNA in bovine digital dermatitis lesions in swabs and fine-needle aspiration versus biopsies. Front. Vet. Sci. 2022, 9, 1040988. [Google Scholar] [CrossRef]
- Marčeková, P.; Mad’ar, M.; Styková, E.; Kačírová, J.; Sondorová, M.; Mudroň, P.; Žert, Z. The presence of Treponema spp. in equine hoof canker biopsies and skin samples from bovine digital dermatitis lesions. Microorganisms 2021, 9, 2190. [Google Scholar] [CrossRef] [PubMed]
- Krull, A.C.; Shearer, J.K.; Gorden, P.J.; Cooper, V.L.; Phillips, G.J.; Plummer, P.J. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 2014, 82, 3359–3373. [Google Scholar] [CrossRef] [PubMed]
- Brodard, I.; Alsaaod, M.; Gurtner, C.; Jores, J.; Steiner, A.; Kuhnert, P. A filter-assisted culture method for isolation of Treponema spp. from bovine digital dermatitis and their identification by MALDI-TOF MS. J. Vet. Diagn. Investig. 2021, 33, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Frosth, S.; Eriksson, H.K.; Rosander, A. Development of a multiplex quantitative PCR assay for simultaneous detection of Treponema phagedenis, Treponema pedis, Treponema medium, and ‘Treponema vincentii’ and evaluation on bovine digital dermatitis biopsies. Vet. Res. Commun. 2023, 47, 1937–1947. [Google Scholar] [CrossRef]
- Klitgaard, K.; Boye, M.; Capion, N.; Jensen, T.K. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 2008, 46, 3012–3020. [Google Scholar] [CrossRef]
- Nordhoff, M.; Moter, A.; Schrank, K.; Wieler, L.H. High prevalence of treponemes in bovine digital dermatitis-a molecular epidemiology. Vet. Microbiol. 2008, 131, 293–300. [Google Scholar] [CrossRef]
- Wilson-Welder, J.H.; Alt, D.P.; Nally, J.E. Digital Dermatitis in Cattle: Current Bacterial and Immunological Findings. Animals 2015, 5, 1114–1135. [Google Scholar] [CrossRef]
- Corlevic, A.T.; Beggs, D.S. Host Factors Impacting the Development and Transmission of Bovine Digital Dermatitis. Ruminants 2022, 2, 90–100. [Google Scholar] [CrossRef]
- Dias, A.P.; Orsel, K.; Gammariello, C.S.; De Buck, J. Sequential emergence and quantitative dynamics of key bacterial species preceding digital dermatitis lesion onset in dairy cattle. Vet. Microbiol. 2025, 302, 110378. [Google Scholar] [CrossRef]
- Wong, N.S.T.; Malmuthge, N.; Gellatly, D.; Nordi, W.M.; Alexander, T.W.; Ortega Polo, R.; Janzen, E.; Schwartzkopf-Genswein, K.; Jelinski, M. Characterization of the hoof bacterial communities in feedlot cattle affected with digital dermatitis, foot rot or both using a surface swab technique. Anim. Microbiome 2024, 6, 2. [Google Scholar] [CrossRef]
- Nischal, U.; Nischal, K.C.; Khopkar, U. Techniques of skin biopsy and practical considerations. J. Cutan. Aesthetic Surg. 2008, 1, 107–111. [Google Scholar] [CrossRef]
- Bell, J.; Crosby-Durrani, H.E.; Blowey, R.W.; Carter, S.D.; Evans, N.J. Survival of bovine digital dermatitis treponemes in conditions relevant to the host and farm environment. Anaerobe 2023, 82, 102766. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, K.; Strube, M.L.; Isbrand, A.; Jensen, T.K.; Nielsen, M.W. Microbiota Analysis of an Environmental Slurry and Its Potential Role as a Reservoir of Bovine Digital Dermatitis Pathogens. Appl. Environ. Microbiol. 2017, 83, e00244-17. [Google Scholar] [CrossRef] [PubMed]
- Zinicola, M.; Lima, F.; Lima, S.; Machado, V.; Gomez, M.; Döpfer, D.; Guard, C.; Bicalho, R. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. PLoS ONE 2015, 10, e0120504. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.P.; Orsel, K.; De Buck, J. Quantifying and mapping digital dermatitis-associated bacteria in lesion and nonlesion body sites and dairy farm environment. J. Dairy Sci. 2024, 107, 3252–3268. [Google Scholar] [CrossRef]
- Evans, N.J.; Timofte, D.; Isherwood, D.R.; Brown, J.M.; Williams, J.M.; Sherlock, K.; Carter, S.D. Host and environmental reservoirs of infection for bovine digital dermatitis treponemes. Vet. Microbiol. 2012, 156, 102–109. [Google Scholar] [CrossRef]
- Potterton, S.L.; Green, M.J.; Millar, K.M.; Brignell, C.J.; Harris, J.; Whay, H.R.; Huxley, J.N. Prevalence and characterisation of, and producers’ attitudes towards, hock lesions in UK dairy cattle. Vet. Rec. 2011, 169, 634. [Google Scholar] [CrossRef]
- Klitgaard, K.; Foix Bretó, A.; Boye, M.; Jensen, T.K. Targeting the treponemal microbiome of digital dermatitis infections by high-resolution phylogenetic analyses and comparison with fluorescent in situ hybridization. J. Clin. Microbiol. 2013, 51, 2212–2219. [Google Scholar] [CrossRef]
- Evans, N.J.; Blowey, R.W.; Timofte, D.; Isherwood, D.R.; Brown, J.M.; Murray, R.; Paton, R.J.; Carter, S.D. Association between bovine digital dermatitis treponemes and a range of ’non-healing’ bovine hoof disorders. Vet. Rec. 2011, 168, 214. [Google Scholar] [CrossRef]
- Roelofs, L.; Frössling, J.; Rosander, A.; Bjerketorp, J.; Belaghi, R.A.; Hansson, I.; Frosth, S. Digital dermatitis in Swedish dairy herds assessed by ELISA targeting Treponema phagedenis in bulk tank milk. BMC Vet. Res. 2024, 20, 168. [Google Scholar] [CrossRef]
- Mellado, M.; Saavedra, E.; Gaytán, L.; Veliz, F.G.; Macías-Cruz, U.; Avendano-Reyes, L.; García, E. The effect of lameness-causing lesions on milk yield and fertility of primiparous Holstein cows in a hot environment. Livest. Sci. 2018, 217, 8–14. [Google Scholar] [CrossRef]
- Robcis, R.; Ferchiou, A.; Berrada, M.; Raboisson, D. Management of digital dermatitis in dairy herds: Optimization and time allocation. Animals 2023, 13, 1988. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence 5′–3′ | Self-Complementarity Index | Melting Temperature | Product Size | References |
|---|---|---|---|---|---|
| T. pedis flaB Fw | GCAAGTTCCGCACAATTTAA | 3 | 58 °C | 121 bp | In this study |
| T. pedis flaB Rev | TTCTTTGGTCCATGTTTGCA | 2 | 58 °C | ||
| T. bren. 16S rRNAFw | CAAAGCAAACGTGATAAGTGT | 3 | 60 °C | 117 bp | |
| T.bren. 16S rRNA Rev | TCGCGTACCATCGAATTAAA | 2 | 58 °C |
| Treponema pedis | Treponema brennaborense | |||
|---|---|---|---|---|
| No. | Real-Time PCR | PCR | Real-Time PCR | PCR |
| 1 | Negative | Positive | Positive | Negative |
| 2 | Positive | Positive | Positive | Negative |
| 3 | Positive | Positive | Negative | Negative |
| 4 | Positive | Positive | Positive | Negative |
| 5 | Positive | Positive | Positive | Negative |
| 6 | Negative | Positive | Positive | Negative |
| 7 | Positive | Positive | Positive | Negative |
| 8 | Positive | Negative | Positive | Negative |
| 9 | Negative | Positive | Positive | Negative |
| 10 | Positive | Positive | Positive | Negative |
| 11 | Positive | Negative | Negative | Negative |
| 12 | Positive | Positive | Negative | Negative |
| 13 | Positive | Positive | Positive | Negative |
| 14 | Positive | Positive | Positive | Negative |
| 15 | Negative | Positive | Positive | Negative |
| 16 | Negative | Negative | Negative | Negative |
| 17 | Positive | Positive | Positive | Negative |
| 18 | Positive | Positive | Positive | Negative |
| 19 | Positive | Positive | Positive | Negative |
| 20 | Negative | Positive | Negative | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekková, S.; Sondorová, M.; Šurín Hudáková, N.; Karaffová, V.; Maďar, M.; Gomulec, P.; Mudroň, P. Distribution of Treponema Species in Active Digital Dermatitis Lesions and Non-Lesional Skin of Dairy Cattle. Microbiol. Res. 2025, 16, 119. https://doi.org/10.3390/microbiolres16060119
Mekková S, Sondorová M, Šurín Hudáková N, Karaffová V, Maďar M, Gomulec P, Mudroň P. Distribution of Treponema Species in Active Digital Dermatitis Lesions and Non-Lesional Skin of Dairy Cattle. Microbiology Research. 2025; 16(6):119. https://doi.org/10.3390/microbiolres16060119
Chicago/Turabian StyleMekková, Simona, Miriam Sondorová, Natália Šurín Hudáková, Viera Karaffová, Marián Maďar, Pavel Gomulec, and Pavol Mudroň. 2025. "Distribution of Treponema Species in Active Digital Dermatitis Lesions and Non-Lesional Skin of Dairy Cattle" Microbiology Research 16, no. 6: 119. https://doi.org/10.3390/microbiolres16060119
APA StyleMekková, S., Sondorová, M., Šurín Hudáková, N., Karaffová, V., Maďar, M., Gomulec, P., & Mudroň, P. (2025). Distribution of Treponema Species in Active Digital Dermatitis Lesions and Non-Lesional Skin of Dairy Cattle. Microbiology Research, 16(6), 119. https://doi.org/10.3390/microbiolres16060119







