Succession Characteristics of Soil Microbial Communities Along Elevational Gradients in the Lhasa River Basin and Analysis of Environmental Driving Factors
Abstract
1. Introduction
2. Study Area and Methodology
2.1. Overview of the Study Area
2.2. Soil Sample Collection
2.3. Determination of Soil Physicochemical Properties
2.4. Soil Microbial DNA Extraction and Sequencing
2.5. Data Analysis
3. Results
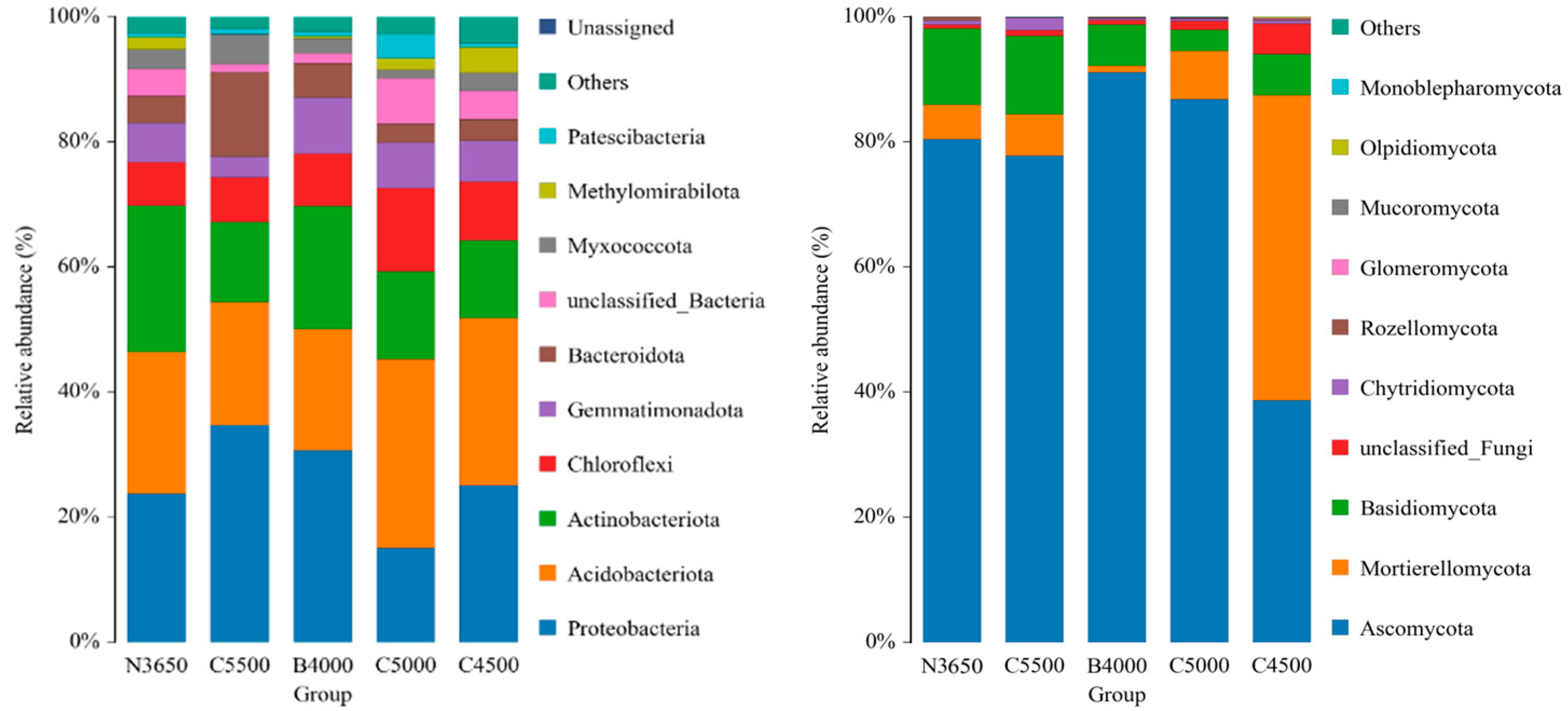
3.1. Composition of Soil Microbial Communities at Different Elevations in the Duilong Qu Basin
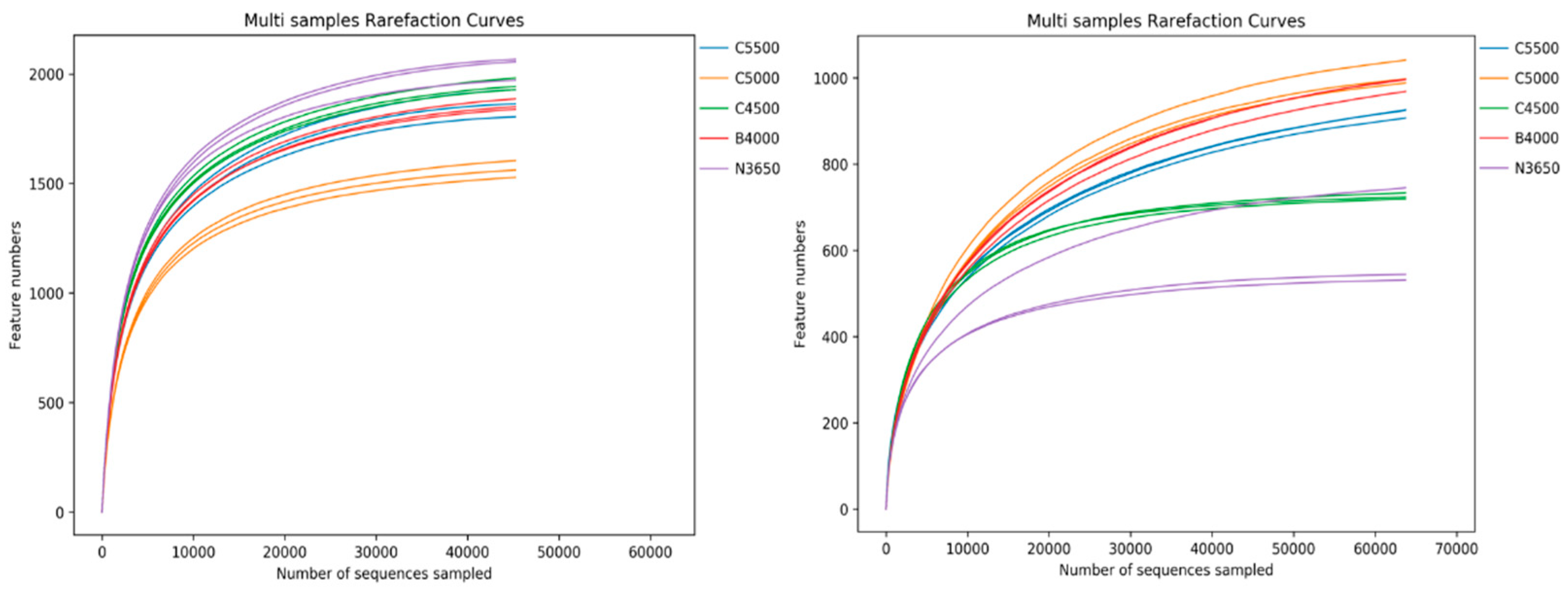
3.2. Diversity of Soil Microbial Communities at Different Elevations in the Duilong Qu Basin
3.3. Structural Characteristics of the Soil Microbial Communities at Different Elevations in the Duilong Qu Basin
3.4. Variations in Soil Physicochemical Properties at Different Elevations in the Duilong Qu Basin
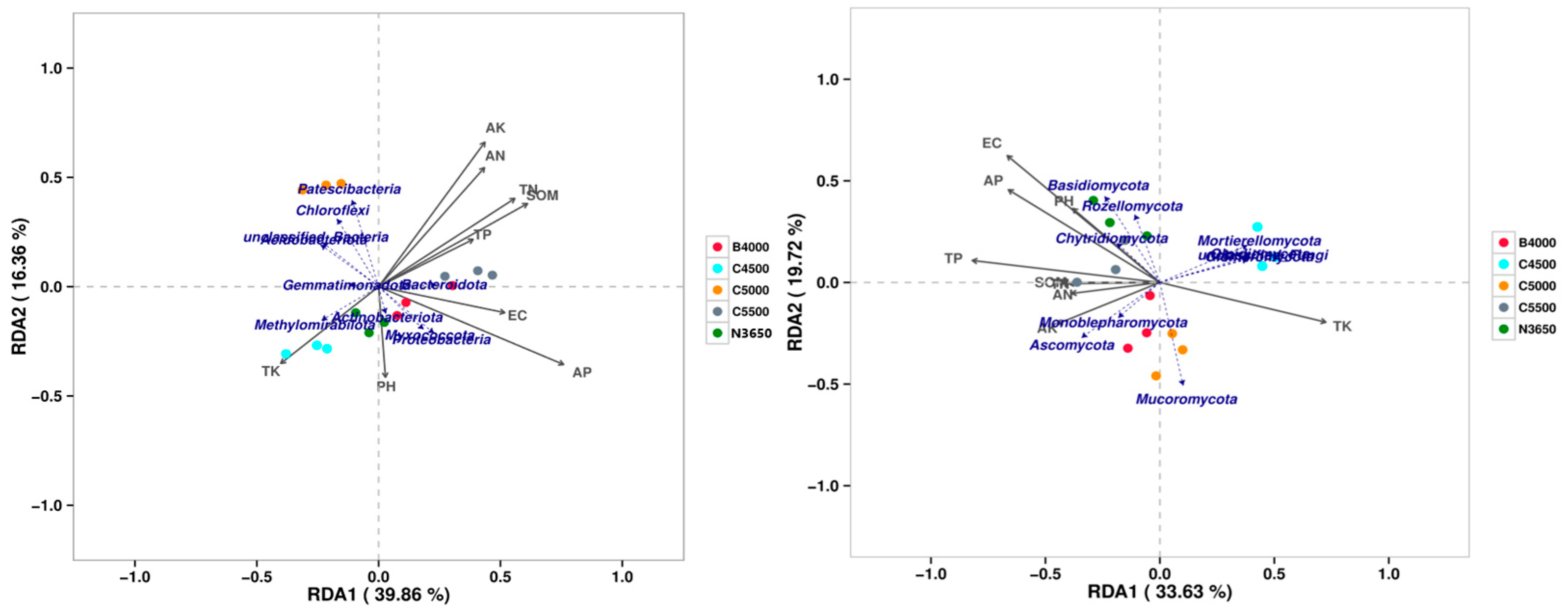
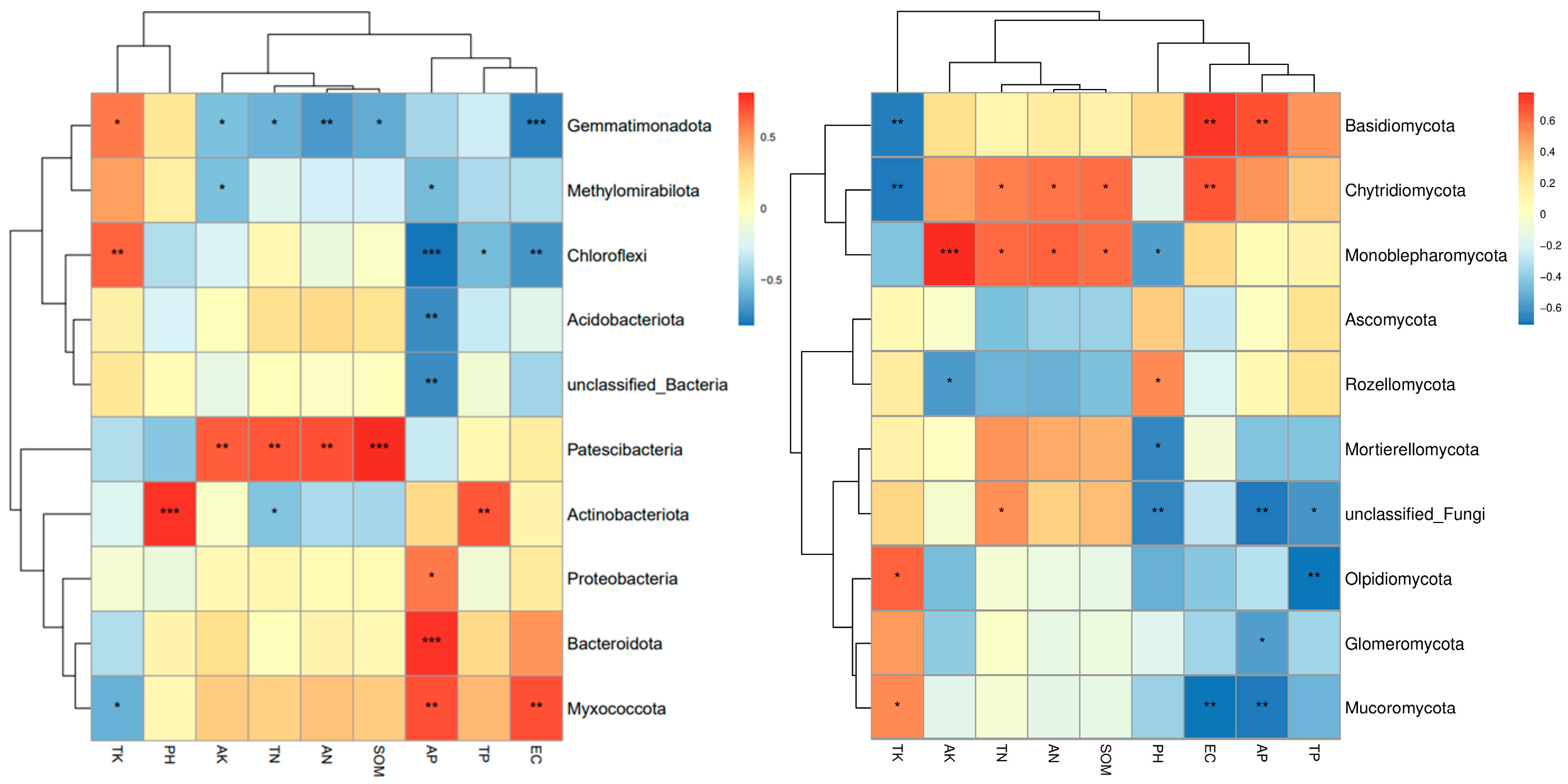
3.5. Relationships Between the Soil Microbial Community Structure and Environmental Factors
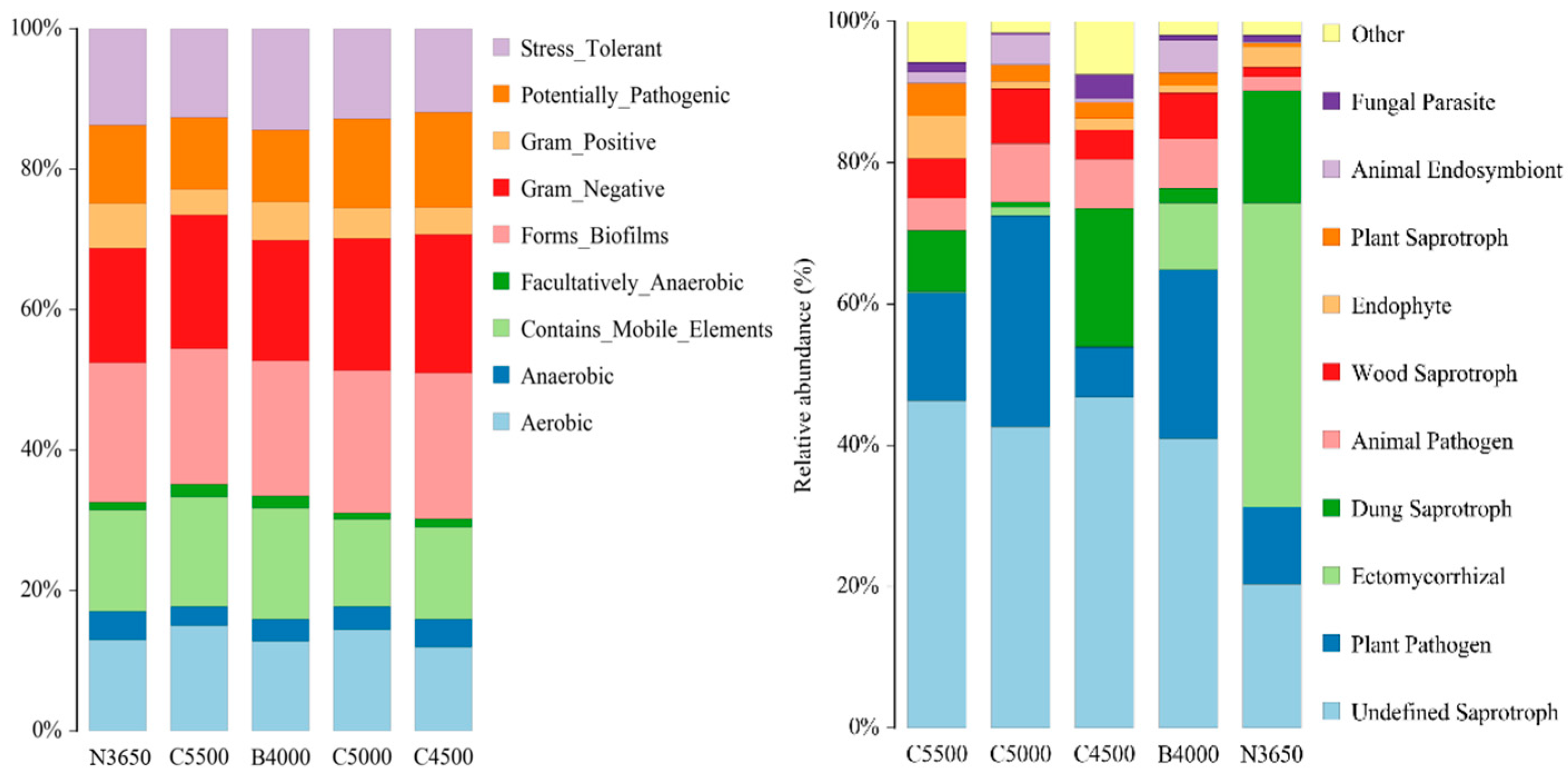
3.6. Functional Prediction of Soil Microbial Communities Along Different Elevations in the Duilong Qu Basin
4. Discussion
4.1. Composition and Diversity of Soil Bacterial Communities at Different Elevations in the Duilong Qu Basin
4.2. Composition and Diversity of Soil Fungal Communities in Different Elevations in the Duilong Qu Basin
5. Conclusions
- (1)
- This research investigated the interplay and ecological roles of soil microbe populations, including bacteria and fungi, across different elevations within the Duilong Qu Basin of the Lhasa River. These findings indicate substantial alterations in the composition of microbial communities at various altitudes. For bacteria, the Chao1 richness index displayed a fluctuating trend, whereas the Shannon diversity index exhibited a distinct V-shaped pattern. In contrast, fungal richness generally increased with altitude, while fungal diversity showed no consistent altitudinal trend. Available phosphorus (AP) was identified as the primary environmental factor influencing bacterial community structure, with soil SOM, TN, AN, and AK also playing significant roles. For fungal communities, EC was the most important factor, followed by total phosphorus (TP), AP, and pH. In terms of functional structure, bacterial communities shifted markedly along the elevation gradient—from being dominated by aerobic and biofilm-forming taxa at lower altitudes to facultative anaerobes, stress-tolerant strains, and potential pathogens at higher altitudes. Conversely, fungal communities gradually transitioned from ectomycorrhizal dominance at low elevations to a community structure increasingly characterized by saprotrophic and plant pathogenic fungi with increasing elevation.
- (2)
- The current altitudinal sampling design presents certain limitations that may affect the resolution of microbial community response analysis. With only five discrete elevation points, the existing scheme lacks sufficient granularity to capture fine-scale changes along the altitudinal gradient. To overcome these limitations, several improvements are recommended for future research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.T.; Zhao, X.Q.; Zi, H.B.; Hu, L.; Ade, L.; Wang, G.X.; Lerdau, M. The effect of simulated warming on root dynamics and soil microbial community in an alpine meadow of the Qinghai–Tibet Plateau. Appl. Soil Ecol. 2017, 116, 30–41. [Google Scholar] [CrossRef]
- Cong, J.; Cong, W.; Lu, H.; Zhang, Y. Distinct Elevational Patterns and Their Linkages of Soil Bacteria and Plant Community in An Alpine Meadow of the Qinghai–Tibetan Plateau. Microorganisms 2022, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- An, B.; Yao, T.; Guo, Y.; Wang, W.; Li, J.; Li, X.; Wang, Z. Demonstration system of protection, restoration, and treatment techniques in typical areas of the Lhasa River Basin. Sci. Bull. 2021, 66, 2775–2784. (In Chinese) [Google Scholar]
- Sun, W.; Li, Z.; Lei, J.; Liu, X. Bacterial Communities of Forest Soils along Different Elevations: Diversity, Structure, and Functional Composition with Potential Impacts on CO2 Emission. Microorganisms 2022, 10, 766. [Google Scholar] [CrossRef]
- Wang, J.J.; Soininen, J.; Zhang, Y.; Wang, B.X.; Yang, X.D.; Shen, J. Contrasting patterns in elevational diversity between microorganisms and macroorganisms. J. Biogeogr. 2011, 38, 595–603. [Google Scholar] [CrossRef]
- Song, C.; Wu, J.; Lu, Y.; Shen, Q.; He, J.; Huang, Q.; Jia, Z.; Leng, S.; Zhu, Y. A ten-year retrospective of soil microbiology research in China. Adv. Earth Sci. 2013, 28, 1087–1105. (In Chinese) [Google Scholar]
- Yin, J.; Yuan, D.; Lu, J.; Li, H.; Luo, S.; Zhang, J.; Xiang, X. Effect of Warming on Soil Fungal Community Along Altitude Gradients in a Subalpine Meadow. Microorganisms 2024, 12, 2527. [Google Scholar] [CrossRef]
- Jiao, K. Characteristics of Deep Soil Microbial Communities in Typical Vegetation Types of the Sejila Mountains. Master’s Thesis, Guizhou University, Guiyang, China, 2021. (In Chinese). [Google Scholar] [CrossRef]
- Singh, D.; Takahashi, K.; Kim, M.; Chun, J.; Adams, J.M. A Hump-Backed Trend in Bacterial Diversity with Elevation on Mount Fuji, Japan. Microb. Ecol. 2012, 63, 429–437. [Google Scholar] [CrossRef]
- Siles, J.A.; Margesin, R. Abundance and Diversity of Bacterial, Archaeal, and Fungal Communities Along an Altitudinal Gradient in Alpine Forest Soils: What Are the Driving Factors? Microb. Ecol. 2016, 72, 207–220. [Google Scholar] [CrossRef]
- Pan, J.W.; Guo, Q.Q.; Li, H.E.; Luo, S.Q.; Zhang, Y.Q.; Yao, S.; Fan, X.; Sun, X.G.; Qi, Y.J. Dynamics of Soil Nutrients, Microbial Community Structure, Enzymatic Activity, and Their Relationships along a Chronosequence of Pinus massoniana Plantations. Forests 2021, 12, 376. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Cong, J.; Lu, H.; Li, G.L.; Xue, Y.D.; Deng, Y.; Li, H.; Zhou, J.Z.; Li, D.Q. Soil bacterial diversity patterns and drivers along an elevational gradient on Shennongjia Mountain, China. Microb. Biotechnol. 2015, 8, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, G.H.; Chen, L.; Wang, J.T.; Zhang, L.M. Soil pH determines fungal diversity along an elevation gradient in Southwestern China. Sci. China-Life Sci. 2018, 61, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.Y.; Cong, W.; Yang, L.S.; Liu, Q.; Zhang, Y.G. Forest Soil Fungal Community Elevational Distribution Pattern and Their Ecological Assembly Processes. Front. Microbiol. 2019, 10, 2226. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Y.; Yang, T.; Xia, S.G.; Yin, T.; Liu, X.; Li, S.P.; Sun, R.B.; Gao, H.J.; Chu, H.Y.; Ma, C. Soil depth exerts stronger impact on bacterial community than elevation in subtropical forests of Huangshan Mountain. Sci. Total Environ. 2022, 852, 158438. [Google Scholar] [CrossRef]
- Singh, D.; Lee-Cruz, L.; Kim, W.S.; Kerfahi, D.; Chun, J.H.; Adams, J.M. Strong elevational trends in soil bacterial community composition on Mt. Ha lla, South Korea. Soil Biol. Biochem. 2014, 68, 140–149. [Google Scholar] [CrossRef]
- Bryant, J.A.; Lamanna, C.; Morlon, H.; Kerkhoff, A.J.; Enquist, B.J.; Green, J.L. Microbes on mountainsides: Contrasting elevational patterns of bacterial and plant diversity. Proc. Natl. Acad. Sci. USA 2008, 105, 11505–11511. [Google Scholar] [CrossRef]
- Bahram, M.; Polme, S.; Koljalg, U.; Zarre, S.; Tedersoo, L. Regional and local patterns of ectomycorrhizal fungal diversity and community structure along an altitudinal gradient in the Hyrcanian forests of northern Iran. New Phytol. 2012, 193, 465–473. [Google Scholar] [CrossRef]
- Li, G.X.; Xu, G.R.; Shen, C.C.; Tang, Y.; Zhang, Y.X.; Ma, K.M. Contrasting elevational diversity patterns for soil bacteria between two ecosystems divided by the treeline. Sci. China-Life Sci. 2016, 59, 1177–1186. [Google Scholar] [CrossRef]
- Corneo, P.E.; Pellegrini, A.; Cappellin, L.; Roncador, M.; Chierici, M.; Gessler, C.; Pertot, I. Microbial community structure in vineyard soils across altitudinal gradients and in different seasons. FEMS Microbiol. Ecol. 2013, 84, 588–602. [Google Scholar] [CrossRef]
- Shen, C.C.; Xiong, J.B.; Zhang, H.Y.; Feng, Y.Z.; Lin, X.G.; Li, X.Y.; Liang, W.J.; Chu, H.Y. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, Y.B.; Nan, Q.; Guo, J.W.; Tan, Z.Y.; Shao, X.M.; Tian, C.F. Barley farmland harbors a highly homogeneous soil bacterial community compared to wild ecosystems in the Qinghai-Xizang Plateau. Front. Microbiol. 2024, 15, 1418161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiao, J.; Chen, T.; Chen, Y.; Lin, H.; Xu, Q.; Cheng, Y.; Zhao, W. Evaluation of soil nutrients in the alluvial fan area of the middle and lower Lhasa River Basin. J. Plant Nutr. Fertil. 2022, 28, 2082–2096. (In Chinese) [Google Scholar]
- Bu, D.; Xu, Z.; Wu, J.; Li, M.; Deji, D. Distribution of mineral processing plants and their environmental impact in the Lhasa River Basin. J. Tibet Univ. 2009, 24, 33–38. (In Chinese) [Google Scholar] [CrossRef]
- Bao, S.D. Soil Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2013; pp. 72–75. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Schloss, P.D.; Gevers, D.; Westcott, S.L. Reducing the Effects of PCR Amplification and Sequencing Artifacts on 16S rRNA-Based Studies. PLoS ONE 2011, 6, e27310. [Google Scholar] [CrossRef]
- Wang, J.J.; Hu, A.; Meng, F.F.; Zhao, W.Q.; Yang, Y.F.; Soininen, J.; Shen, J.; Zhou, J.Z. Embracing mountain microbiome and ecosystem functions under global change. New Phytol. 2022, 234, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Li, M.S.; Dai, G.H.; Mu, L.Q. Composition and diversity of soil bacterial communities under identical vegetation along an elevational gradient in Changbai Mountains, China. Front. Microbiol. 2022, 13, 1065412. [Google Scholar] [CrossRef]
- Zhang, F.; Lv, F.; Chen, M. Biodiversity and Structure of Microbial Community in Glacial Melts and Soil in the High Arctic Ny-Ålesund, Svalbard. Microorganisms 2022, 10, 1941. [Google Scholar] [CrossRef]
- Chu, H.Y.; Sun, H.B.; Tripathi, B.M.; Adams, J.M.; Huang, R.; Zhang, Y.J.; Shi, Y. Bacterial community dissimilarity between the surface and subsurface soils equals horizontal differences over several kilometers in the western Tibetan Plateau. Environ. Microbiol. 2016, 18, 1523–1533. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, D.; Sun, K.; Wang, N. Structure and diversity of soil microbial communities in Berberis diaphana habitats along altitudinal gradients in alpine regions. Acta Bot. Boreal.-Occident. Sin. 2021, 41, 1036–1050. (In Chinese) [Google Scholar]
- Kong, L.; Zhang, L.; Wang, Y.; Huang, Z. Impact of Ecological Restoration on the Physicochemical Properties and Bacterial Communities in Alpine Mining Area Soils. Microorganisms 2024, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Jian, S.L.; Li, K.M.; Wu, Z.B.; Guan, H.T.; Hao, J.W.; Wang, S.Y.; Lin, Y.Y.; Wang, G.J.; Li, A.H. Community structure of bacterioplankton and its relationship with environmental factors in the upper reaches of the Heihe River in Qinghai Plateau. Environ. Microbiol. 2021, 23, 1210–1221. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, Y.; Yin, Y. Response of soil bacterial communities to alpine meadow degradation. Biodiversity 2021, 29, 53–64. (In Chinese) [Google Scholar]
- Wu, J.; Zhi, X.; Li, Y.; Guan, T.; Tang, S.; Xu, L.; Li, W. Comparative study on uncultured actinomycetes in sediments from Jiangcheng and Heijing salt mines in Yunnan. Microbiol. China 2008, 35, 1550–1555. (In Chinese) [Google Scholar]
- Liu, W.; Guo, S.; Zhang, H.; Chen, Y.; Shao, Y.; Yuan, Z. Effect of Altitude Gradients on the Spatial Distribution Mechanism of Soil Bacteria in Temperate Deciduous Broad-Leaved Forests. Microorganisms 2024, 12, 1034. [Google Scholar] [CrossRef]
- Pushkareva, E.; Barrantes, I.; Leinweber, P.; Karsten, U. Microbial Diversity in Subarctic Biocrusts from West Iceland following an Elevation Gradient. Microorganisms 2021, 9, 2195. [Google Scholar] [CrossRef]
- Lin, L.; Li, G.; Yu, H.; Ma, K. pH Nonlinearly Dominates Soil Bacterial Community Assembly along an Altitudinal Gradient in Oak-Dominant Forests. Microorganisms 2024, 12, 1877. [Google Scholar] [CrossRef]
- Rodrigues, Y.F.; Andreote, F.D.; Silva, A.M.M.; Dias, A.C.F.; Taketani, R.G.; Cotta, S.R. Disentangling the role of soil bacterial diversity in phosphorus transformation in the maize rhizosphere. Appl. Soil Ecol. 2023, 182, 104739. [Google Scholar] [CrossRef]
- Chen, J.; Xu, M.; Zou, X.; Yang, Y.; Zhang, J.; Zhang, J. Soil bacterial community structure in Pinus massoniana forests in central Guizhou. J. Microbiol. 2021, 41, 12–22. (In Chinese) [Google Scholar]
- Lei, H.; Yin, Y.; Liu, Y.; Wan, X.; Ma, H.; Gao, R.; Yang, Y. Effects of Chinese fir litter and its biochar on soil microbial community structure. Acta Pedol. Sin. 2016, 53, 790–799. (In Chinese) [Google Scholar]
- Zeng, Z.F.; Huang, R.L.; Li, W. Elevation Determines Fungal Diversity, and Land Use Governs Community Composition: A Dual Perspective from Gaoligong Mountains. Microorganisms 2024, 12, 2378. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, M.; Zhang, H.; Zhang, Y.; Huang, P.; Wu, Y.; Zou, T.; Wang, N.; Zeng, H. Interactive effects of vegetation and soil factors on microbial communities during alpine grassland degradation. Environ. Sci. 2024, 45, 4251–4265. (In Chinese) [Google Scholar] [CrossRef]
- Wen, Y.; Zhao, B.; Luo, Q.; Jia, Y.; Feng, T.; Wang, Q. Distribution of arbuscular mycorrhizal fungi in alpine grasslands of the Qinghai-Tibet Plateau and their ecological role in quasinatural restoration. Mycosystema 2021, 40, 2562–2578. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, J.; Song, M. Role of plants and soil microorganisms in regulating ecosystem nutrient cycling. Chin. J. Plant Ecol. 2010, 34, 979–988. (In Chinese) [Google Scholar]
- Quinteros-Urquieta, C.; Francois, J.-P.; Aguilar-Muñoz, P.; Orellana, R.; Villaseñor, R.; Moreira-Muñoz, A.; Molina, V. Microbial Diversity of Soil in a Mediterranean Biodiversity Hotspot: Parque Nacional La Campana, Chile. Microorganisms 2024, 12, 1569. [Google Scholar] [CrossRef]
- Romanowicz, K.J.; Freedman, Z.B.; Upchurch, R.A.; Argiroff, W.A.; Zak, D.R. Active microorganisms in forest soils differ from the total community yet are shaped by the same environmental factors: The influence of pH and soil moisture. FEMS Microbiol. Ecol. 2016, 92, fiw149. [Google Scholar] [CrossRef]
- Sheng, Y.; Cong, J.; Lu, H.; Yang, K.; Yang, L.; Wang, M.; Zhang, Y. Soil fungal diversity in the forest line ecotone of Shennongjia National Park. Acta Ecol. Sin. 2018, 38, 5322–5330. (In Chinese) [Google Scholar]
- Nguyen, N.H.; Song, Z.W.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
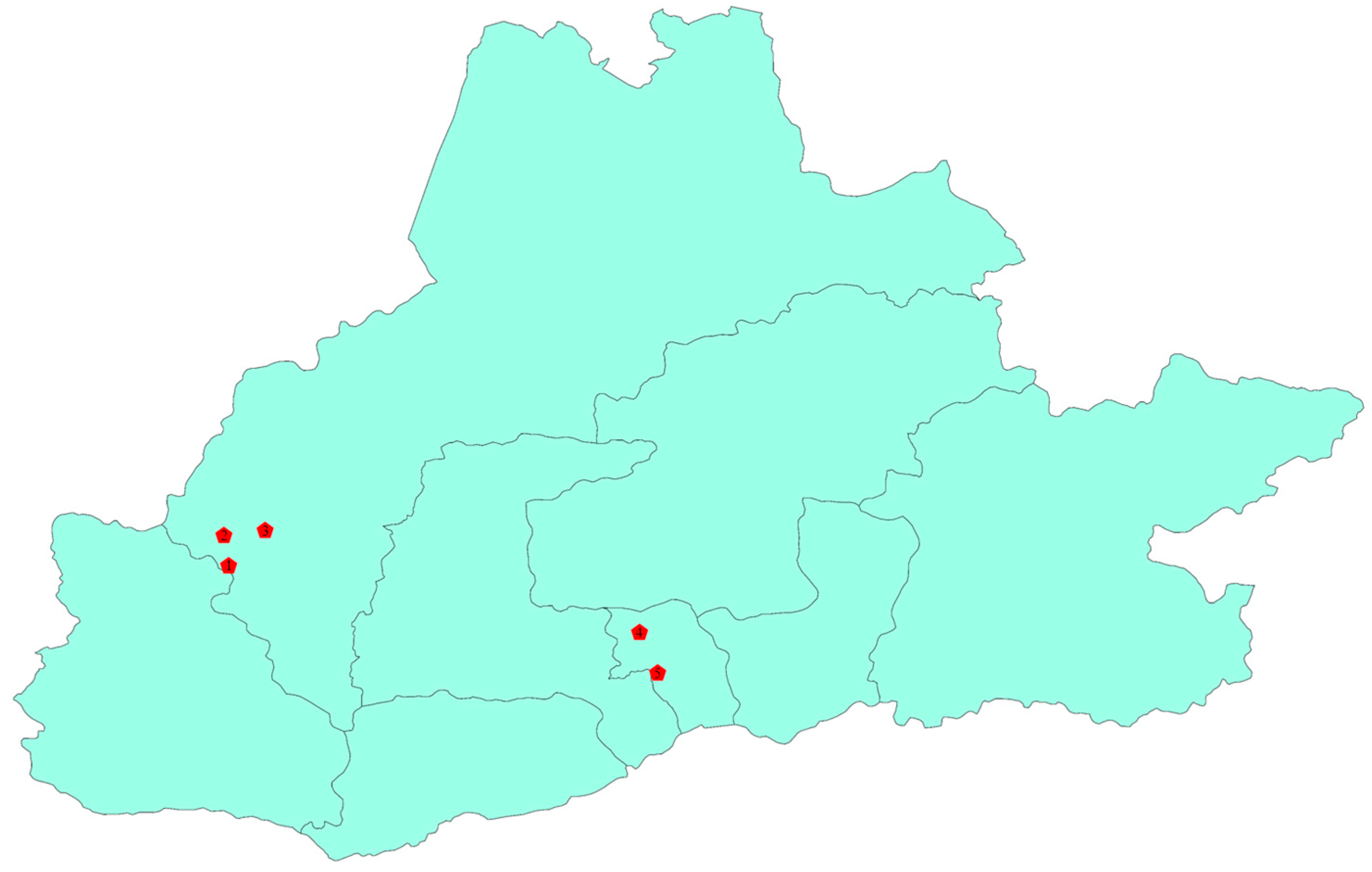


| Plot | Vegetation Type | Ecological Types | Latitude and Longitude | Elevation (m) |
|---|---|---|---|---|
| 1 | Alpine Meadow | Alpine Meadow Soil | 29°86′93″ N, 90°21′03″ E | 5522.1 |
| 2 | Alpine Meadow | Alpine Meadow Soil | 29°93′38″ N, 90°20′02″ E | 4989.7 |
| 3 | Alpine Desert | Desert soil | 29°94′44″ N, 90°28′77″ E | 4594.4 |
| 4 | Natural Grassland | Grassland soil | 29°72′70″ N, 91°08′44″ E | 3996.8 |
| 5 | Mixed Forest | Planted forest soil | 29°64′11″ N, 91°12′28″ E | 3683.9 |
| Altitude | Soil Bacteria | Soil Fungi | ||||
|---|---|---|---|---|---|---|
| ASV | Chao1 | Shannon | ASV | Chao1 | Shannon | |
| 3650 m | 5100 | 2039.16 ± 51.90 a | 9.91 ± 0.03 a | 1427 | 611.22 ± 124.20 c | 6.30 ± 0.05 b |
| 4000 m | 4472 | 1872.75 ± 27.86 b | 9.90 ± 0.01 a | 2183 | 1022.15 ± 11.15 a | 6.16 ± 0.04 b,c |
| 4500 m | 4762 | 1965.00 ± 28.98 a | 9.04 ± 0.05 c | 1376 | 730.76 ± 6.13 b | 6.08 ± 0.09 c,d |
| 5000 m | 3516 | 1591.17 ± 40.12 c | 9.58 ± 0.03 b | 2148 | 1031.30 ± 32.17 a | 5.98 ± 0.09 d |
| 5500 m | 4401 | 1868.00 ± 63.41 b | 9.62 ± 0.03 b | 1895 | 956.17 ± 16.05 a | 6.88 ± 0.10 a |
| Altitude | pH | EC μs·cm −1 | AK mg·kg −1 | AP mg·kg −1 | AN mg·kg −1 | TK g·kg −1 | TP g·kg −1 | TN g·kg −1 | SOM g·kg −1 |
|---|---|---|---|---|---|---|---|---|---|
| 5500 | 6.52 ± 0.29 c | 147.00 ± 10 a | 167.70 ± 59.27 a | 26.16 ± 1.49 a | 256.70 ± 14.15 a | 35.47 ± 0.42 c | 0.27 ± 0.02 b | 4.23 ± 0.07 a | 108.20 ± 1.88 a |
| 5000 | 6.58 ± 0.25 c | 48.30 ± 2.5 b | 121.00 ± 2.00 b | 5.86 ± 1.58 b | 165.7 ± 15.78 b | 39.27 ± 0.70 b | 0.26 ± 0.02 b | 2.00 ± 0.09 b | 42.22 ± 1.30 b |
| 4500 | 6.70 ± 0.07 c | 41.00 ± 9.75 b | 25.00 ± 1.73 a | 10.32 ± 2.52 b | 67.70 ± 8.80 c | 47.10 ± 2.48 a | 0.13 ± 0.04 c | 0.96 ± 0.04 c | 15.60 ± 0.98 c |
| 4000 | 7.30 ± 0.37 b | 41.30 ± 13 b | 28.30 ± 5.13 d | 19.79 ± 6.42 a | 43.17 ± 4.04 d | 44.70 ± 3.25 a | 0.25 ± 0.02 b | 0.64 ± 0.04 d | 11.20 ± 2.50 d |
| 3650 | 7.90 ± 0.7 a | 141.00 ± 3 a | 43.30 ± 1.53 c | 25.56 ± 3.98a | 77.00 ± 18.20 c | 35.87 ± 0.50 b,c | 0.35 ± 0.026 a | 0.90 ± 0.07 c | 15.80 ± 1.20 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sun, X.; An, B.; Li, S.; Li, J.; Wang, C. Succession Characteristics of Soil Microbial Communities Along Elevational Gradients in the Lhasa River Basin and Analysis of Environmental Driving Factors. Microbiol. Res. 2025, 16, 117. https://doi.org/10.3390/microbiolres16060117
Li X, Sun X, An B, Li S, Li J, Wang C. Succession Characteristics of Soil Microbial Communities Along Elevational Gradients in the Lhasa River Basin and Analysis of Environmental Driving Factors. Microbiology Research. 2025; 16(6):117. https://doi.org/10.3390/microbiolres16060117
Chicago/Turabian StyleLi, Xiaoyu, Xiangyang Sun, Baosheng An, Suyan Li, Jiule Li, and Chuanfei Wang. 2025. "Succession Characteristics of Soil Microbial Communities Along Elevational Gradients in the Lhasa River Basin and Analysis of Environmental Driving Factors" Microbiology Research 16, no. 6: 117. https://doi.org/10.3390/microbiolres16060117
APA StyleLi, X., Sun, X., An, B., Li, S., Li, J., & Wang, C. (2025). Succession Characteristics of Soil Microbial Communities Along Elevational Gradients in the Lhasa River Basin and Analysis of Environmental Driving Factors. Microbiology Research, 16(6), 117. https://doi.org/10.3390/microbiolres16060117




