HIV-1 and Antiretroviral Therapy Modulate HERV Pol and Syncytin Gene Expression in Mothers and Newborns
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Samples Collection
2.2. RNA Extraction
2.3. Reverse Transcription and Real Time PCR
2.4. Relative Quantification with ΔΔCT Method
2.5. Statistical Analysis
3. Results
3.1. Expression of Pol Genes of HERV-K, HERV-W, and HERV-H and of Syncytin 1 and Syncytin 2 Genes in HIV-1-Negative Mothers and Their Newborns
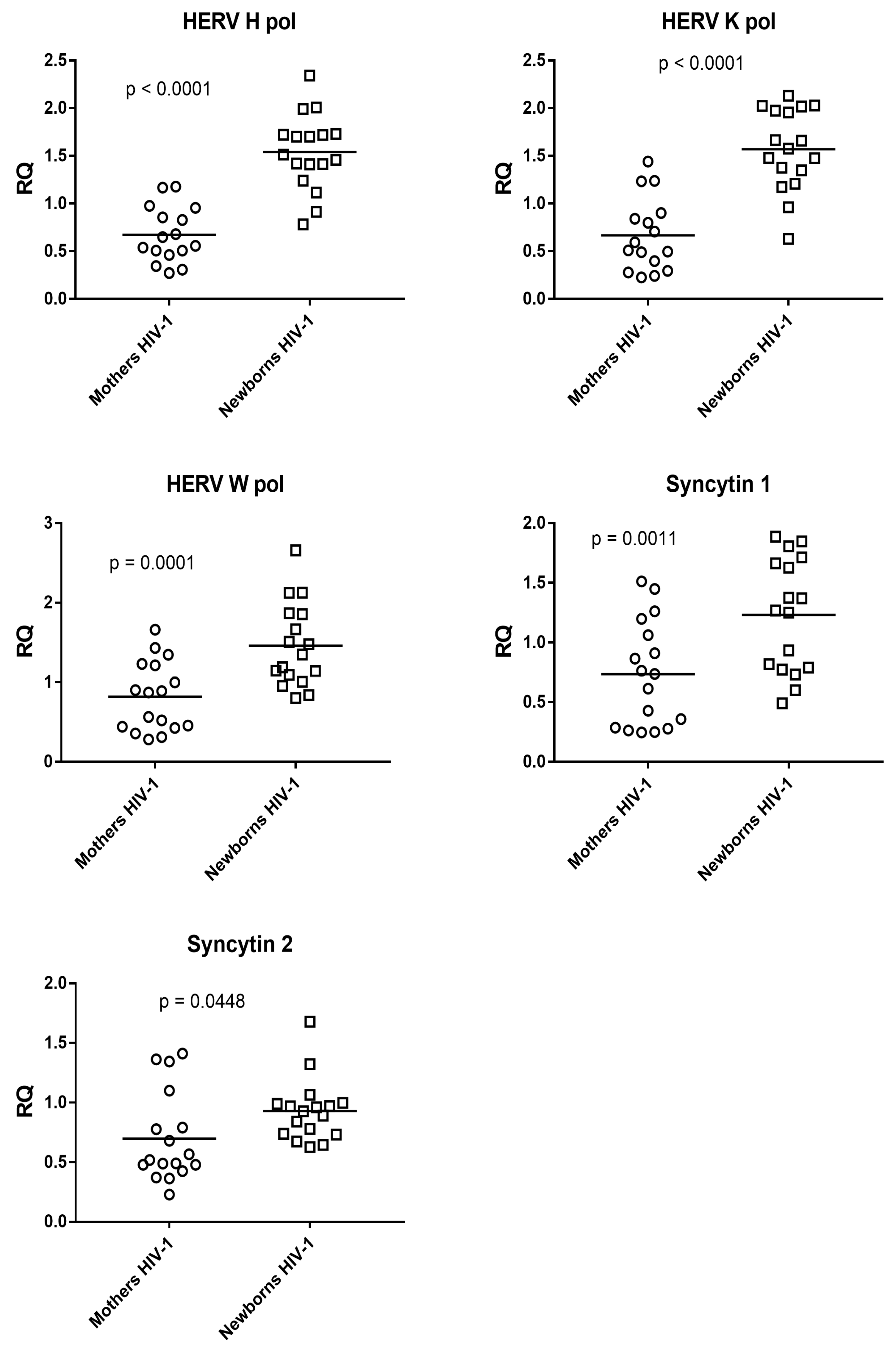
3.2. Expression of Pol Genes of HERV-K, HERV-W, and HERV-H and of Syncytin 1 and Syncytin 2 Genes in HIV-1-Positive Mothers with Antiretroviral Therapy and Their Newborns
3.3. Expression of Pol Genes of HERV-K, HERV-W, and HERV-H and of Syncytin 1 and Syncytin 2 Genes in HIV-1-Infected Mothers on Antiretroviral Therapy and in Healthy Mothers
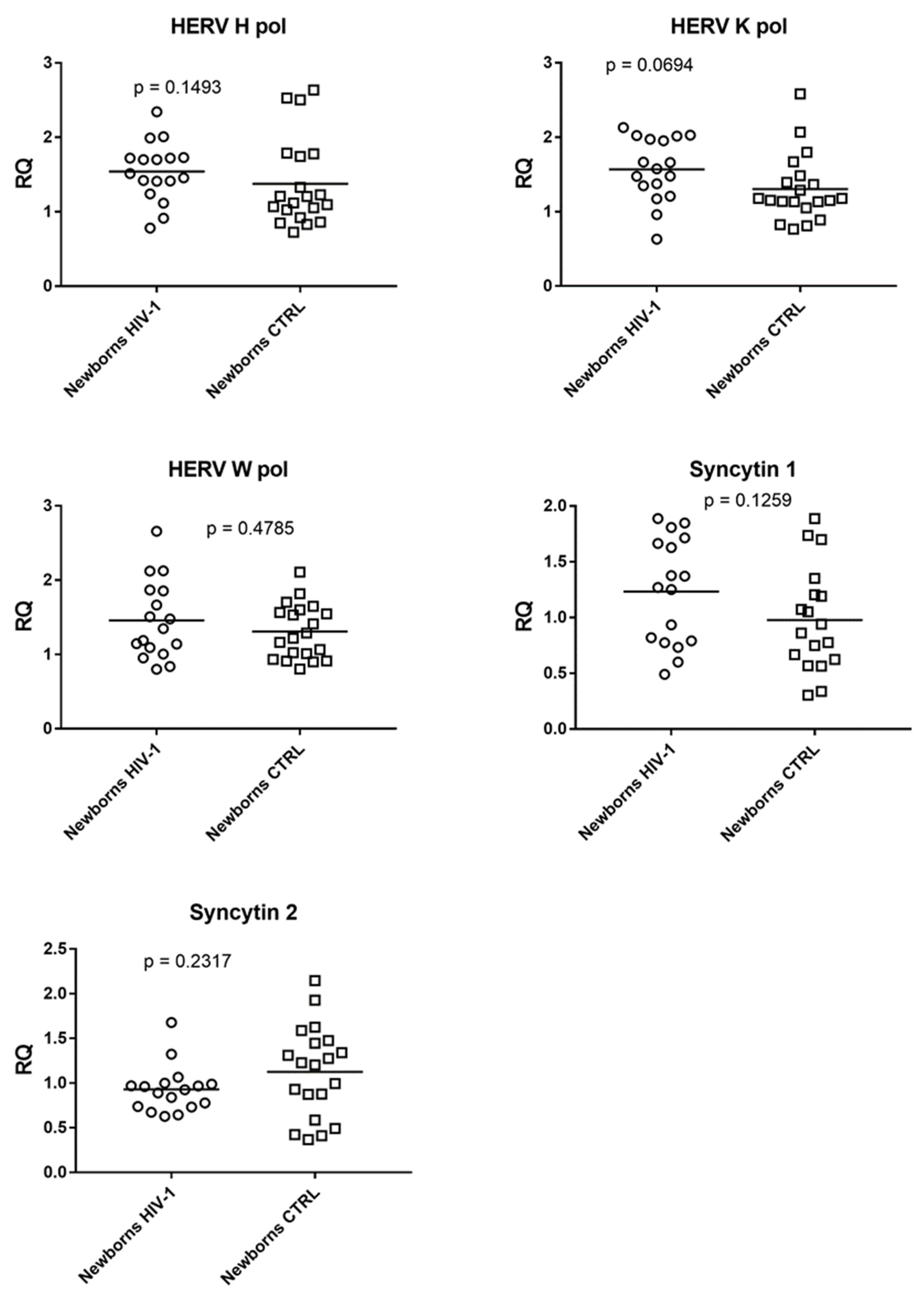
3.4. Expression of Pol Genes of HERV-K, HERV-W, and HERV-H and of Syncytin 1 and Syncytin 2 Genes in Infants Exposed to Antiretroviral Therapy and in Not-Exposed Infants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, W.E. Origins and evolutionary consequences of ancient endogenous retroviruses. Nat. Rev. Microbiol. 2019, 17, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. Human endogenous retroviruses are ancient acquired elements still shaping innate immune responses. Front. Immunol. 2018, 9, 2039. [Google Scholar] [CrossRef] [PubMed]
- Chabukswar, S.; Grandi, S.; Tramontano, E. Prolonged activity of HERV-K(HML2) in Old World Monkeys accounts for recent integrations and novel recombinant variants. Front Microbiol. 2022, 13, 1040792. [Google Scholar] [CrossRef]
- Xue, B.; Sechi, L.A.; Kelvin, D.J. Human Endogenous Retrovirus K (HML-2) in Health and Disease. Front Microbiol. 2020, 11, 1690. [Google Scholar] [CrossRef] [PubMed]
- Bolze, P.A.; Mommert, M.; Mallet, F. Contribution of Syncytins and Other Endogenous Retroviral Envelopes to Human Placenta Pathologies. Prog. Mol. Biol. Transl. Sci. 2017, 145, 111–162. [Google Scholar]
- Dopkins, N.; Singh, B.; Michael, S.; Zhang, P.; Marston, J.L.; Fei, T.; Singh, M.; Feschotte, C.; Collins, N.; Bendall, M.L.; et al. Ribosomal profiling of human endogenous retroviruses in healthy tissues. BMC Genom. 2024, 25, 5. [Google Scholar] [CrossRef]
- Fan, J.; Qin, Z. Roles of Human Endogenous Retrovirus-K-Encoded Np9 in Human Diseases: A Small Protein with Big Functions. Viruses 2024, 16, 581. [Google Scholar] [CrossRef]
- Nishihara, H. Transposable elements as genetic accelerators of evolution: Contribution to genome size, gene regulatory network rewiring and morphological innovation. Genes Genet Syst. 2020, 94, 269–281. [Google Scholar] [CrossRef]
- Lavialle, C.; Cornelis, G.; Dupressoir, A.; Esnault, C.; Heidmann, O.; Vernochet, C.; Heidmann, T. Paleovirology of ‘syncytins’, retroviral env genes exapted for a role in placentation. Philos. Trans. R Soc. Lond B Biol. Sci. 2013, 368, 1626. [Google Scholar] [CrossRef]
- Gholami Barzoki, M.; Shatizadeh Malekshahi, S.; Heydarifard, Z.; Mahmodi, M.J.; Soltanghoraee, H. The important biological roles of Syncytin-1 of human endogenous retrovirus W (HERV-W) and Syncytin-2 of HERV-FRD in the human placenta development. Mol. Biol. Rep. 2023, 50, 7901–7907. [Google Scholar] [CrossRef]
- Holder, B.S.; Tower, C.L.; Abrahams, V.M.; Aplin, J.D. Syncytin 1 in the human placenta. Placenta 2012, 33, 460–466. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, C.; Wang, P.; Yang, W.; Zhu, H.; Zhang, S. Regulators involved in trophoblast syncytialization in the placenta of intrauterine growth restriction. Front. Endocrinol. 2023, 14, 1107182. [Google Scholar] [CrossRef] [PubMed]
- Göke, J.; Ng, H.H. Retrotransposons and their contribution to regulation and innovation of the transcriptome. EMBO Rep. 2016, 17, 1131–1144. [Google Scholar] [CrossRef]
- Chisca, M.; Larouche, J.D.; Xing, Q.; Kassiotis, G. Antibodies against endogenous retroviruses. Immunol. Rev. 2024, 328, 300–313. [Google Scholar] [CrossRef]
- Van der Kuyl, A.C. HIV infection and HERV expression: A review. Retrovirology 2012, 9, 6. [Google Scholar] [CrossRef]
- Towler, E.M.; Gulnik, S.V.; Bhat, T.N.; Xie, D.; Gustschina, E.; Sumpter, T.R.; Robertson, N.; Jones, C.; Sauter, M.; Mueller-Lantzsch, N.; et al. Functional characterization of the protease of human endogenous retrovirus, K10: Can it complement HIV-1 protease? Biochemistry 1998, 37, 17137–17144. [Google Scholar] [CrossRef]
- Padow, M.; Lai, L.; Fisher, R.J.; Zhou, J.C.; Wu, X.; Kappes, J.C.; Towler, E.M. Analysis of human immunodeficiency virus type 1 containing HERV-K protease. AIDS Res. Hum. Retroviruses 2000, 16, 1973–1980. [Google Scholar] [CrossRef]
- Ogata, T.; Okui, N.; Sakuma, R.; Kobayashi, N.; Kitamura, Y. Integrase of human endogenous retrovirus K-10 supports the replication of replication-incompetent Int- human immunodeficiency virus type 1 mutant. Jpn. J. Infect. Dis. 1999, 52, 251–252. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Y.; Zhang, Y.; Yang, D.; Sun, Y. Functions of the fusogenic and non-fusogenic activities of Syncytin-1 in human physiological and pathological processes. Biochem. Biophys. Res. Commun. 2025, 761, 151746. [Google Scholar] [CrossRef] [PubMed]
- An, D.S.; Xie, Y.; Chen, I.S.Y. Envelope Gene of the Human Endogenous Retrovirus HERV-W Encodes a Functional Retrovirus Envelope. J. Virol. 2001, 75, 3488–3489. [Google Scholar] [CrossRef]
- Contreras-Galindo, R.; Almodóvar-Camacho, S.; González-Ramírez, S.; Lorenzo, E.; Yamamura, Y. Short communication: Comparative longitudinal studies of HERV-K and HIV-1 RNA titers in HIV-1-infected patients receiving successful versus unsuccessful highly active antiretroviral therapy. AIDS Res. Hum. Retroviruses 2007, 23, 1083–1086. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bergallo, M.; Marozio, L.; Botta, G.; Tancredi, A.; Daprà, V.; Galliano, I.; Montanari, P.; Coscia, A.; Benedetto, C.; Tovo, P.A. Human Endogenous Retroviruses Are Preferentially Expressed in Mononuclear Cells from Cord Blood Than from Maternal Blood and in the Fetal Part of Placenta. Front Pediatr. 2020, 14, 244. [Google Scholar] [CrossRef]
- Nali, L.H.S.; Oliveira, A.C.S.; Alves, D.O.; Caleiro, G.S.; Nunes, C.F.; Gerhardt, D.; Succi, R.C.M.; Romano, C.M.; Machado, D.M. Expression of human endogenous retrovirus K and W in babies. Arch. Virol. 2017, 162, 857–861. [Google Scholar] [CrossRef]
- Garcia-Montojo, M.; Fathi, S.; Norato, G.; Smith, B.R.; Rowe, D.B.; Kiernan, M.C.; Vucic, S.; Mathers, S.; van Eijk, R.P.A.; Santamaria, U.; et al. Inhibition of HERV-K (HML-2) in amyotrophic lateral sclerosis patients on antiretroviral therapy. J. Neurol. Sci. 2021, 15, 117358. [Google Scholar] [CrossRef]
- Li, M.; Yu, F.; Zhu, B.; Xiao, J.; Yan, C.; Yang, X.; Liang, X.; Wang, F.; Zhang, H.; Zhang, F. Interactions between human immunodeficiency virus and human endogenous retroviruses. J. Virol. 2025, 99, e02319-24. [Google Scholar] [CrossRef]
- Serrao, E.; Wang, C.-H.; Frederick, T.; Lee, C.-L.; Anthony, P.; Arribas-Layton, D.; Baker, K.; Millstein, J.; Kovacs, A.; Neamati, N. Alteration of select gene expression patterns in individuals infected with HIV-1. J. Med. Virol. 2014, 86, 678–686. [Google Scholar] [CrossRef]
- Morandi, E.; Tanasescu, R.; Tarlinton, R.E.; Constantin-Teodosiu, D.; Gran, B. Do antiretroviral drugs protect from multiple sclerosis by inhibiting expression of MS-associated retrovirus? Front. Immunol. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Contreras-Galindo, R.; González, M.; Almodovar-Camacho, S.; González-Ramírez, S.; Lorenzo, E.; Yamamura, Y. A new Real-Time-RT-PCR for quantitation of human endogenous retroviruses type K (HERV-K) RNA load in plasma samples: Increased HERV-K RNA titers in HIV patients with HAART non-suppressive regimens. J. Virol. Methods 2006, 136, 51–57. [Google Scholar] [CrossRef]
- Tyagi, R.; Li, W.; Parades, D.; Bianchet, M.A.; Nath, A. Inhibition of human endogenous retrovirus-K by antiretroviral drugs. Retrovirology 2017, 14, 21. [Google Scholar] [CrossRef]
- Lennox, J.L.; Landovitz, R.J.; Ribaudo, H.J.; Ofotokun, I.; Na, L.H.; Godfrey, C.; Kuritzkes, D.R.; Sagar, M.; Brown, T.T.; Cohn, S.E.; et al. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naïve volunteers infected with HIV-1: A Randomized, controlled equivalence trial. Ann. Intern. Med. 2014, 161, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Fauci, A.S. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009, 9, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bergallo, M.; Galliano, I.; Pirra, A.; Daprà, V.; Licciardi, F.; Montanari, P.; Coscia, A.; Bertino, E.; Tovo, P.-A. Transcriptional activity of human endogenous retroviruses is higher at birth in inversed correlation with gestational age. Infect. Genet. Evol. 2019, 68, 273–279. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pau, A.; Galliano, I.; Gambarino, S.; Clemente, A.; Montanari, P.; Calvi, C.; Tovo, P.-A.; Bergallo, M. HIV-1 and Antiretroviral Therapy Modulate HERV Pol and Syncytin Gene Expression in Mothers and Newborns. Microbiol. Res. 2025, 16, 116. https://doi.org/10.3390/microbiolres16060116
Pau A, Galliano I, Gambarino S, Clemente A, Montanari P, Calvi C, Tovo P-A, Bergallo M. HIV-1 and Antiretroviral Therapy Modulate HERV Pol and Syncytin Gene Expression in Mothers and Newborns. Microbiology Research. 2025; 16(6):116. https://doi.org/10.3390/microbiolres16060116
Chicago/Turabian StylePau, Anna, Ilaria Galliano, Stefano Gambarino, Anna Clemente, Paola Montanari, Cristina Calvi, Pier-Angelo Tovo, and Massimiliano Bergallo. 2025. "HIV-1 and Antiretroviral Therapy Modulate HERV Pol and Syncytin Gene Expression in Mothers and Newborns" Microbiology Research 16, no. 6: 116. https://doi.org/10.3390/microbiolres16060116
APA StylePau, A., Galliano, I., Gambarino, S., Clemente, A., Montanari, P., Calvi, C., Tovo, P.-A., & Bergallo, M. (2025). HIV-1 and Antiretroviral Therapy Modulate HERV Pol and Syncytin Gene Expression in Mothers and Newborns. Microbiology Research, 16(6), 116. https://doi.org/10.3390/microbiolres16060116





